Abstract
Purpose
To determine the prevalence of insulin-like growth factor-1 (IGF-1) normalization with long-term multimodality therapy in a pituitary center and to assess changes over time.
Methods
Patients with acromegaly (N = 409), with ≥1 year of data after surgery and at least 2 subsequent clinic visits were included in long-term analysis (N = 266). Biochemical data, clinical characteristics, and therapeutic interventions were reviewed retrospectively.
Results
At diagnosis, mean [standard deviation] age was 43.4 [14.3] years, body mass index was 28.5 (24.9–32.1) kg/m2 (median, interquartile range), serum IGF-1 index (IGF-1 level/upper limit of normal) was 2.3 [1.7–3.1], and 80.5% had macroadenomas. Patients with transsphenoidal surgery after 2006 were older [46.6 ± 14.3 vs 40.0 ± 13.4 years; P < 0.001]. Age and tumor size correlated inversely. Overall (N = 266), 93.2% achieved a normal IGF-1 level during 9.9 [5.0–15.0] years with multimodality therapy. The interval to first normal IGF-1 level following failed surgical remission was shorter after 2006: 14.0 (95% confidence interval, 10.0–20.0) versus 27.5 (22.0–36.0) months (P = 0.002). Radiation therapy and second surgery were rarer after 2006: 28 (22%) versus 62 (47.0%); P < 0.001 and 12 (9.4%) versus 28 (21.2%); P = 0.010, respectively. Age at diagnosis increased over time periods, possibly reflecting increased detection of acromegaly in older patients with milder disease. Male gender, older age, smaller tumor and lower IGF-1 index at diagnosis predicted long-term sustained IGF-1 control after surgery without adjuvant therapies.
Conclusion
The vast majority of patients with acromegaly can be biochemically controlled with multimodality therapy in the current era. Radiotherapy and repeat pituitary surgery became less frequently utilized over time. Long-term postoperative IGF-1 control without use of adjuvant therapies has improved.
Keywords: Acromegaly, medical therapy, IGF-1, pituitary center, pituitary surgery, radiation therapy
Acromegaly is caused by excess secretion of growth hormone (GH), typically arising from tumors located in the pituitary gland (1). Its prevalence has been estimated at 2.8 to 13.7 per 100 000 and an incidence of 0.2 to 1.1 cases per 100 000 individuals annually (2). Excess GH increases the secretion of insulin-like growth factor-1 (IGF-1), and together elevated GH and IGF-1 levels cause multisystem comorbidities such as metabolic changes, cardiovascular disease, hypertension, sleep apnea, colonic polyps, joint and skeletal abnormalities, among many others including possibly increased risk of other neoplasms (3). Epidemiologic studies have identified demographic factors that may be predictive of outcomes (4,5). Older patients with smaller tumors, milder disease, and lower GH levels have been more frequently diagnosed over the last decade compared to the prior one (6) and are associated with better response to medical therapies (4,5,7,8). In addition, younger individuals with acromegaly tend to have more aggressive tumors. For example, genetic mutations are more commonly found in patients diagnosed with gigantism or in patients at a younger age who are more likely to develop recurrences and often are resistant to somatostatin analog therapy (1,9,10).
Experienced dedicated pituitary neurosurgeons have been reported to achieve a higher rate of biochemical remission with primary surgery than those with less experience, ranging from 85% to 91% of patients with microadenomas and 40% to 66% for patients with macroadenomas, results that are similar comparing different surgical techniques (3,11–15). Nevertheless, persistent disease after transsphenoidal surgery (TSS) or subsequent recurrences cause an extensive economic burden (16) as well as decreased quality of life (17,18). Among options for patients with persistent disease, consecutive surgeries and/or radiation therapy may result in biochemical control as well as reduction or stabilization of tumor remnants (19). Medical therapies such as somatostatin analogs (SSAs), dopamine agonists, and the GH receptor antagonist have been widely studied in the management of patients with persistent or recurrent acromegaly after surgery and also may be used as an alternative to primary TSS (20). First-generation long-acting SSAs (octreotide long-acting release and lanreotide depot) are considered first-line options in patients with recurrent or persistent acromegaly and in selected cases as primary medical therapy, showing variable disease control that range from 38% to 85% depending on study design, dose, and frequency of administration, with similar findings when comparing both formulations (21,22). Pegvisomant (PEG) has been shown to achieve IGF-1–level normalization in 89% to 100% of patients in initial clinical trials (23–25) and 73% in more recent real-life cohorts (26). Dopamine agonists, and specifically cabergoline, can be used as a monotherapy or in combination with SSAs or PEG (or both) in order to improve disease control, resulting in normalization of IGF-1 levels in 34% to 39% of patients as a monotherapy and 37% to 52% in combination with SSAs (27–29). Pasireotide is a second-generation SSA that has demonstrated benefits in 15% to 20% of patients resistant to first-generation SSAs but is also approved as first-line therapy (30,31). While better efficacy has been shown with pasireotide, adverse events such as hyperglycemia have been shown to occur in more than 50% of patients (30,31).
Clinical characteristics of patients with acromegaly and their associated comorbidities have been reported in large cohorts (6,32). Information on the efficacy of different treatment modalities in acromegaly has been widely published, but long-term outcomes after many years of multimodality therapies and the baseline prognostic factors associated with such outcomes have been scarcely reported (4,33,34). Hence, this report evaluates the proportion of patients achieving IGF-1–level normalization among a large cohort of patients with acromegaly who received multimodality therapies and long-term management at a pituitary center.
Primary objectives were to determine 1) prevalence of normalization of IGF-1 levels in patients with acromegaly and comparison across different time periods; 2) prevalence of use of second surgery, radiation therapy, and medical therapies and comparison of the frequency of administration across different time periods; and 3) the time interval to normalization of IGF-1 levels in patients who did not achieve surgical remission and comparison across different time periods. Secondary goals were to evaluate 1) the association of baseline characteristics with long-term IGF-1–level normalization and 2) the association between baseline characteristics and the use of repeat surgery and/or radiation therapy.
Material and Methods
Subjects
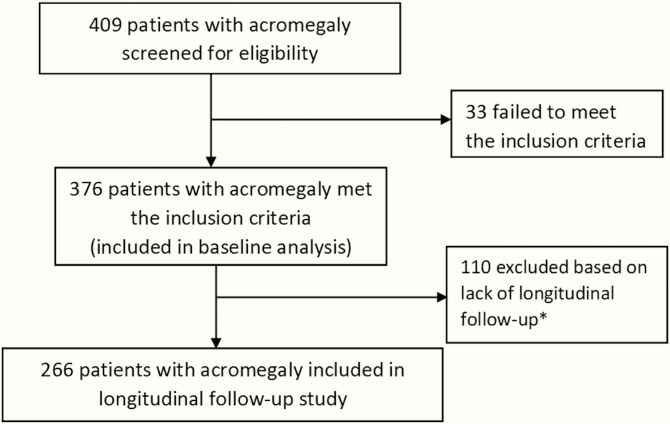
The study was approved by the Institutional Review Board of Partners Healthcare. Consecutive records (N = 409) of patients with a clinical diagnosis of acromegaly seen at a single pituitary center from January 1980 through July 2019 were reviewed (Fig. 1). The criteria for inclusion included confirmed biochemical diagnosis of acromegaly (elevated IGF-1 level and/or nonsuppressed GH after a 75-g oral glucose tolerance test [OGTT]), neuroimaging compatible with a pituitary adenoma, available medical records, and, in those treated surgically, a pathologically confirmed GH-secreting adenoma. These inclusion criteria were fulfilled by 376 individuals. Data were extracted, when available, from electronic records including demographics, biochemical parameters, radiographic findings, and relevant clinical information regarding treatment. Tumor size was recorded as stated either by official magnetic resonance imaging (MRI) reports by a neuroradiologist and/or clinical experts at the pituitary center.
