Abstract
Diabetic nephropathy (DN) is the leading cause of kidney failure, with an increasing incidence worldwide. Mitochondrial dysfunction is known to occur in DN and has been implicated in the underlying pathogenesis of disease. These complex organelles have an array of important cellular functions and involvement in signaling pathways, and understanding the intricacies of these responses in health, as well as how they are damaged in disease, is likely to highlight novel therapeutic avenues. A key cell type damaged early in DN is the podocyte, and increasing studies have focused on investigating the role of mitochondria in podocyte injury. This review will summarize what is known about podocyte mitochondrial dynamics in DN, with a particular focus on bioenergetic pathways, highlighting key studies in this field and potential opportunities to target, enhance or protect podocyte mitochondrial function in the treatment of DN.
Keywords: diabetic nephropathy, podocyte injury, bioenergetics, mitochondria, lipotoxicity, mitophagy
Diabetes mellitus is a common chronic metabolic disease and major global healthcare burden. According to the World Health Organization, in 2019 diabetes accounted for over 1.5 million deaths and its incidence continues to increase (1). Diabetic nephropathy (DN) is 1 of the most frequent complications of diabetes, affecting around 30% of individuals with diabetes. Not only does DN often require dialysis or transplantation in its advanced stages, but also the increased risk of all-cause and cardiovascular mortality in diabetes is predominantly seen in individuals with kidney disease (2, 3). Despite extensive research into the pathogenesis of DN, current treatment options are limited. As such, there is still a desperate need to identify novel therapeutic targets, which requires further understanding of the molecular mechanisms underlying DN development and progression.
An increase in urinary albumin excretion is often the first clinical sign of renal injury in diabetes (4). A key driver of albuminuria is podocyte loss, occurring as a consequence of their detachment and apoptosis or injury (which can also manifest as hypertrophy, cell flattening, and foot process effacement) (5-8). Podocytes are highly specialized, terminally differentiated cells, with a limited capacity for renewal and thus rely on their ability to maintain their complex structure via regulation and organization of the actin cytoskeleton and extracellular matrix. This imposes a significant energy demand on podocytes, which in turn requires maintaining an adequate mitochondrial number and their proper function. The interest in understanding the mechanisms behind podocyte damage in DN has highlighted the crucial role of mitochondria (9-11). Mitochondria are key intracellular organelles, which control energy metabolism and are essential for the production and generation of cellular adenosine triphosphate (ATP), through oxidative phosphorylation. An efficient mitochondrial turnover—biogenesis, fusion, fission, and mitophagy—is essential for proper energy supply and targeted distribution of the mitochondrial metabolites throughout the cell. Apart from energy supply, mitochondria modulate various intracellular processes such as cell proliferation, apoptosis, calcium homeostasis, oxidative stress, and lipid metabolism (12, 13). Their dysfunction is linked to the pathogenesis of various chronic and metabolic conditions, including DN (14, 15). However, the complex changes in podocyte mitochondrial metabolism in DN, and whether mitochondrial dysfunction is a direct cause rather than a consequence of podocyte injury, are not fully understood.
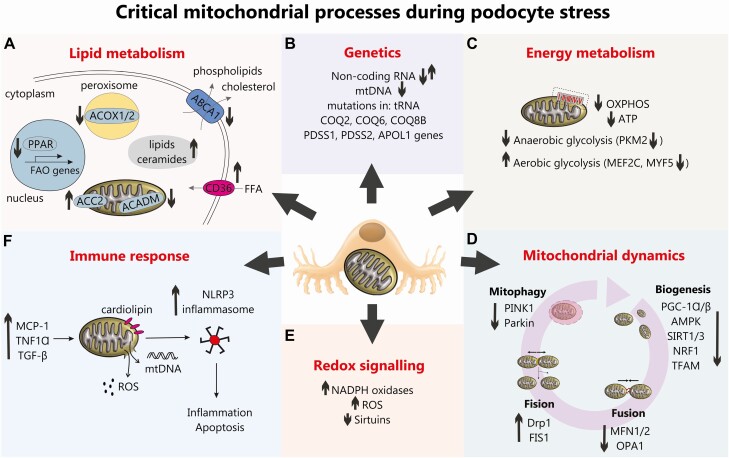
Here, we will focus on the role of mitochondria in podocyte bioenergetics in diabetes, review the recent advances in our understanding of mitochondrial dysfunction in podocytes, and give perspectives on the direct relationship between changes of the mitochondrial metabolism and the development of DN. The critical mitochondrial processes known to be regulated in conditions of podocyte stress are summarized in Fig. 1.
Figure 1.
An overview of critical mitochondrial processes during podocyte stress. (A) Lipid metabolism. An upregulated CD36 in DN promotes free fatty acids (FFA) uptake, whereas downregulated ABCA1 hampers extracellular transport of phospholipids and cholesterol. Dysregulated lipid metabolism in podocytes in DN is also associated with the decreased fatty acids β-oxidation (FAO), which results from the reduced expression of peroxisome proliferator-activated receptor (PPAR), peroxisomal acyl-CoA oxidase (ACOX1/2) and mitochondrial acyl-CoA dehydrogenase (ACADM), as well as the increased ACC2 activity. All this results in accumulation of lipids and ceramides in cytoplasm. (B) Genetics. Mutations in mitochondrial genes COQ2, COQ6, COS8B, PDSS1, PDSS2, and APOL1 have all been associated with DN and noncoding RNAs (eg, miRNA-21 and long-noncoding RNAs Tug1 and Meg3) have been shown to regulate podocyte bioenergetics and fusion/fission in DN. (C) Energy metabolism. A reduction in oxidative phosphorylation (OXPHOS) and ATP production has been demonstrated in DN in addition to podocyte “glycolytic switch.” (D) Mitochondrial dynamics. A reduction in podocyte mitochondrial biogenesis pathways are observed in DN alongside a reduction in mitochondrial fusion, an increase in podocyte mitochondrial fission (22, 70) and a reduction in podocyte mitophagy (81). (E) Altered podocyte redox signaling in DN is evidenced by an increase in NADPH oxidases (32), an increase in reactive oxygen species (34), a reduction in sirtuins which can lead to a reduction in FOXO1 (62). (F) Increased immune signaling (eg, increased MCP-1, tumor necrosis factor-α and TGF-β) in DN can damage podocyte mitochondria and cause an increase in ROS production and release of mtDNA which along with oxidized cardiolipin accumulated in mitochondrial outer membrane, can trigger NLRP3-inflammasome assembly leading to inflammation and apoptosis.
Podocyte Bioenergetics: Role of Mitochondrial Metabolism
Cells generate energy from glucose by 2 pathways: glycolysis in the cytoplasm and oxidative phosphorylation (OXPHOS) in mitochondria. In general, OXPHOS is by far the most efficient energy-producing pathway, generating up to 36 ATPs per glucose molecule (vs the 2 ATP molecules generated during glycolysis) and has the capacity to utilize all nutrients available to cells. In podocytes, there is yet no consensus on the prevalence of aerobic vs anaerobic metabolism and a crucial role of mitochondria in podocyte bioenergetics is currently debated. There are some contradictory studies, showing that podocyte energy status is not altered due to mitochondrial imbalance, whereas others demonstrate opposing results (16-19). These discrepancies may arise for various reasons, including the use of different cellular and animal models, as well as employing distinct experimental conditions. Below, we discuss the views on the role of mitochondria in podocyte bioenergetics, referring to the contentious issues in this area.
Due to their unique morphology, mitochondria are not evenly distributed throughout the cell. Like most organelles, mitochondria are located in the cytosol around the nucleus and not found in the foot processes, where energy metabolism is probably powered by glycolysis (16). In support of this predominant role of glycolysis in podocyte foot processes, blocking glycolysis with 2-deoxy-D-glucose has been shown to prevent the formation of lamellipodia and reduce podocyte migration (16). Others have also demonstrated that podocyte-specific knockout of genes responsible for mitochondrial biogenesis and dynamics (ie, PGC-1α, Tfam, DRP1) does not result in glomerular or renal injury in mice under physiological conditions (17). Although this may suggest glycolysis has the capacity to compensate for a loss of mitochondrial respiration, or a predominant role for glycolysis in podocytes, these studies do not address whether these changes can be deleterious in conditions of podocyte stress, for example in DN. Others have also demonstrated that mitochondrial respiration accounts for around 77% of podocyte respiration (15, 18). Moreover, podocytes have limited ability to increase ATP levels by enhancing glycolysis or OXPHOS after partial suppression of mitochondrial function or glycolytic flux (18), providing evidence that both pathways are involved in the maintenance of cell energy balance. Overall, whether glycolysis or mitochondria play a predominant role in podocyte bioenergetics likely depends on context (eg, stage of differentiation, extracellular environment, subcellular localization). The question remains as to which pathway plays a predominant role in podocytes exposed to stressful environments, such as in DN.
It is clear, however, that both glycolysis and OXPHOS are dysregulated in podocytes in DN and recently, the role of increased interactions between Smad4 and the rate-limiting glycolytic enzyme pyruvate kinase M2 (PKM2) and ATPase inhibitory factor 1 (20) in this process has been demonstrated. This suggests the depletion of Smad4 could be a potential therapeutic target to maintain podocyte bioenergetics in DN. Podocyte bioenergetic status also seems to be dependent on the stage of differentiation. Glycolysis has been shown as the main source of ATP production before differentiation, while OXPHOS seems to predominate during and after differentiation. A stepwise activation of oxidative metabolism during podocyte differentiation depends on the peroxisome proliferator activated receptor (PPAR) gamma coactivator-1 (PGC-1)α-dependent stimulation of mitochondrial biogenesis and function, with simultaneous reduction of the glycolytic enzyme content (19). Thus, mature podocytes could be more sensitive to an alteration of oxidative metabolism (16).
Interestingly, a novel mechanism of podocyte “bioenergetic de-differentiation” has been observed in diabetic conditions, showing that a high glucose (HG) environment induces an aerobic “glycolytic switch” in podocytes (ie, preferential glycolysis and increased lactate production despite oxygen abundance), by inhibiting MEF2C and MYF5 (19). The downregulation of these transcription factors reduces the expression of respiratory chain complex IV, decreases mitochondrial biogenesis, along with enhancing the expression of pyruvate dehydrogenase kinase 1, the OXPHOS inhibitor (19). This mechanism, resembling the Warburg effect which is mainly attributed to cancer cell bioenergetics, could be essential in maintaining efficient energy supply especially in podocyte foot processes. Changes in podocyte bioenergetic homeostasis toward glycolysis have also been observed in patients with DN, characterized by an increased expression of PKM2 and reduced expression of MEF2C, MYF5, and PGC-1α in renal biopsy samples, compared with tissue from healthy individuals (19).
In summary, though some studies have suggested that glycolysis can partially compensate impaired OXPHOS function and the loss of some mitochondrial proteins may not be detrimental for podocytes, there is strong evidence that molecular components of mitochondrial metabolism play an essential role in podocyte bioenergetics and physiology, particularly in DN.
Mitochondrial Injury Underlies the Development of Diabetic Nephropathy
Mitochondria are highly dynamic organelles and constantly change their shape and intracellular localization. Alteration of their morphology is associated with many pathological conditions, including diabetes (21) and there is a wealth of literature highlighting mitochondrial dysfunction in DN. Although many studies emphasize the key role of mitochondrial metabolism in podocyte biology, the mechanisms by which primary mitochondrial impairment contributes to renal damage in DN remain unknown. Fully understanding the involvement of mitochondria in podocyte injury and the unique bioenergetics of these cells, particularly in diabetes, is essential for determining whether directly targeting podocyte mitochondria could be a therapeutic option for DN.
Impairment of podocyte mitochondrial homeostasis has been observed both in vitro, for example after exposure to high glucose, and in vivo, in patients with DN (19, 22). An increased amount of small, fragmented mitochondria with abnormal cristae characterizes glomerular endothelial cell, podocytes, and proximal tubular epithelial cells from diabetic animal models and patients with DN (23, 24). Urinary metabolomic studies have shown decreased and abnormal mitochondria in DN (25) and evidence also suggests restoring mitochondrial activity can improve renal function in diabetes (26).
Crucial findings have come from studying individuals who have had diabetes for a duration of over 50 years and yet have no evidence of kidney damage. Compared with those with DN, “DN-protected” individuals had elevated enzymes in the glycolytic, sorbitol, methylglyoxal, and mitochondrial pathways. These seminal results (from both type 1 and type 2 diabetes) suggest that the ability to enhance or preserve these enzymes and pathways may reduce the toxic effects of glucose and preserve kidney function (27, 28). Conversely, we can infer that a disruption to glycolytic and mitochondrial pathways in the kidney is a major driving factor underlying DN. A particular emphasis has been placed on PKM expression and activity, which was highly upregulated the glomeruli of individuals with long-standing diabetes but without nephropathy. Mechanistically, a podocyte-specific reduction in PKM2 promoted mitochondrial dysfunction and glomerular damage and, interestingly, the pharmacological activation of PKM2 reversed mitochondrial dysfunction and kidney pathology in experimental models and increased glycolytic flux in podocytes in vitro (28).