Figure 1.
Flow diagram of study inclusion criteria included symptoms and signs of acromegaly, pituitary adenoma on magnetic resonance imaging, biochemical confirmation of acromegaly and pathological confirmation in patients who had surgery. *Exclusion criteria included less than 2 follow-up visits and/or less than 1 year of follow-up after initial therapy
Exclusion criteria consisted of less than a year of follow-up after treatment initiation and/or less than 2 follow-up visits at the pituitary center. Based on these exclusion criteria, 110 patients were excluded from long-term analyses but included in the baseline characterization of the cohort. Based on the exclusion criteria and fulfillment of inclusion criteria, 266 patients were included in the long-term follow-up analysis.
In order to understand how diagnosis and treatment of acromegaly have changed over time, patients in this study were categorized in 2 groups: those diagnosed before 2006 (group 1) and those diagnosed after 2006 (group 2). The year 2006 was chosen as the dividing point between the eras of therapy because this center had previously reported clinical features at diagnosis in patients with acromegaly diagnosed from 1980 to 2005 (32). Furthermore, the number of patients diagnosed with acromegaly before and after 2006 were similar, allowing for comparisons of roughly equal numbers. Also, new medical therapies became available just prior to that year and afterward, including PEG and lanreotide long-acting release that were approved by the Food and Drug Administration in the United States in 2003 and 2007, respectively.
Biochemical definitions
Biochemical confirmation of acromegaly was determined by an IGF-1 level above the upper limit of normal, expressed as an IGF-1 index > 1 and/or nonsuppressed GH after standard OGTT, defined as a GH nadir > 1 ng/mL. In order to account for assay differences, the IGF-1 index was calculated by dividing IGF-1 levels by the age and gender-adjusted upper limit of normal determined at each laboratory. Assays used for measuring IGF-1 levels varied over time. After 2013,liquid chromatography mass spectrometry by Quest Diagnostics (200 Forest St, Marlborough) was used to measure IGF-1 levels. Between 2005 and 2013, Immulite (333 Coney St, East Walpole, MA 02032) 2000 immunoassay system by Siemens Medical Systems (333 Coney St, East Walpole, MA 02032) was used. Between 2003 and 2005, Nichols Advantage was used. Before 2003, measurements were performed at outside institutions using immunoradiometric assays, radioimmunoassays, and immunochemiluminometric assays. Other assays were used in some cases, including liquid chromatography mass spectrometry by Mayo Labs (3050 Superior Dr NW, Rochester, MN 55901), and an immunochemiluminometric assay by LabCorp (531 South Spring Street Burlington, North Carolina 27215). GH levels were assessed using an immunoassay by Quest Diagnostics from 2014 onward; Immulite was used before 2014. As most patients did not have glucose-suppressed GH levels available at diagnosis, fasting, random, or nadir levels were pooled for analysis.
MRI scans, or in rare cases, computed tomography in those diagnosed before MRI or with contraindications to MRI, were available and were analyzed by experienced neuroradiologists, neurosurgeons, or neuroendocrinologists to assess maximal tumor diameter at baseline and in long-term follow-up. Biochemical control was defined as a normal IGF-1 index and, when available, a normal GH suppression after OGTT (ie, GH < 1 ng/mL) at least 3 months after the first surgery. Time to first normal IGF-1 index was determined. Recurrence was defined as biochemical control sustained for at least 6 months after initial TSS followed by IGF-1 index elevation and/or abnormal GH suppression after OGTT in long-term follow-up.
Statistical analysis
Data were analyzed using JMP Statistical Discoveries (version 12; SAS Institute, Inc., Cary, NC). For continuous variables, data were plotted and tested for normal distribution using the Shapiro-Wilk test. In variables with normal distributions, mean and standard deviation (SD) were reported. Since most variables were not normally distributed, median and interquartile range (IQR; 25th and 75th percentiles) are shown unless otherwise specified. Data spreads are presented using boxplots with medians and IQRs, with whiskers showing 1.5 times the IQR. Statistical comparisons were performed using t tests and Mann-Whitney tests for normally and not normally distributed continuous variables, respectively. Nominal variables were compared using the Chi-square and Fisher’s exact test. A log-rank test was used to assess the time to IGF-1 normalization in patients who did not achieve IGF-1 normalization after first-line surgery and required second-line therapy. Logistic regression analyses were used to assess predictive factors for long-term outcomes. For all analyses, a two-tailed P value of less than 0.05 was considered statistically significant. A Bonferroni test was used to adjust the P values when performing multiple comparisons. P values reported as adjusted were based on a Bonferroni correction.
Results
Baseline clinical characteristics at diagnosis
Mean [SD] age at diagnosis of patients who met inclusion criteria (N = 376) was 43.4 [14.3] years, ranging from 9 to 80 (10 [2.7%] patients were under age 18 years), and 208 (55.3%) were female. Median body mass index at diagnosis was 28.5 (24.9–32.1) kg/m2. Median IGF-1 index at diagnosis was 2.3 (1.7–3.1), with a GH of 10.2 (5.6–21.6) ng/mL (including both random [N = 234] and nadir GH [N = 87]). Macroadenomas were present in 80.5% of the patients (N = 359), with a median maximum tumor diameter of 1.5 (1.1–2.4) cm. Age, tumor size, and GH level at diagnosis did not differ by gender. Females had significantly lower median body mass index, IGF-1 index, and IGF-1 level at diagnosis than males (27.0 [23.5–31.1] vs 30.2 [26.8–32.8] kg/m2, P = 0.002; 2.1 [1.6–2.8] vs 2.5 [1.9–3.3], P < 0.001; and 741 [552–949] vs 887 [649–1110] ng/mL, P < 0.001, respectively).
Mean age at diagnosis was significantly higher in patients diagnosed after 2006 compared to the earlier cohort (46.6 ± 14.3 vs 40.0 ± 13.4 years; P < 0.001) even when comparing males and females separately. While IGF-1 levels were higher in group 1 (868 [633–1099] vs 745 [547–989] ng/mL; P = 0.002), IGF-1 index was not significantly different between groups (2.2 [1.7–2.8] vs 2.5 [1.7–3.2]; P = 0.079). The remaining variables did not show significant differences between groups.