The diabetic environment is associated with a dysregulation of many metabolites causing disruptions to podocyte homeostasis, including glucose, insulin, and lipids. Many studies have focused on the role of hyperglycemia in promoting podocyte damage, which may result from an elevated concentration of intracellular glucose. Podocytes uptake glucose via a number of glucose transporters (GLUTs) (29), including insulin-sensitive GLUT1 and GLUT4 (30). Notably, the podocyte-specific depletion of GLUT4 has been found to protect from DN (31), suggesting that limiting intracellular glucose in diabetes could be beneficial.
A major mechanism by which hyperglycemia promotes podocyte damage is via the increased production of reactive oxygen species (ROS) (32, 33), from the 2 main sources: mitochondrial (complex I and complex III of respiratory chain), and nonmitochondrial (membrane bound NADPH oxidases, mainly NOX4) (32, 34). Although ROS are inevitable by-products of mitochondrial respiration, which (in health) are neutralized by efficient intracellular antioxidant mechanisms, an increase in ROS generation and/or defects of the antioxidant defense causes oxidative modification of intracellular molecules (eg, DNA, proteins, lipids) leading to an impairment of cell function via a cascade of downstream mechanisms. Since podocytes are very metabolically active cells, they are particularly susceptible to oxidative stress. Increases in ROS can promote proinflammatory responses, via oxidized cardiolipin, a mitochondria-specific phospholipid, in the mitochondrial outer membrane, which serves as a docking molecule for NLRP3-inflammasome assembly (35). Interestingly, treatment with a selective cardiolipin peroxidase inhibitor, elamipretide (SS-31), can reverse high-fat diet–induced pathological changes in podocytes and renal tissue in mice (36). HG also induces NOX4-dependent generation of H2O2, which inhibits a mitochondrial enzyme, fumarate hydratase, leading to an increased level of fumarate (37). Fumarate is a potent inhibitor of prolyl hydroxylase, and its downregulation results in hypoxia-inducible factor (HIF)-1α accumulation and activation of HIF-1 signaling pathways, such as upregulation of profibrotic (transforming growth factor [TGF]-β) or proinflammatory cytokines (monocyte chemoattractant protein 1 [MCP-1], tumor necrosis factor-1α) in podocytes (37, 38). There is also evidence that NOX4 partially localizes to mitochondria in podocytes and its downregulation ameliorates HG-induced injury of podocytes and prevents glomerular damage in diabetic mice (39).
The early stages of DN are typically associated with hyperglycemia and hyperinsulinemia, along with progressive podocyte insulin resistance (40). Insulin signaling is essential for normal podocyte and kidney function (41). We have previously shown that insulin stimulates NOX4-dependent generation of ROS and induces reorganization of the actin cytoskeleton in podocytes, which leads to the increased glomerular filtration barrier permeability to albumin. This suggests excessive cellular insulin signaling (eg, in conditions of hyperinsulinemia as observed in type 2 diabetes) may also have negative effects on podocyte function via promoting ROS, potentially prior to the development of insulin resistance (42). There is also evidence of direct crosstalk between insulin resistance and mitochondrial dysfunction in podocytes (43).
Mitochondrial bioenergetics is tightly linked with lipid metabolism, and dyslipidemia is another hallmark of DN. Lipids are also an important energy source for the kidney and a major component of mitochondrial membranes. However, a systemic dysregulation of lipid metabolism occurs in diabetes, and may lead to an excessive and damaging accumulation of lipids in tissues, including in the kidney. Importantly, excessive lipid accumulation and dysfunctional lipid metabolism can also damage mitochondria in DN and protecting mitochondrial function in such situations can overcome lipotoxicity in the kidney in experimental models (36, 44).
Intracellular lipid homeostasis is maintained by balanced synthesis, degradation and/or storage, which can also be disrupted in diabetes. In podocytes, high glucose downregulates β-oxidation of fatty acids via several mechanisms including: decreased expression of peroxisome proliferator-activated receptor (PPAR)-α, Acyl-CoA dehydrogenase medium chain (ACADM), or acyl-CoA oxidase 1/2 (ACOX1/2) (45); upregulated acetyl-CoA carboxylase 2 (ACC2) activity in the mitochondrial surface (46); overexpression of cluster differentiation 36 (CD36); and accumulation of ceramides (47, 48). There is also evidence that therapeutic modulation of these pathways may be beneficial in DN, providing further potential targets for clinical intervention. The pharmacological activation of PPAR signaling (eg, by thiazolidinediones) is a common treatment strategy in type 2 diabetes, improving insulin sensitivity (49). PPARγ can also activate the enzyme Klotho, which can attenuate HG-induced oxidative stress and decrease podocyte apoptosis in vitro and in vivo (50). The antioxidant effects of Klotho may in part be via activation of nuclear respiratory factor (NRF)-2 signaling (51). HG-induced Klotho deficiency can also lead to reduced DNA repair capacity in podocytes, which is, importantly, accompanied with mitochondrial dysfunction and cell injury (52).
CD36 is an example of a fatty acid translocase which is overexpressed in diabetic kidney tissue with a potential role in DN pathogenesis. In podocytes, a CD36-dependent increase in free fatty acids (FFA) uptake promotes intracellular accumulation of triglyceride-enriched lipid droplets, which activates NLRP3 inflammasome assembly at the mitochondrial surface via cardiolipin interaction, leading to apoptosis (45, 53).
Ceramides are a class of lipids, elevated in the plasma of patients with type 2 diabetes, which have also been shown to play a significant role in podocyte biology (54). The accumulation of ceramides in podocyte mitochondria is associated with increased cell death (47). Furthermore, the key role of acid ceramidase activation in mitigating ATP release through the membrane channel pannexin-1 has been demonstrated in podocytes, preventing NLRP3-induced apoptosis and inflammation (55).