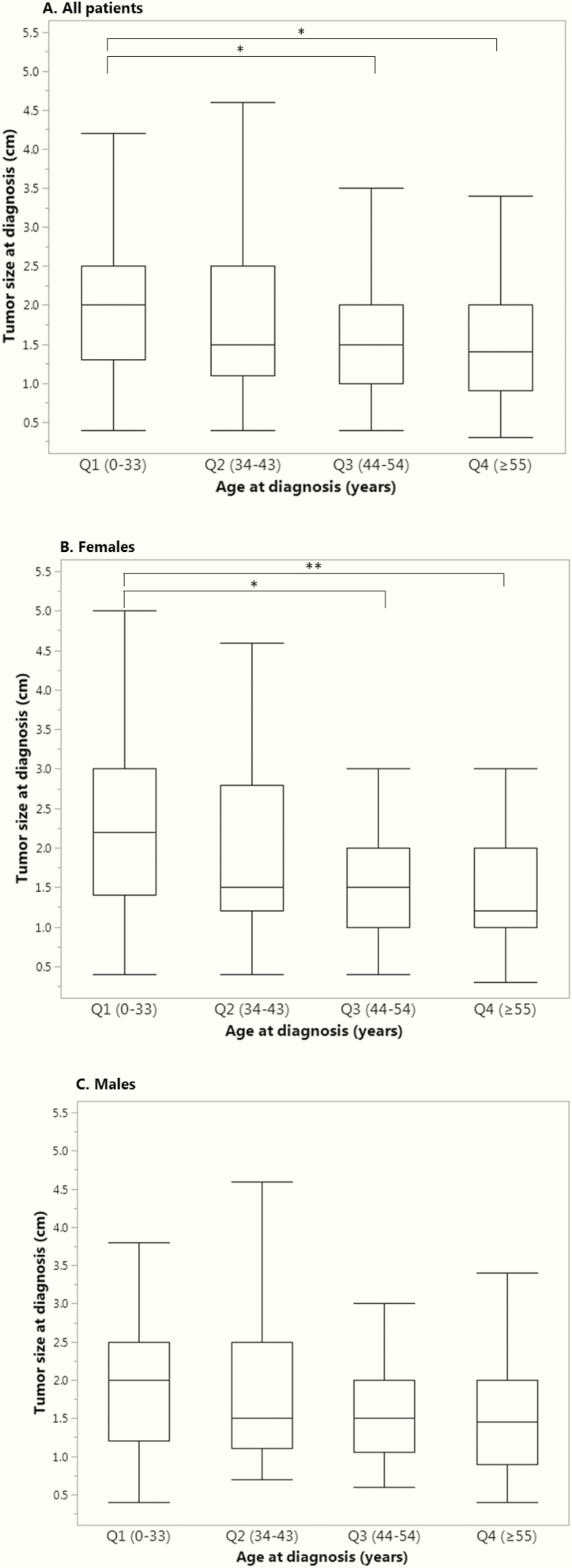
When divided by quartiles (Qs) of age, pairwise analysis showed that median tumor size (Fig. 2A) was significantly smaller among older patients (Q4 1.4 [0.9–2.0] vs Q1 2.0 [1.3–2.5] cm, adjusted P < 0.001; and Q3 1.5 [1.0–2.0] cm vs Q1, adjusted P < 0.001). When analyzing female patients alone (Fig. 2B), median tumor size was smaller in older women (Q4 1.2 [1.0–2.0] vs Q1 2.2 [1.4–3.0] cm, adjusted P = 0.004; and Q3 1.5 [1.0–2.0] vs Q1 cm, adjusted P = 0.002). However, when analyzing male patients alone (Fig. 2C), median tumor size was not significantly different between age subgroups. Regarding GH levels, older patients had significantly lower median values compared with younger patients (Q4 9.0 [3.3–18.3] vs Q1 14.2 [8.4–36.3] ng/mL, adjusted P = 0.017). Similarly, median IGF-1 levels decreased with older-age quartiles (Q1 918.0 [709.5–1096.0] vs Q4 730.0 [583.3–904.5] cm, adjusted P = 0.002; and Q1 vs Q3 732.0 [539.3–997.8] cm, adjusted P = 0.010). In contrast, IGF-1 index increased significantly with older age (Q4 2.8 [2.1–3.6] vs Q1 1.9 [1.4–2.5] cm, adjusted P < 0.001; and Q3 2.4 [1.7–3.1] cm vs Q1, adjusted P = 0.022; and Q4 vs Q2 2.3 [1.6–3.0], adjusted P = 0.003), likely reflecting changes in age-based normative ranges. Patients with microadenomas had significantly lower median GH and IGF-1 levels at diagnosis compared to macroadenomas (5.9 [2.9–10.0] vs 13.0 [6.6–25.5] ng/mL, P < 0.001; and 622.0 [456.3- 777.3] vs 845.0 [632.0–1030.0] ng/mL, P < 0.001, respectively). Regarding IGF-1 index, a trend toward lower values in microadenomas was observed (2.1 [1.3–3.0] vs 2.4 [1.7–3.1]; P = 0.093). Smaller tumor size was correlated with lower IGF-1 levels (R2 = 0.032; P = 0.002). In summary, overall at baseline, older patients had smaller tumors (Fig. 2A) and lower IGF-1 levels.
Figure 2.
Clinical characteristics and age of diagnosis.Tumor size was defined as maximal diameter (cm) on magnetic resonance imaging at time of diagnosis. Age groups shown include quartiles 1 through 4 corresponding to age Q1 (0–33), Q2 (34–43), Q3 (44–54), Q4 (≥55) years old, respectively.
A. Tumor size and age of diagnosis for all the patients (N = 325), *P < 0.001
B. Tumor size and age of diagnosis in women (N = 178), *P = 0.004, **P = 0.002.
C. Tumor size and age of diagnosis in men (N = 147) (no significant difference between groups).
Twenty-two patients were tested for an multiple endocrine neoplasia type 1 gene mutation, and all were negative. Twenty-three patients were tested for an aryl hydrocarbon receptor-interacting protein gene mutation; such a mutation was identified in 1 patient. Fourteen (3.9%) patients had a family history of a pituitary tumor and 7 (1.9%) had a history of acromegaly in any relative (N = 360 available).
Baseline clinical characteristics in long-term follow-up cohort
Baseline characteristics were also analyzed within the subset of patients with sufficient follow-up data as shown in Table 1 (N = 266 patients). The differences in baseline characteristics between group 1 and group 2 in this subset with long-term follow-up were the same as described above for all patients that met the inclusion criteria regarding a lower mean age and a higher median IGF-1 level in group 1. Similar to the group as a whole, the subset with long-term follow-up demonstrated no differences in baseline median tumor size or IGF-1 index stratified by the era of diagnosis.
Table 1.
Baseline Characteristics of Patients Included in Long-term Follow-Up (N = 266)
| Baseline characteristics | All (N = 266) | Group 1 (N = 136) | Group 2 (N = 130) | P-value |
|---|---|---|---|---|
| Age at diagnosis (years) | 42.5 ± 14.1 | 39.8 ± 13.9 | 45.3 ± 13.9 | 0.001 |
| BMI at diagnosis (kg/m2) | 28.3 (25.3–32.0) | 28.4 (24.2–31.3) | 28.1 (25.3–32.1) | 0.429 |
| Female (%) | 155 (58.3%) | 84 (61.8%) | 71 (54.6%) | 0.237 |
| GH at diagnosis (ng/mL)b | 10.3 (5.5–22.8) | 11.1 (5.9–23.6) | 10.0 (4.4–21.5) | 0.710 |
| IGF-1 level at diagnosis (ng/mL) | 782 (601–1024) | 848 (634–1099) | 739 (542–1002) | 0.012 |
| IGF-1 index at diagnosis | 2.3 (1.6–3.0) | 2.2 (1.6–2.7) | 2.4 (1.6–3.2) | 0.262 |
| Maximum tumor size at diagnosis (cm) | 1.6 (1.1–2.5) | 1.8 (1.2–2.5) | 1.5 (1.0–2.4) | 0.062 |
| Macroadenoma (%) | 208 (82.5%)a | 105 (84.7%) | 103 (80.5%) | 0.379 |
Age is described as mean ± standard deviation due to normal distribution; all other variables are median [Q1-Q3]. Random and fasting GH are pooled (nadir GH is used in some patients). Maximum tumor size refers to the single largest diameter on MRI. BMI: body mass index; GH: growth hormone; IGF-1: insulin-like growth factor-1; MRI, magnetic resonance imaging; OGTT, oral glucose tolerance test; Q, quartile. aN = 252 with available information on tumor size category. bGH includes nadir levels on OGTT and fasting or random levels based on availability.
Therapeutic modalities
Different therapeutic modalities are depicted in Table 2. First-line surgery was performed in 259 (97.7%) individuals, similarly distributed in both groups. Some patients had surgery in an outside hospital before ongoing management at the pituitary center, but most, 188 (72.6%) patients, underwent TSS at the pituitary center. Preoperative medical therapy was administered in 45 (17.4%) patients, which was usually prescribed with the intention of reducing perioperative risk in those with severe comorbidities. This modality was significantly more common among patients in group 2 compared with group 1 (32 [25.2%] vs 13 [9.8%]; P = 0.001). Of those who received preoperative medical therapy, 36 (80%) were treated with SSA, 5 (11.1%) with PEG, 1 (2.2%) with bromocriptine, and 7 (15.6%) with cabergoline, either alone or in combination. No differences were found between patients who received preoperative medical therapy and those who did not in terms of median tumor size, median IGF-1 index, mean age at diagnosis, and in achieving and sustaining IGF-1 normalization after first-line surgery. Among patients who fulfilled inclusion criteria (N = 376), 11 (2.9%) had primary medical therapy without surgery. Four of these 11 (36.4%) met criteria for exclusion from long-term analysis. Seven patients, 2.6% of the long-term follow-up cohort (N = 266), received only medical therapy with no surgery due to patients’ preference and/or high risk of perioperative complications.
Table 2.
Treatment Used and Normalization of IGF-1 Index During Long-term Follow-up
| All (N = 266) | Group 1 (N = 136) | Group 2 (N = 130) | P | |
|---|---|---|---|---|
| Initial treatment modality | ||||
| Surgery | 259 (97.7%) | 132 (97.1%) | 127 (97.7%) | 1.000 |
| Preoperative medical therapy | 45 (17.4%) | 13 (9.8%) | 32 (25.2%) | 0.001 |
| Medical therapy only (no surgery) | 7 (2.6%) | 4 (2.9%) | 3 (2.3%) | 1.000 |
| Treatment after surgery | ||||
| Observation (no second-line therapy)a | 78 (30.1%) | 30 (22.7%) | 48 (37.8%) | 0.008 |
| Repeat surgery (%)b | 40 (15.4%) | 28 (21.1%) | 12 (9.4%) | 0.010 |
| Radiation therapy (%)b | 90 (34.7%) | 62 (47.0%) | 28 (22.0%) | <0.001 |
| Medical therapy (any time after surgery (%) | 165 (63.7%) | 88 (66.7%) | 77 (60.6%) | 0.312 |
| Medical therapy subgroup | ||||
| SSA | 151 (91.5%) | 81 (92.0%) | 70 (90.9%) | 0.794 |
| Dopamine agonist | 75 (45.4%) | 36 (40.9%) | 39 (50.6%) | 0.210 |
| Cabergoline | 69 (41.8%) | 30 (34.1%) | 39 (50.6%) | 0.031 |
| Bromocriptine | 6 (3.6%) | 6 (6.8%) | 0 | 0.030 |
| Pegvisomant | 69 (41.8%) | 38 (43.2%) | 31 (40.3%) | 0.704 |
| Normalization of IGF-1 index over time | ||||
| Prevalence of normal IGF-1 at last follow-up | 248 (93.2%) | 130 (95.6%) | 118 (90.8%) | 0.118 |
| Time to last follow-up (years) | 9.9 [5.0–15.0] | 15.0 [11.0–20.0] | 6.0 [3.0–8.6] | <0.001 |
Abbreviations: IGF-1, insulin-like growth factor-1; SSA, somatostatin analogs. aAll patients who received no treatment after surgery had a normal IGF-1 index at last follow-up. bOverall 27 (10.4%) patients had both repeat surgery and radiation therapy after first surgery, 21 (15.9%) in group 1 vs 6 (4.7%) in group 2; P = 0.003.
Among patients with sufficient follow-up to be included in the longitudinal analysis, 78 (30.1%) patients received no further therapy after first surgery and maintained a normal IGF-1 index at time of last follow-up. The absence of use of any therapy after surgery was more common among patients in group 2 compared with group 1 (48 [37.8%] vs 30 [22.7%]; P = 0.008). In those patients who underwent first-line surgery, postoperative radiation therapy and repeat surgery were used in 34.7% and 15.4%, respectively. In patients diagnosed in the latest era, these modalities were used less frequently than in those diagnosed before 2006 (radiation therapy was used in 47.0% of patients in group 1 vs 22% in group 2, P < 0.001; and 21.1% vs 9.4% required a second surgery, respectively, P = 0.01).
Among 259 patients who underwent surgery and had sufficient follow-up for longitudinal analysis, 165 (63.7%) received postoperative medical therapy, as shown in Table 2. SSAs were the most commonly used class of medications, either alone or in combination with other medical therapies. At the time of last follow-up, no differences between the 2 groups were found when analyzing the type of medication used (monotherapy or combination) except for cabergoline, which was slightly more frequent in the group diagnosed after 2006 (8.5% vs 1.5%; P = 0.009).
Among the subset of patients who did not achieve normal IGF-1 index after first surgery (N = 157), the percentage of patients who received medical therapy of any class was higher in group 2 than in group 1 (70 [98.6%] vs 75 [87.2%]; P = 0.013) (data not shown in table). Radiation therapy (26 [36.6%] vs 57 [66.3%]; P < 0.001) and second surgery (10 [14.1%] vs 24 [27.9%]; P = 0.034) remained significantly less frequent in this subset. In summary, for patients who did not achieve biochemical remission after initial surgery, second surgery and/or radiation therapy were used more frequently in the prior era, while medical therapy was more commonly used in the recent era.
Time to IGF-1 index normalization
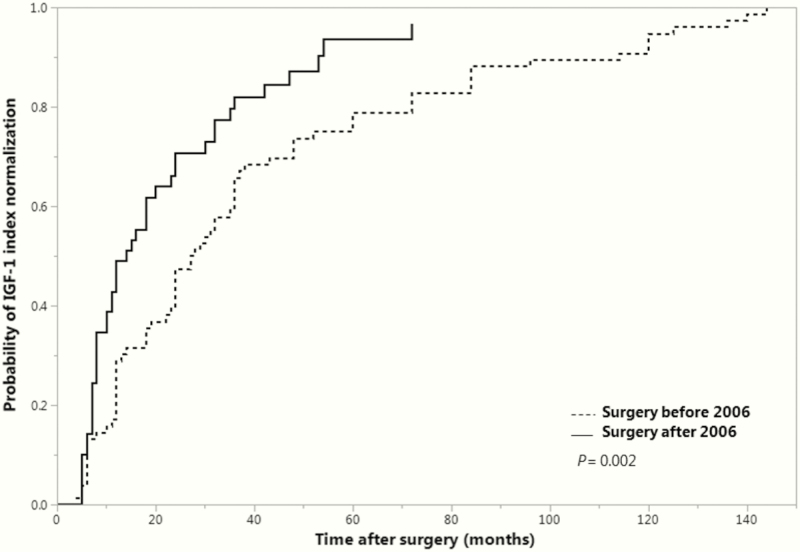
Time to first normal IGF-1 index was assessed using Kaplan-Meier analysis and the log-rank test in patients who did not achieve IGF-1 index normalization after first-line surgery and required any second-line therapy (Fig. 3). Patients with preoperative medical therapy and/or normal IGF-1 index before surgery were excluded from this analysis. Significantly less time was needed for normalization of IGF-1 index among patients diagnosed after 2006 compared with those diagnosed before (χ 2(1) = 9.567; P = 0.002), with a median of 14.0 months (95% confidence interval, 10.0–20.0) versus 27.5 months (22.0–36.0), respectively, (group 1, N = 76; group 2, N = 49 [5 censored]). The type of drug used at first normal IGF-1 index was not significantly different between patients who had surgery before or after 2006. SSAs were the drug most often used, either alone or in combination in both periods.