A dysregulation of cholesterol metabolism is also observed in podocytes in diabetic milieu (56). A key recent study has demonstrated the mechanism by which suppression of a transmembrane protein regulating cholesterol and phospholipid efflux, ATP-binding cassette A1 (ABCA1), may drive mitochondrial dysfunction and increase podocyte susceptibility to injury in diabetes (45). The deletion of ABCA1 specifically in podocytes increased cardiolipin accumulation, disrupted mitochondrial respiratory chain function and contributed to the progression of DN in mice (45). Interestingly, the pharmacological induction of ABCA1 ameliorated podocyte injury and reduced albuminuria (45), highlighting another promising therapeutic approach related to protecting mitochondrial function.
Mitochondrial Biogenesis in Diabetes
Another mechanism of mitochondrial damage in DN is the alteration of mitochondrial turnover, related to biogenesis–fusion–fission–mitophagy cycle. Mitochondrial biogenesis is a process of forming new organelles, which requires a coordinated synthesis of proteins encoded by both nuclear DNA (nDNA) and mitochondrial DNA (mtDNA), membrane biosynthesis and the proper targeting and folding of OXPHOS complexes (57). This is important for maintaining cellular energy production as it enables the replacement of mitochondria severely injured under hyperglycemia with new functional organelles.
Mitochondria encompass over 1000 different proteins, but only 13 (subunits of complexes I, III, IV, and V) are encoded by the mitochondrial genome (58). Furthermore, nDNA transcription factors (regulated by hormones and growth factors) also control the transcription and translation of mtDNA, suggesting that most biogenesis processes are mtDNA independent. The peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 (PGC-1) family has a key role in mitochondrial biogenesis, with PGC-1α and PGC-1β being the most important players (57). PGC-1α is a cotranscriptional regulation factor that induces mitochondrial biogenesis by activating different transcription factors, such as NRF-1 and NRF-2, which in turn promote the expression of transcription factor A mitochondrial (TFAM) (59). PGC-1α is capable of driving almost all processes of mitochondrial formation, including transcription of OXPHOS subunits and fatty acid oxidation genes. One of the main downstream effects of PGC-1α is the activation of NRF-1 and NRF-2, which in turn direct transcription of the nuclear encoded mitochondrial proteins, the mitochondrial protein import machinery and cofactors required for assembly of the OXPHOS complexes, as well as the regulatory factors required for mtDNA transcription and translation, among which TFAM is the most important (12).
Several studies support the hypothesis that a close relationship exists between PGC-1α function, insulin sensitivity and type 2 diabetes. Impaired mitochondrial biogenesis and elevated mtDNA damage have been observed in diabetic kidneys (60). There are various mechanisms of PGC-1α regulation at the transcriptional and post-translational levels (61). Among others, PGC-1α is activated through phosphorylation by the major cellular energy sensor, AMPK, and through deacetylation by SIRT1 in low nutrients or high NAD+ conditions (61). A decreased level of PGC-1α and reduced phosphorylation of AMPK correlate with DN, and furthermore, pharmacological activation of AMPK not only stimulates PGC-1α, ameliorates mitochondrial respiratory chain function and increases mitochondrial density in kidneys, but also reverses diabetes-induced renal changes, involving increased fibronectin and TGF-β production (60). In podocytes, HG decreases the levels of mitochondrial proteins, including subunits of OXPHOS complexes and some markers of mitochondrial biogenesis (PGC-1α, NRF1, TFAM) (19). The restoration of PGC-1α in HG-treated podocytes alleviates lipotoxicity, improves insulin sensitivity and decreases cell injury (46). A secreted glycoprotein, progranulin, can activate SIRT1/PGC-1α/FOXO1 signaling in podocytes exposed to HG, which protected against podocyte injury in mice with DN (62). Recent finding emphasized the importance of mitochondrial glycerol 3-phosphate dehydrogenase in inducing mitochondrial biogenesis in podocytes, which protected the cells against hyperglycemia-induced impairment of mitochondrial bioenergetics and increased ROS production (63).
Overall, there is a consensus that enhancing mitochondrial biogenesis in podocytes in the diabetic milieu is beneficial for renal function (64, 65). Interestingly, thiazolidinediones are PGC-1α activators (59). Moreover, pharmacological activation of the master regulators of mitochondrial biogenesis (ie, AMPK, SIRT1, PGC-1α, NRF-1), by a selective agonist of G protein coupled bile acid receptor TGR5 (INT-777), increases fatty acid β-oxidation and reduces lipid accumulation in podocytes (64). Treatment of diabetic mice with INT-777 not only improves podocyte injury, but also alleviates proteinuria, renal fibrosis and CD68 macrophage infiltration (64). Even more promising therapeutic effects have been observed after the treatment of diabetic mice and mice with diet-induced obesity with dual farnesoid X receptor/TGR5 agonist INT-767 (66). The renoprotective impact of INT-767 was partially due to increased mitochondrial biogenesis and activation of mitochondrial fatty acid β-oxidation (66).
Mitochondrial Fusion and Fission in Diabetes
Mitochondrial fusion is the process of physical merging of 2 mitochondria together, while fission is separating 1 into 2. Mitochondrial fusion generally promotes OXPHOS, rescues mitochondria from degradation through mitophagy and allows biodistribution of fatty acids under nutrient-limited conditions (67). The fusion process is crucial for an equal distribution of the mitochondrial molecular machinery across the entire mitochondrial network and plays an important role in the efficiency improvement of most of reactions, which occur inside the organelles (68). On the contrary, mitochondrial fission is often associated with metabolic stress and leads to a degradation of the defective mitochondria or apoptosis in cases of severe damage (67). Perturbation of the proper regulation of mitochondrial fusion–fission processes affects cellular bioenergetics and inevitably leads to an impairment of cell function. There is also a link between an alteration in mitochondrial dynamics and diabetes (24, 69). The reduced levels of mitofusins-1/2, responsible for mitochondrial fusion, and upregulation of the fission protein, dynamin-related protein 1 (DRP1), are observed in podocytes in diabetic mice, along with mitochondrial fragmentation (22, 70). It has been shown that pharmacological restoration of the mitofusin-2 and DRP1 receptor FIS1 can reverse pathological changes in kidneys, such as podocyte loss, foot processes fusion and effacement in diabetic rats (71). Importantly, defects in mitochondrial fusion machinery in podocytes leads to a hyperactivation of the insulin/IGF-1 signaling pathway and progressive proteinuria in mice, resulting in kidney failure and death of the animals (72).
Several pharmacological agents directly targeting mitochondria and regulating the fusion-fission process have been studied in podocytes and in animal models of diabetes. For example, the Drp-1 inhibitor, Mdivi-1, has renoprotective effects in db/db mice and podocytes by inhibiting mitochondrial fragmentation and lowering ROS levels (73). Similar beneficial effects on mitochondrial dynamics, alongside the suppression of renal injury, have been observed in STZ-induced mice treated with other agents, such as dipeptidyl peptidase-4 inhibitors, antioxidant mitochondrial-targeted protein (SS-31), or SGLT2 inhibitors (74).
The exact role of either mitochondrial fusion or fission in the podocyte in DN and conditions of insulin resistance is still unclear.
Mitophagy in Diabetes
Closely related to mitochondrial fission is the process of mitophagy. Mitophagy is a specific type of autophagy involving a range of intracellular pathways destined to selectively recognize, neutralize, and destroy defective mitochondria. Similar to autophagy, the mitophagy process is based on a series of the following steps: initiation with the recruitment of autophagy machinery, sequestration of mitochondria into autophagosomes, fusion of autophagosomes with lysosomes, and release of digested products into the cytoplasm. The major pathway for the injury-induced mitophagy involves PTEN-induced kinase 1 (PINK1) and Parkin proteins. In depolarized mitochondria, PINK1 accumulates in the mitochondrial outer membrane and recruits and phosphorylates the E3 ubiquitin-protein ligase Parkin, which in turn ubiquitinates numerous mitochondrial outer membrane proteins (eg, FIS1, Mfn1/2, VDAC1, TOM) (75), ultimately leading to the formation of the isolation membrane around damaged mitochondria (76). Autophagosome formation and closure are regulated by different upstream factors, including ULK1 kinase (unc-51-like kinase), which regulates mitochondrial shape and have a role in mitophagy by direct phosphorylation of mitophagy receptor FUNDC1 (FUN14 domain-containing protein 1) (77).
It is becoming increasingly clear that both autophagy and mitophagy are inhibited in diabetic kidneys (78-81). Dysregulation of mitophagy has been shown to contribute to the development of cellular insulin resistance (82-84). The downregulation of PINK1 was found to correlate with palmitate-induced insulin resistance in hepatocytes and in livers from mice maintained on a high-fat diet (82). Upregulation of podocyte autophagy ameliorates HG-induced cell injury and prevents insulin resistance (85, 86). Moreover, activation of PINK1/Parkin1 signaling in HG-exposed podocytes and in renal cortex in diabetic animal models improves cellular and glomerular injury (87). Further studies characterizing the molecular crosstalk between mitophagy and insulin signaling in podocytes in DN are warranted.
The balance of mitochondrial degradation and biogenesis determines cellular adaptation to physiological and pathological stressors and is essential for maintaining mitochondrial homeostasis and normal cellular function and survival. Both mitophagy and mitochondrial biogenesis are coordinated by several common pathways, which promote biogenesis by activating PGC-1α and inhibit mitophagy by phosphorylation of LC3 and DRP1 proteins. Both PINK1 and Parkin not only play a key role in mitophagy, but also positively regulate mitochondrial biogenesis (57). Likewise, AMPK, is involved in the initiation of mitophagy (eg, through phosphorylation of ULK1), and also stimulates biogenesis through PGC-1α activation in kidney (88). However, we still do not know whether such molecular crosstalk exists in podocyte and how it is regulated in diabetes. Mitophagy seems to be 1 of the defense mechanisms in podocytes aiming to reduce oxidative stress and spare cells from apoptosis. It may therefore be particularly important during the early stages of DN and metabolic stress, and strategies to therapeutically target these pathways may be important for maintaining proper mitochondrial dynamics and effective for treating or slowing the progression of DN.
Genetics of Mitochondrial Dysfunction in DN
Though genome-wide association studies have failed to reveal robust DN-associated loci, there is evidence for a genetic basis of mitochondrial dysfunction in DN, involving both mtDNA and nDNA mutations. MtDNA encodes 13 subunits of OXPHOS complexes I, III, IV, and V, 2 rRNAs, and 22 tRNAs, whereas the remaining genes, encoding other structural subunits of respiratory chain complexes, proteins and enzymes involved in mtDNA replication and maintenance, cytochrome c and enzymes for coenzyme Q (CoQ) biosynthesis are products of nDNA (89). The A3243G mutation in the tRNALeu gene is 1 of the most frequent point mutation in mtDNA associated with diabetes and glomerular defects (90). A correlation between the existence of the A3243 in mtDNA and podocyte foot processes effacement has been described in individuals with diabetic and nondiabetic kidney disease (91). Noteworthy, a significant alteration in the urinary proteome pattern has been discovered in patients carrying A3243G mutation, characterized by a reduced level of lysosomal proteins, calcium-binding proteins, and antioxidative enzymes in urine samples (92). Aside from A3243G mutation, the mitochondrial dysfunction associated with glomerular diseases can result from the genetic alterations of the CoQ10 biosynthesis pathway genes such as COQ2, COQ6, COQ8B, PDSS1, PDSS2 (89). Although CoQ10 has its primary role in the electron transport chain, the mechanism of podocyte injury due to CoQ10 deficiency involves disturbed polyunsaturated fatty acid metabolism and is independent of ATP production (93). The early diagnosis of patients with CoQ10 defects is particularly important since oral supplementation with CoQ10 can prevent irreversible neurological lesions and renal damage (89). Targeting CoQ10 pathway may have broad therapeutic potential to treat renal diseases (94). For example, in Pdss2kd/kd mice with global CoQ10 depletion treatment with Braf/Mapk-targeting compound, GDC-0879, restored podocyte lipid peroxidation, and ameliorated albuminuria (93).
Recently, incisive research has been published showing that Apoliprotein L1 gene (APOL1) variants, G1 (S342G and I384M) and G2 (del388N389Y), are associated with mitochondrial dysfunction and cytotoxicity in podocytes, and contribute to lipotoxicity-induced renal injury in mice with focal segmental glomerulosclerosis (95). High-glucose–cultured podocytes have lower expression of APOL1 protein (96); however, the effect of the presence of APOL1 risk variants in podocytes in diabetic kidney has not yet been investigated.