Figure 3.
Time to first normal IGF-1 after surgery in patients who did not achieve normal IGF-1 after surgery. IGF-1, insulin-like growth factor-1.
Recurrences
Twenty-four patients presented with recurrent disease after a period of IGF-1–level normalization (9.0% of the total cohort with enough follow-up), 16 (66.7%) were female, with a median time to recurrence of 48 (24–96) months. Six patients had their first surgery at outside hospitals. Sixteen of them (66.7%) had their first surgeries before 2006. Median IGF-1 index at recurrence was 1.2 (1.1–1.3), and GH values, either nadir or random, were available for 14 patients with a median of 1.0 (0.5–1.3) ng/mL. Stable remnant tissue was observed in 29.2% of patients at time of recurrence, whereas 33.3% had tumor growth. Absence of tumor on imaging was observed at the time of biochemical recurrence in 37.5% of patients. Six (25%) patients with recurrent disease underwent repeat TSS in order to excise remnant tumor, and 7 received radiation therapy (2 patients underwent both modalities). Medical therapy was used in 20 of them (16 with SSA, 6 with PEG, and 10 with dopamine agonists, either alone or in combination with each other). At the time of last follow-up, 21 out of 24 (87.5%) patients had normal IGF-1 index. A logistic regression analysis was performed to ascertain the effects of age, gender, tumor size, and IGF-1 index at diagnosis as well as family history of pituitary disease and era of surgery on the likelihood that patients develop a high IGF-1 after initial surgical remission. None of these factors were significant predictors.
Outcomes at last follow-up
Overall, normalization of IGF-1 index in patients with sufficient long-term follow-up at the pituitary center was achieved in 248 (93.2%) patients, with similar results in those diagnosed before and after 2006 (130 [95.6%] vs 118 [90.8%]; P = 0.118), demonstrating biochemical control in the vast majority of patients. Of the 248 patients with a normal IGF-1 level at last follow-up, 212 had an available MRI within 1 year. All of these scans revealed stable or absent tumor. No patient with a normal IGF-1 index had evidence of tumor growth at last follow-up. The median follow-up time was 9.9 [5.0–15.0] years (Table 2). Seven patients received primary medical therapy only, and 5 had a normal IGF-1 index at last follow-up. In summary, at last follow-up (a median of 10 years after surgery) almost all patients had a normal IGF-1 with controlled tumor.
At last follow-up, 50 of 157 (31.8%) patients who had not achieved normal IGF-1 index after surgery were not receiving medication. All these patients underwent radiation therapy (N = 47) and/or repeat surgery (N = 18) and had IGF-1 levels within the normal range at the time of last assessment. Abnormal levels of IGF-1 were recorded at last follow-up in 18 (6.8%) patients. The primary reasons for suboptimal biochemical control included noncompliance (N = 8), lack of access to medication or insurance coverage (N = 4), or side effects/intolerance (N = 3). Additionally, 2/18 were still undergoing adjustment of therapy at the time of last follow-up (1 patient was waiting for radiation therapy and 1 was undergoing medical therapy change). The remaining patient (1/18) had only mildly elevated IGF-1 levels, a normal GH nadir on OGTT, and no clinical symptoms of acromegaly at last follow-up after a surgical remission a decade before.
Overall, hypopituitarism, including at least 1 deficient axis was present in 55.6% of patients (95/171) with available data on pituitary function and with a significantly higher rate of any pituitary deficiency before 2006 compared to after 2006 (67.0% [61/91] vs 42.5% [34/80], respectively, P = 0.001). Two or more axes were deficient in 25.7% (44/171) of patients, with a higher prevalence before versus after 2006 (33.0% [30/91] vs 17.5% [14/80; P = 0.021, respectively). Only 6 patients had deficiencies of all 4 pituitary hormonal axes, and all of these patients were diagnosed before 2006. Among patients with radiation therapy, 86.4% (51/59) had at least 1 hormonal axis deficiency at last follow-up versus 39.3% (44/112) in those who never received radiation therapy (P < 0.001). At least 2 hormonal deficiencies were present at last follow-up in 55.9% (33/59) of those who had received radiation therapy versus 9.8% (11/112) among those who did not (P < 0.001). In patients who had radiation therapy, there was no significant difference in the prevalence of any hypopituitarism before versus after 2006 (90.2% [37/41] vs 77.8% [14/18]; P = 0.232). In addition, among patients with no history of radiation therapy, there were no significant differences in the prevalence of hypopituitarism between eras. Regarding mortality, 6.0% (16/266) of patients had died at time of last follow-up at a mean [SD] age of 67.1 [18.0] years. Causes of death could not be ascertained from available records. All patients diagnosed after 2006 remained alive at time of last follow-up.
Predictors of long-term biochemical control
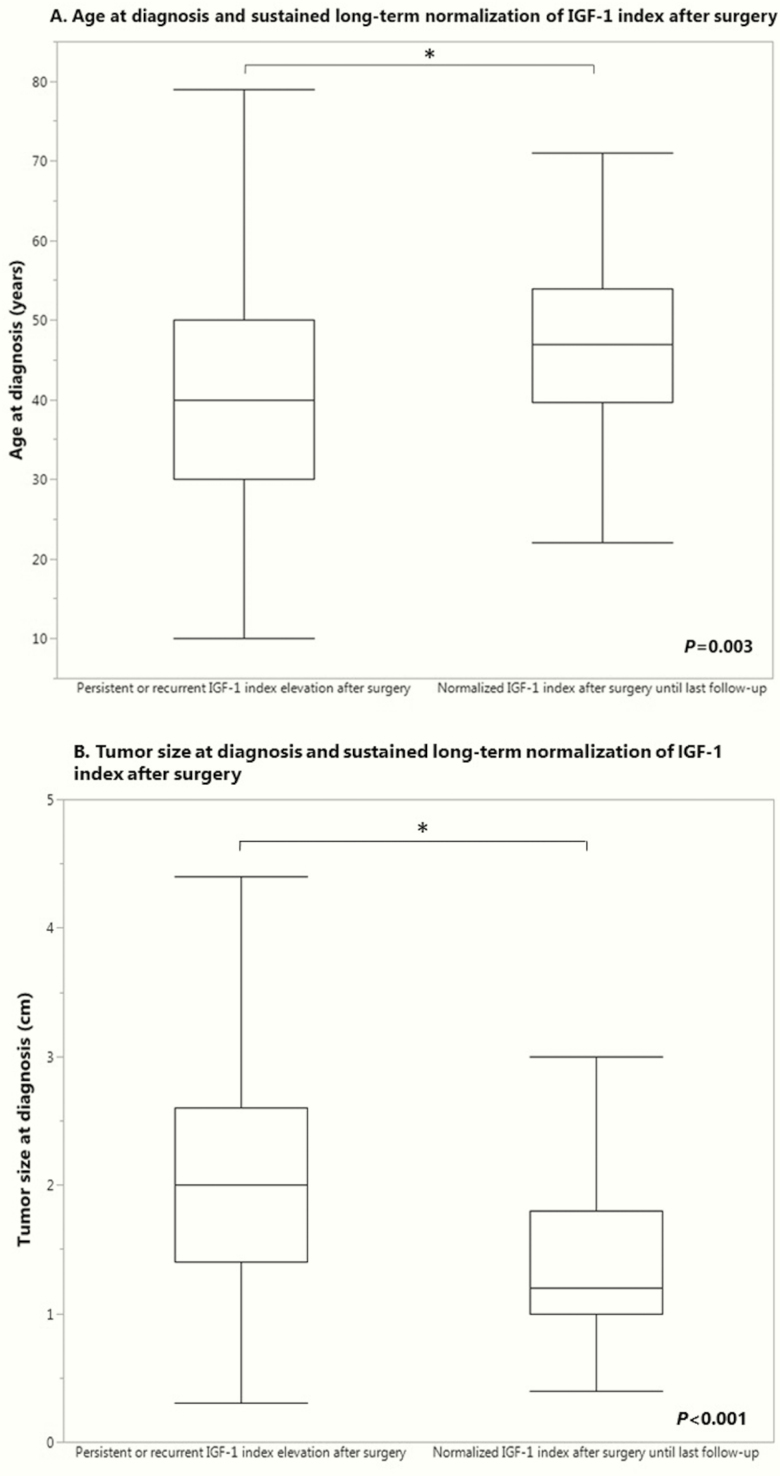
In order to determine the clinical features associated with the increased likelihood that patients achieve and remain with a normal IGF-1 index after surgery without use of other modalities, a logistic regression analysis was performed adjusting for age at diagnosis, gender, tumor size at diagnosis, IGF-1 index, family history of pituitary disease, and era of surgery. The logistic regression model was statistically significant, χ 2(6) = 60.440; P < 0.001. The model explained 35.3% (generalized R2) of the variance in achieving and sustaining a normal IGF-1 index after surgery and correctly classified 73.4% of cases. In multivariate analysis, male gender, older age (Fig. 4A), smaller tumor size (Fig. 4B), and lower IGF-1 index at diagnosis were associated with an increased likelihood of long-term sustained normalization of IGF-1 index after surgery without use of other modalities (P = 0.014, P = 0.003, P < 0.001, and P < 0.001, respectively). Males were 2.5 (95% confidence interval, 1.2–5.2) times more likely than females to achieve and remain with a normal IGF-1 after primary surgery without other therapies.
Figure 4.
Predictors of sustained postoperative normalization of IGF-1. IGF-1, insulin-like growth factor.
In order to determine the clinical features associated with the increased likelihood of requiring radiation therapy and/or repeat surgery in patients who did not achieve normal IGF-1 index after first surgery, logistic regression analysis was performed adjusting for age at diagnosis, gender, tumor size at diagnosis, IGF-1 index, family history of pituitary disease, and whether the surgery was before or after 2006. The multivariate logistic regression model was statistically significant, χ 2(6) = 31.481; P < 0.001. The model explained 31.3% (generalized R2) of the variance in achieving and maintaining a normal IGF-1 after surgery and correctly classified 72.0% of cases. Lower age at diagnosis (P = 0.027), positive history of a pituitary tumor in any relative (P = 0.038), and surgery before 2006 (P < 0.001) were found to increase the likelihood of treatment with radiation or repeat surgery. A larger tumor size at diagnosis was a borderline significant determinant (P = 0.053). A similar analysis was done to determine predictors of receiving medical therapy after surgery, and none of the factors were statistically significant.
Discussion
This study spans a long follow-up period of more than 2000 patient-years at a single pituitary center, which allows the identification of factors that ultimately contribute to IGF-1 control in acromegaly after multimodality therapies. An important finding is that with a multidisciplinary approach and long-term follow-up in a pituitary center, the vast majority of patients (93.2%) will ultimately achieve normal IGF-1 levels. This is higher than reported in prior cohorts (34–37) and shows that the disease can be controlled effectively in most cases. The continued follow-up within a multidisciplinary pituitary center may have optimized care, such that this cohort had a better outcome and/or a higher likelihood of IGF-1 control than previously described. In addition, studies with shorter follow-up periods or that focused only on a single therapeutic modality or were published in an era with fewer available medical therapies may reveal a lower rate of IGF-1 normalization than reported here (35).
Another important finding is the rate of recurrence of elevated IGF-1 levels after confirmed normalization (for at least 6 months). Nine percent of patients had a biochemical recurrence at some point during follow-up. Among those patients who recurred, most of them (66.7%) had no tumor growth or no visible remnant at the time that IGF-1 became elevated. It is also important to highlight that at the time of last follow-up, all patients with a normal IGF-1 had no visible tumor or stable tumor remnants. The fact that a subset of patients after sustained normalization of IGF-1, even with stable or absent tumor remnant, develop an elevated IGF-1 suggests the importance of ongoing monitoring of IGF-1 over time. Since no patient with a normal IGF-1 index had evidence of tumor growth at last follow-up, it seems the vast majority of patients who have had long-term normalization of IGF-1 levels and stable structural disease may not require routine ongoing serial pituitary imaging as long as IGF-1 levels remain normal. It might be argued that after some period of tumoral stability, follow-up pituitary MRI could be reserved only for those who develop elevated IGF-1 levels, using a reliable assay with validated age-based normative ranges. This strategy may help prevent unnecessary gadolinium exposure since concerns about long-term retention of the compound have become a topic of emerging potential concern (38).
An average of a 10-year follow-up period was achieved in this cohort, which provided the opportunity to identify predictors of sustained normalization of IGF-1. When analyzing patients that underwent surgery, in the absence of other modalities such as radiation therapy, repeat surgery or medical therapy, baseline smaller tumor size, older age at diagnosis, lower IGF-1 index at diagnosis and male gender were found to be significant determinants, similar to published predictors of initial remission after first surgery (4,39–41).
Surprisingly, after regression analysis, men were 2.5 times more likely than women to achieve and remain with a normal IGF-1 level after first surgery without other subsequent adjuvant therapies. Furthermore, older women were found to have smaller tumors, an association specific to female patients and not seen among males. These gender differences at diagnosis and outcomes of acromegaly have not been studied much (42); however, there is prior evidence that women with acromegaly, despite having lower baseline IGF-1 and GH levels, have an increased risk of complications such as heart failure and diabetes mellitus compared with men with acromegaly (5).
It has been shown that in more recent years there have been changes in the epidemiologic features of patients with acromegaly at diagnosis as well as in changes in treatment modalities used. Similar to findings from a large European multi-center database, it appears that the age at diagnosis has actually increased over time, possibly reflecting an increase in the detection of acromegaly in older patients with milder disease (6). A decrease in the use of radiation therapy before 1995 compared to the decade afterward through 2005 was previously reported at the site of the present study (32). Likewise, the analysis of the cohort presented in this study shows that in the subsequent era there has again been a decrease in use of radiation therapy after 2006. Furthermore, this study shows that not only the use of radiotherapy but also the intervention with repeat surgery has decreased over time. Since it is known that radiation therapy increases the risk of hypopituitarism (19), it is not surprising that patients in the earlier era who had a higher rate of radiation therapy also had more hypopituitarism. In addition, the longer duration of follow-up in the earlier era provides a greater opportunity for the development of hypopituitarism over time and may skew the data accordingly.