Epigenetic modifications drive changes in gene expression independent of changes to the underlying DNA sequence and can encompass modifications to DNA (eg, methylation), histones (eg, acetylation), and noncoding RNAs (eg, microRNAs, long noncoding [lnc]RNAs). The interest in epigenetic modifications in DN has in part been driven by their potential role in the phenomenon of “metabolic memory,” whereby individuals with diabetes and long-term normoglycemia (>25 years) still have an increased risk of DN due to poor glucose control in the past (97). There is increasing evidence that epigenetic changes are associated with DN (98). Although the majority of epigenetic studies concern nuclear DNA modifications, mitoepigenetics also has an important role in a variety of diseases, including cardiovascular disease, obesity, and diabetes (99). For example, an increased methylation of D-loop region of mtDNA and subsequent reduction in the mtDNA copy number in leukocytes has been associated with insulin resistance in obesity (100), and decreased methylation of mtDNA has been linked with the reduced expression of PGC-1α and p-AMPK, and the accumulation of fumarate in diabetic kidney (101).
Noncoding RNAs are involved in the regulation of various mitochondrial pathways in DN (102). For example, miRNA-21 is downregulated in animal models of DN and CKD patients and can suppress the expression of PPAR-α, thereby regulating fatty acid β-oxidation (103). miRNA-34a is increased in plasma and urine of DN patients and can suppress SIRT1, which in turn regulates autophagy, apoptosis, and mitochondrial biogenesis (104). SIRT1 activity is also regulated by miRNA-150-5p, and silencing miRNA-150-5p prevents HG-induced podocyte injury and STZ-induced DN in mice (105). The lncRNA “taurine upregulated gene 1” (Tug1) appears to modulate PGC-1α transcription and mitochondrial bioenergetics in DN (106). Tug1 expression is significantly decreased in the podocytes of diabetic mice and its overexpression leads to alleviated albuminuria, improved podocyte morphology and reduced mesangial matrix expansion (106). Along with regulation of mitochondrial metabolism, lncRNAs have been identified which can regulate mitochondrial dynamics. For example, the lncRNA “Meg3” is increased in podocytes in diabetic conditions and correlates with an excessive mitochondrial fission and podocyte injury in DN (102).
Future studies of mitochondrial genetics and epigenetics will highlight further mechanisms underlying mitochondrial dysfunction in DN.
Therapeutic Potential and Future Perspectives
As described throughout this review, there is increasing evidence that mitochondrial damage is a driving factor toward podocyte dysfunction and DN, and there is evidence that therapeutic strategies in experimental models of DN have beneficial effects on mitochondria. Such examples include stimulation of AMPK-PGC-1α signaling via AMPK activators (metformin, AICAR) or inhibition of mTOR (sirolimus). Furthermore, mechanistic studies of SGLT2 and dipeptidyl peptidase-4 inhibitors have demonstrated they can have beneficial effects on mitochondrial function in DN (107-109). Though indeed, such agents have a primary role in maintaining glucose homeostasis, so any effects on mitochondrial protection may arise from lowering glycaemia opposed to directly targeting podocytes, it does not discount that mitochondrial disturbance could be the driving intermediate between hyperglycemia and podocyte dysfunction. Interestingly, glycemia-independent renoprotective effects of the SGLT2 inhibitor dapagliflozin have been demonstrated in a model of albumin overload, alongside protection against podocyte dysfunction and loss (110), and protective effects of the SGLT2 inhibitor Ertugliflozin on cardiac mitochondrial function have recently been described in the absence of diabetes (111), suggesting additional (albeit debated) mechanisms of action for such SGLT2 inhibitors should be explored.
There are also several reports demonstrating that agents specifically targeting mitochondria could be therapeutically beneficial, such as mitochondrial division inhibitor (Mdivi-1), elamipretide (SS-31), or triphenylphosphonium–CoQ conjugate (MitoQ) (112). Importantly, SS-31 is an example of a synthetic peptide that improves mitochondrial function which is currently undergoing clinical trials for treatment of heart failure as well as primary mitochondrial diseases. Preclinical studies have shown beneficial effects of this compound in models of DN, protecting mitochondrial structure in all kidney cell types (36, 113). This suggests that therapeutic strategies directly targeting mitochondria have vast potential in prevention of the renal injury in diabetes and in DN treatment.
It is also important to note that upregulation of both glycolytic (PKM1, PKM2) and mitochondrial (MTCO2) enzymes have positive effects on retaining renal function and preventing glomerular pathology in diabetes (27). Pharmacological activation of PKM2 ameliorates mitochondrial dynamics in podocytes and improves renal function in diabetic animal models (28) providing a promising clinical target in the future.
Conclusions
The pathogenesis of DN is complex, involving a variety of molecular and biochemical pathways. Podocytes are especially vulnerable to the alterations in the extracellular environment and hemodynamic disturbance caused by diabetes and numerous studies using disease models and samples from individuals with DN have indicated that unbalanced mitochondrial homeostasis underlies pathological changes in podocytes in DN. Though not covered in detail in present manuscript, there is also evidence that mitochondrial injury is a major driving factor behind the damage of other renal cells in DN, especially proximal tubule cells, which are particularly rich in mitochondria and almost exclusively oxygen dependent (79, 114, 115). Importantly, proximal tubule cells are considered the primary cellular target for SGLT2 inhibitors (116). However, whether mitochondrial dysfunction is always a cause (rather than consequence) of podocyte damage in DN is not understood and the mechanisms by which primary mitochondrial injury affects podocyte function are still not fully elucidated. Although, studies directly targeting mitochondrial proteins have shown renoprotective effects, which is encouraging to further research in this area. Additional studies are needed to identify the pivotal mechanisms which are truly driving the bioenergetic changes in podocytes in diabetes, providing potential therapeutic targets, which could be pharmacologically or genetically modulated in order to prevent the development of the DN and to establish more effective treatments of diabetes.
Acknowledgments
Financial Support : The authors would like to acknowledge support from the National Science Center in Poland for I.A. (no. 2016/23/D/NZ5/01449), Kidney Research UK for A.B. and the Society for Endocrinology UK, for A.C.L.
Glossary
Abbreviations
- ABCA1
ATP-binding cassette A1
- ATP
adenosine triphosphate
- CD36
cluster differentiation 36
- CoQ
coenzyme Q
- DN
diabetic nephropathy
- DRP
dynamin-related protein
- GLUT
glucose transporter
- HG
high glucose
- HIF
hypoxia-inducible factor
- NRF
nuclear respiratory factor
- OXPHOS
oxidative phosphorylation
- PKM2
pyruvate kinase M2
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- TFAM
transcription factor A mitochondrial
- TGF
transforming growth factor
Additional Information
Disclosures: The authors declare no conflict of interests.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. World Health Organization: diabetes fact sheets, 2021. www.who.int/news-room/fact-sheets/detail/diabetes. Accessed September 9, 2021.