Other management changes that varied based on the era of the diagnosis of these patients included the increased use of preoperative medical therapy. In the more recent era, the higher prevalence of sustained normalization of IGF-1 levels after surgery only and in the absence of any adjuvant therapy suggests that in the later cohort initial surgical management of these patients is associated with a higher rate of normalization of IGF-1 than in the past, perhaps reflecting improved surgical techniques, the use of intraoperative MRI, or the inclusion of patients with less aggressive tumors. Of note, most patients (12/18) with high IGF-1 at last follow-up had failure to normalize related to noncompliance or access issues rather than lack of efficacy, suggesting that normalization of IGF-1 is now possible in nearly all patients with acromegaly.
Finally, in patients who fail to achieve normal IGF-1 after initial surgery, the time to normalization of IGF-1 level has become shorter in the more recent time period, compared with those diagnosed prior to 2006. Though the median unadjusted IGF-1 level at baseline was higher in the group diagnosed before 2006, this finding is less likely to explain the decreased time to normalization of the IGF-1 level among those diagnosed later, since the IGF-1 index did not differ between groups, and variations in unadjusted IGF-1 concentrations would be expected based on assay differences. These findings may reflect new and improved medical therapies and/or better access to therapy.
Limitations of this study include lack of growth hormone suppression tests on many patients as well as some unavailable random and fasting growth hormone levels in this cohort. However, as the main objective of this study was to report long-term IGF-1–level normalization, the lack of GH assessment in some patients is not a major limitation. Even though there are patients with acromegaly who have discrepancies between IGF-1 and GH levels (5,43,44) and remission criteria includes both GH and IGF-1 (45), it is known that IGF-1 levels better reflect signs and symptoms of acromegaly (46), and its normalization has been associated with improved mortality and lower complication rates (14). In addition, GH suppression testing after glucose loading is not a reliable metric for monitoring therapy with SSAs, and GH levels are not used in monitoring patients being treated with PEG (3,47). Genetic testing is not routinely done in patients with sporadic disease, so the exact prevalence of genetic mutations cannot be assessed. MRI tumor size was determined by experts and reported in medical records; analysis of volumetric or radiologic characteristics in T2-weighted sequences, the latter of which has been shown to be predictive of biochemical response to SSAs (48), was not performed since it was not the focus of this study. Accurate surgical cure rates cannot be assessed from the data obtained from this retrospective cohort since many patients who were placed into biochemical remission after surgery were not followed at this center and were referred to local endocrinology practices for continuing care. In addition, the actual center’s surgical rate is not reflected here because this cohort includes some patients who had initial surgery at outside institutions. This center’s previous overall surgical remission rate was reported before, achieving control in 58% of patients (14). Notably, since patients with surgical cure were less likely to have been included, the potential bias would be to lower the rate of IGF-1–level normalization at last follow-up in this study. Comprehensive pituitary function testing was not available on all patients. The prevalence of hypopituitarism is not reflective of the rate of hypopituitarism due to surgery at our center since 1) many patients had initial surgery at an outside center; 2) patients with hypopituitarism may have been more likely to continue long-term follow-up, confounding the prevalence in this cohort; and 3) most patients had macroadenomas and preexisting hypopituitarism prior to surgery may have been present. The purpose of this study was specifically to report on those patients with ongoing follow-up at a pituitary center. The strength of the study and novelty include the focus on the multimodality therapy over a long period of time in a large group that has detailed long-term follow-up.
Important next steps include improving the identification and stratification of patients who will benefit most from early multimodality therapy, allowing a shorter interval time to remission. Access and compliance are limiting factors for those who do not achieve IGF-1–level normalization; identifying and addressing these risk factors early after diagnosis may help decrease these barriers.
Conclusion
This study reveals that in the more recent era, in association with advances in medical therapies and improved surgical techniques, biochemical control without use of adjuvant therapies has become more prevalent, and time to normalization of IGF-1 levels in those with persistent elevation of IGF-1 levels after surgery has decreased. The vast majority of patients with acromegaly achieve normal IGF-1 levels with multimodality therapy and management in a pituitary center. In fact, 93.2 % of patients achieve long-term control of IGF-1 over the course of up to 38 years and a median of 10 years of postoperative follow-up. Among other factors, gender predicted long-term outcomes, with women more likely to require adjuvant therapy after surgery to control IGF-1 levels. Finally, the recurrence of high IGF-1 levels many years after achieving control, even with stable or nonvisible tumors, suggests the need for ongoing long-term monitoring of IGF-1 levels in all patients with acromegaly. These data show that the normalization of IGF-1 levels is achievable in almost all patients with acromegaly with available therapies and that access, compliance, and time are the limiting factors in those who do not achieve control.
Acknowledgments
Financial Support: The authors received no financial support.
Glossary
Abbreviations
- GH
growth hormone
- IGF-1
insulin-like growth factor
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OGTT
oral glucose tolerance test
- PEG
pegvisomant
- Q
quartile
- SD
standard deviation
- SSA
somatostatin analog
- TSS
transsphenoidal surgery
Additional Information
Disclosure Summary: A.G., P.J., F.J.G., A.F., and B.S. have nothing to declare. L.B.N. received honoraria for consulting from Pfizer and Ipsen and research funds from Ipsen and Chiasma. N.A.T. received institution-directed research support from Ipsen and Novartis. K.K.M. has received investigator-initiated grants from Pfizer and Ipsen and study medication from Pfizer.