- 2. Afkarian M, Sachs MC, Kestenbaum B, et al. . Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groop PH, Thomas MC, Moran JL, et al. ; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pyram R, Kansara A, Banerji MA, Loney-Hutchinson L. Chronic kidney disease and diabetes. Maturitas. 2012;71(2):94-103. [DOI] [PubMed] [Google Scholar]
- 5. Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. . Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steffes MW, Schmidt D, McCrery R, Basgen JM; International Diabetic Nephropathy Study Group . Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59(6):2104-2113. [DOI] [PubMed] [Google Scholar]
- 7. Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest. 2004;34(12):785-796. [DOI] [PubMed] [Google Scholar]
- 8. Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13(12):3005-3015. [DOI] [PubMed] [Google Scholar]
- 9. Lin Q, Ma Y, Chen Z, et al. . Sestrin‐2 regulates podocyte mitochondrial dysfunction and apoptosis under high‐glucose conditions via AMPK. Int J Mol Med. 2020;45(5):1361-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei X, Wei X, Lu Z, et al. . Activation of TRPV1 channel antagonizes diabetic nephropathy through inhibiting endoplasmic reticulum-mitochondria contact in podocytes. Metabolism. 2020;105:154182. [DOI] [PubMed] [Google Scholar]
- 11. Chen Z, Ma Y, Yang Q, et al. . AKAP1 mediates high glucose-induced mitochondrial fission through the phosphorylation of Drp1 in podocytes. J Cell Physiol. 2020;235(10):7433-7448. [DOI] [PubMed] [Google Scholar]
- 12. Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2017;284(2):183-195. [DOI] [PubMed] [Google Scholar]
- 13. Soria LR, Marrone J, Calamita G, Marinelli RA. Ammonia detoxification via ureagenesis in rat hepatocytes involves mitochondrial aquaporin-8 channels. Hepatology. 2013;57(5):2061-2071. [DOI] [PubMed] [Google Scholar]
- 14. Su J, Ye D, Gao C, Huang Q, Gui D. Mechanism of progression of diabetic kidney disease mediated by podocyte mitochondrial injury. Mol Biol Rep. 2020;47(10):8023-8035. [DOI] [PubMed] [Google Scholar]
- 15. Gujarati NA, Vasquez JM, Bogenhagen DF, Mallipattu SK. The complicated role of mitochondria in the podocyte. Am J Physiol Renal Physiol. 2020;319(6):F955-F965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ozawa S, Ueda S, Imamura H, et al. . Glycolysis, but not Mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci Rep. 2015;5:18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinkkoetter PT, Bork T, Salou S, et al. . Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep. 2019;27(5):1551-1566.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol. 2010;299(2):C464-C476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imasawa T, Obre E, Bellance N, et al. . High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J. 2017;31(1):294-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Sun YBY, Chen W, et al. . Smad4 promotes diabetic nephropathy by modulating glycolysis and OXPHOS. EMBO Rep. 2020;21(2):e48781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14(3):439-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Wang Y, Long J, et al. . Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coughlan MT, Nguyen TV, Penfold SA, et al. . Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci (Lond). 2016;130(9):711-720. [DOI] [PubMed] [Google Scholar]
- 24. Ma Y, Chen Z, Tao Y, et al. . Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr Connect. 2019;8(8):1206-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma K, Karl B, Mathew AV, et al. . Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24(11):1901-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallan S, Sharma K. The role of mitochondria in diabetic kidney disease. Curr Diab Rep. 2016;16(7):61. [DOI] [PubMed] [Google Scholar]
- 27. Gordin D, Shah H, Shinjo T, et al. . Characterization of glycolytic enzymes and pyruvate kinase M2 in type 1 and 2 diabetic nephropathy. Diabetes Care. 2019;42(7):1263-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi W, Keenan HA, Li Q, et al. . Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23(6):753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schiffer M, Susztak K, Ranalletta M, Raff AC, Böttinger EP, Charron MJ. Localization of the GLUT8 glucose transporter in murine kidney and regulation in vivo in nondiabetic and diabetic conditions. Am J Physiol Renal Physiol. 2005;289(1):F186-F193. [DOI] [PubMed] [Google Scholar]
- 30. Coward RJ, Welsh GI, Yang J, et al. . The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54(11):3095-3102. [DOI] [PubMed] [Google Scholar]
- 31. Guzman J, Jauregui AN, Merscher-Gomez S, et al. . Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes. 2014;63(2):701-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225-233. [PubMed] [Google Scholar]
- 33. Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446-1454. [DOI] [PubMed] [Google Scholar]
- 34. Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112(6):1661-1672. [DOI] [PubMed] [Google Scholar]
- 35. Elliott EI, Miller AN, Banoth B, et al. . Cutting edge: mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. J Immunol. 2018;200(9):3047-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016;90(5):997-1011. [DOI] [PubMed] [Google Scholar]
- 37. You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol. 2016;27(2):466-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang H, Fan Y, Gao Z, et al. . HIF-1α contributes to Ang II-induced inflammatory cytokine production in podocytes. BMC Pharmacol Toxicol. 2019;20(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liang Y, Liu H, Fang Y, et al. . Salvianolate ameliorates oxidative stress and podocyte injury through modulation of NOX4 activity in db/db mice. J Cell Mol Med. 2021;25(2):1012-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lay AC, Hurcombe JA, Betin VMS, et al. . Prolonged exposure of mouse and human podocytes to insulin induces insulin resistance through lysosomal and proteasomal degradation of the insulin receptor. Diabetologia. 2017;60(11):2299-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welsh GI, Hale LJ, Eremina V, et al. . Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lay AC, Hale LJ, Stowell-Connolly H, et al. . IGFBP-1 expression is reduced in human type 2 diabetic glomeruli and modulates β1-integrin/FAK signalling in human podocytes. Diabetologia. 2021;64(7):1690-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ising C, Brinkkoetter PT. Prohibitin signaling at the kidney filtration barrier. Adv Exp Med Biol. 2017;982:563-575. [DOI] [PubMed] [Google Scholar]
- 44. Ducasa GM, Mitrofanova A, Fornoni A. Crosstalk between lipids and mitochondria in diabetic kidney disease. Curr Diab Rep. 2019;19(12):144. [DOI] [PubMed] [Google Scholar]
- 45. Ducasa GM, Mitrofanova A, Mallela SK, et al. . ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest. 2019;129(8):3387-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Q, Zhao B, Zhang J, et al. . Faster lipid β-oxidation rate by acetyl-CoA carboxylase 2 inhibition alleviates high-glucose-induced insulin resistance via SIRT1/PGC-1α in human podocytes. J Biochem Mol Toxicol. 2021;35(7):e22797. [DOI] [PubMed] [Google Scholar]