References
- 1. Colao A, Grasso LFS, Giustina A, et al. Nat Rev Dis Primers. 2019;5(1):20. [DOI] [PubMed] [Google Scholar]
- 2. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katznelson L, Laws ER Jr, Melmed S, et al. ; Endocrine Society . Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez-Rodriguez E, Casanueva FF, Bernabeu I. Update on prognostic factors in acromegaly: Is a risk score possible? Pituitary. 2015;18(3):431–440. [DOI] [PubMed] [Google Scholar]
- 5. Dal J, Feldt-Rasmussen U, Andersen M, et al. Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur J Endocrinol. 2016;175(3):181–190. [DOI] [PubMed] [Google Scholar]
- 6. Petrossians P, Daly AF, Natchev E, et al. Acromegaly at diagnosis in 3173 patients from the Liège Acromegaly Survey (LAS) database. Endocr Relat Cancer. 2017;24(10):505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Lely AJ, Harris AG, Lamberts SW. The sensitivity of growth hormone secretion to medical treatment in acromegalic patients: influence of age and sex. Clin Endocrinol (Oxf). 1992;37(2):181–185. [DOI] [PubMed] [Google Scholar]
- 8. Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev. 2011;32(2):247–271. [DOI] [PubMed] [Google Scholar]
- 9. Gadelha MR, Kasuki L, Korbonits M. The genetic background of acromegaly. Pituitary. 2017;20(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rostomyan L, Daly AF, Petrossians P, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22(5):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CJ, Ironside N, Pomeraniec IJ, et al. Microsurgical versus endoscopic transsphenoidal resection for acromegaly: a systematic review of outcomes and complications. Acta Neurochir (Wien). 2017;159(11):2193–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zahr R, Fleseriu M. Updates in diagnosis and treatment of acromegaly. Eur Endocrinol. 2018;14(2):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013;98(8):3190–3198. [DOI] [PubMed] [Google Scholar]
- 14. Swearingen B, Barker FG 2nd, Katznelson L, et al. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998;83(10):3419–3426. [DOI] [PubMed] [Google Scholar]
- 15. Gittoes NJ, Sheppard MC, Johnson AP, Stewart PM. Outcome of surgery for acromegaly–the experience of a dedicated pituitary surgeon. QJM. 1999;92(12):741–745. [DOI] [PubMed] [Google Scholar]
- 16. Kimmell KT, Weil RJ, Marko NF. Multi-modal management of acromegaly: a value perspective. Pituitary. 2015;18(5):658–665. [DOI] [PubMed] [Google Scholar]
- 17. Postma MR, Netea-Maier RT, van den Berg G, et al. Quality of life is impaired in association with the need for prolonged postoperative therapy by somatostatin analogs in patients with acromegaly. Eur J Endocrinol. 2012;166(4):585–592. [DOI] [PubMed] [Google Scholar]
- 18. Adelman DT, Liebert KJ, Nachtigall LB, Lamerson M, Bakker B. Acromegaly: the disease, its impact on patients, and managing the burden of long-term treatment. Int J Gen Med. 2013;6:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hannon MJ, Barkan AL, Drake WM. The role of radiotherapy in acromegaly. Neuroendocrinology. 2016;103(1):42–49. [DOI] [PubMed] [Google Scholar]
- 20. Polat Korkmaz O, Gurcan M, Nuhoglu Kantarci FE, et al. The effects of pre-operative somatostatin analogue therapy on treatment cost and remission in acromegaly. Pituitary. 2019;22(4):387–396. [DOI] [PubMed] [Google Scholar]
- 21. Fleseriu M. Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review. Pituitary. 2011;14(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmichael JD, Bonert VS, Nuño M, Ly D, Melmed S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab. 2014;99(5):1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neggers SJ, van Aken MO, Janssen JA, Feelders RA, de Herder WW, van der Lely AJ. Long-term efficacy and safety of combined treatment of somatostatin analogs and pegvisomant in acromegaly. J Clin Endocrinol Metab. 2007;92(12):4598–4601. [DOI] [PubMed] [Google Scholar]
- 24. Trainer PJ, Drake WM, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171–1177. [DOI] [PubMed] [Google Scholar]
- 25. van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754–1759. [DOI] [PubMed] [Google Scholar]
- 26. Buchfelder M, van der Lely AJ, Biller BMK, et al. Long-term treatment with pegvisomant: observations from 2090 acromegaly patients in ACROSTUDY. Eur J Endocrinol. 2018;179(6):419–427. [DOI] [PubMed] [Google Scholar]
- 27. Kuhn E, Chanson P. Cabergoline in acromegaly. Pituitary. 2017;20(1):121–128. [DOI] [PubMed] [Google Scholar]
- 28. Abs R, Verhelst J, Maiter D, et al. Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab. 1998;83(2):374–378. [DOI] [PubMed] [Google Scholar]
- 29. Sandret L, Maison P, Chanson P. Place of cabergoline in acromegaly: a meta-analysis. J Clin Endocrinol Metab. 2011;96(5):1327–1335. [DOI] [PubMed] [Google Scholar]
- 30. Colao A, Bronstein MD, Freda P, et al. ; Pasireotide C2305 Study Group . Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99(3):791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gadelha MR, Bronstein MD, Brue T, et al. ; Pasireotide C2402 Study Group . Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–884. [DOI] [PubMed] [Google Scholar]
- 32. Nachtigall L, Delgado A, Swearingen B, Lee H, Zerikly R, Klibanski A. Changing patterns in diagnosis and therapy of acromegaly over two decades. J Clin Endocrinol Metab. 2008;93(6):2035–2041. [DOI] [PubMed] [Google Scholar]
- 33. Sagvand BT, Khairi S, Haghshenas A, et al. Monotherapy with lanreotide depot for acromegaly: long-term clinical experience in a pituitary center. Pituitary. 2016;19(4):437–447. [DOI] [PubMed] [Google Scholar]
- 34. Carmichael JD, Broder MS, Cherepanov D, et al. Long-term treatment outcomes of acromegaly patients presenting biochemically-uncontrolled at a tertiary pituitary center. BMC Endocr Disord. 2017;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mateos CF, García-Uria M, Morante TL, García-Uria J. Erratum to: Acromegaly: surgical results in 548 patients. Pituitary. 2017;20(5):529–530. [DOI] [PubMed] [Google Scholar]
- 36. Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3) . Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf). 2013;79(5):689–699. [DOI] [PubMed] [Google Scholar]
- 37. Anagnostis P, Efstathiadou ZA, Polyzos SA, et al. Acromegaly: presentation, morbidity and treatment outcomes at a single centre. Int J Clin Pract. 2011;65(8):896–902. [DOI] [PubMed] [Google Scholar]
- 38. Nachtigall LB, Karavitaki N, Kiseljak-Vassiliades K, et al. Physicians’ awareness of gadolinium retention and MRI timing practices in the longitudinal management of pituitary tumors: a “Pituitary Society” survey. Pituitary. 2019;22(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998;83(10):3411–3418. [DOI] [PubMed] [Google Scholar]
- 40. Kreutzer J, Vance ML, Lopes MB, Laws ER Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86(9):4072–4077. [DOI] [PubMed] [Google Scholar]
- 41. Park SH, Ku CR, Moon JH, Kim EH, Kim SH, Lee EJ. Age- and sex-specific differences as predictors of surgical remission among patients with acromegaly. J Clin Endocrinol Metab. 2018;103(3):909–916. [DOI] [PubMed] [Google Scholar]
- 42. Freda PU, Landman RE, Sundeen RE, Post KD. Gender and age in the biochemical assessment of cure of acromegaly. Pituitary. 2001;4(3):163–171. [DOI] [PubMed] [Google Scholar]
- 43. Kanakis GA, Chrisoulidou A, Bargiota A, et al. The ongoing challenge of discrepant growth hormone and insulin-like growth factor I results in the evaluation of treated acromegalic patients: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016;85(5):681–688. [DOI] [PubMed] [Google Scholar]
- 44. Sherlock M, Aragon Alonso A, Reulen RC, et al. Monitoring disease activity using GH and IGF-I in the follow-up of 501 patients with acromegaly. Clin Endocrinol (Oxf). 2009;71(1):74–81. [DOI] [PubMed] [Google Scholar]
- 45. Giustina A, Chanson P, Bronstein MD, et al. ; Acromegaly Consensus Group . A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141–3148. [DOI] [PubMed] [Google Scholar]
- 46. Freda PU. Monitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant? Clin Endocrinol (Oxf). 2009;71(2):166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carmichael JD, Bonert VS, Mirocha JM, Melmed S. The utility of oral glucose tolerance testing for diagnosis and assessment of treatment outcomes in 166 patients with acromegaly. J Clin Endocrinol Metab. 2009;94(2):523–527. [DOI] [PubMed] [Google Scholar]
- 48. Tortora F, Negro A, Grasso LFS, et al. Pituitary magnetic resonance imaging predictive role in the therapeutic response of growth hormone-secreting pituitary adenomas. Gland Surg. 2019;8(Suppl 3):S150–S158. [DOI] [PMC free article] [PubMed] [Google Scholar]