- 47. Woo CY, Baek JY, Kim AR, et al. . Inhibition of ceramide accumulation in podocytes by myriocin prevents diabetic nephropathy. Diabetes Metab J. 2020;44(4):581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fucho R, Casals N, Serra D, Herrero L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017;31(4):1263-1272. [DOI] [PubMed] [Google Scholar]
- 49. Agrawal S, He JC, Tharaux PL. Nuclear receptors in podocyte biology and glomerular disease. Nat Rev Nephrol. 2021;17(3):185-204. [DOI] [PubMed] [Google Scholar]
- 50. Xing L, Fang J, Zhu B, et al. . Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 2021;269:119068. [DOI] [PubMed] [Google Scholar]
- 51. Xing L, Guo H, Meng S, et al. . Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem Biophys Res Commun. 2021;534:450-456. [DOI] [PubMed] [Google Scholar]
- 52. Chen Z, Zhou Q, Liu C, Zeng Y, Yuan S. Klotho deficiency aggravates diabetes-induced podocyte injury due to DNA damage caused by mitochondrial dysfunction. Int J Med Sci. 2020;17(17):2763-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang X, Wu Y, Li Q, et al. . CD36 promotes podocyte apoptosis by activating the pyrin domain-containing-3 (NLRP3) inflammasome in primary nephrotic syndrome. Med Sci Monit. 2018;24:6832-6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mitrofanova A, Sosa MA, Fornoni A. Lipid mediators of insulin signaling in diabetic kidney disease. Am J Physiol Renal Physiol. 2019;317(5):F1241-F1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li G, Zhang Q, Hong J, Ritter JK, Li PL. Inhibition of pannexin-1 channel activity by adiponectin in podocytes: Role of acid ceramidase activation. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(10):1246-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitrofanova A, Burke G, Merscher S, Fornoni A. New insights into renal lipid dysmetabolism in diabetic kidney disease. World J Diabetes. 2021;12(5):524-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9(11):1663-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gottlieb RA, Bernstein D. Mitochondrial remodeling: rearranging, recycling, and reprogramming. Cell Calcium. 2016;60(2):88-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dugan LL, You YH, Ali SS, et al. . AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123(11):4888-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S-8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou D, Zhou M, Wang Z, et al. . PGRN acts as a novel regulator of mitochondrial homeostasis by facilitating mitophagy and mitochondrial biogenesis to prevent podocyte injury in diabetic nephropathy. Cell Death Dis. 2019;10(7):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qu H, Gong X, Liu X, et al. . Deficiency of mitochondrial glycerol 3-phosphate dehydrogenase exacerbates podocyte injury and the progression of diabetic kidney disease. Diabetes. 2021;70(6):1372-1387. [DOI] [PubMed] [Google Scholar]
- 64. Wang XX, Edelstein MH, Gafter U, et al. . G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. 2016;27(5):1362-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mise K, Galvan DL, Danesh FR. Shaping up mitochondria in diabetic nephropathy. Kidney360. 2020;1(9):982-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang XX, Wang D, Luo Y, et al. . FXR/TGR5 dual agonist prevents progression of nephropathy in diabetes and obesity. J Am Soc Nephrol. 2018;29(1):118-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Toyama EQ, Herzig S, Courchet J, et al. . Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351(6270):275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Haroon S, Vermulst M. Linking mitochondrial dynamics to mitochondrial protein quality control. Curr Opin Genet Dev. 2016;38:68-74. [DOI] [PubMed] [Google Scholar]
- 69. Geto Z, Molla MD, Challa F, Belay Y, Getahun T. Mitochondrial dynamic dysfunction as a main triggering factor for inflammation associated chronic non-communicable diseases. J Inflamm Res. 2020;13:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hongbo M, Yanjiao D, Shuo W, Kun S, Yanjie L, Mengmeng L. Podocyte RNF166 deficiency alleviates diabetic nephropathy by mitigating mitochondria impairment and apoptosis via regulation of CYLD signal. Biochem Biophys Res Commun. 2021;545:46-53. [DOI] [PubMed] [Google Scholar]
- 71. Su J, Chen X, Xiao Y, et al. . Bruceae fructus oil inhibits triple-negative breast cancer by restraining autophagy: dependence on the gut microbiota-mediated amino acid regulation. Front Pharmacol. 2021;12:727082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ising C, Koehler S, Brähler S, et al. . Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol Med. 2015;7(3):275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ayanga BA, Badal SS, Wang Y, et al. . Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol. 2016;27(9):2733-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim K, Lee EY, Excessively enlarged mitochondria in the kidneys of diabetic nephropathy. Antioxidants (Basel) 2021;10(5):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125(Pt 4):795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rüb C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017;367(1):111-123. [DOI] [PubMed] [Google Scholar]
- 77. Wu H, Wei H, Sehgal SA, Liu L, Chen Q. Mitophagy receptors sense stress signals and couple mitochondrial dynamic machinery for mitochondrial quality control. Free Radic Biol Med. 2016;100:199-209. [DOI] [PubMed] [Google Scholar]
- 78. Fang L, Zhou Y, Cao H, et al. . Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One 2013;8(4):e60546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171(8):1917-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Audzeyenka I, Rogacka D, Piwkowska A, Angielski S, Jankowski M. Viability of primary cultured podocytes is associated with extracellular high glucose-dependent autophagy downregulation. Mol Cell Biochem. 2017;430(1-2):11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Audzeyenka I, Rachubik P, Typiak M, et al. . Hyperglycemia alters mitochondrial respiration efficiency and mitophagy in human podocytes. Exp Cell Res. 2021;407(1):112758. [DOI] [PubMed] [Google Scholar]
- 82. Cang X, Wang X, Liu P, et al. . PINK1 alleviates palmitate induced insulin resistance in HepG2 cells by suppressing ROS mediated MAPK pathways. Biochem Biophys Res Commun. 2016;478(1):431-438. [DOI] [PubMed] [Google Scholar]
- 83. Su Z, Nie Y, Huang X, et al. . Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol. 2019;10:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu HY, Han J, Cao SY, et al. . Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284(45):31484-31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xin W, Li Z, Xu Y, et al. . Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism. 2016;65(9):1307-1315. [DOI] [PubMed] [Google Scholar]
- 86. Li Z, Yuan Y, Meng Y, Rong Y, Bai H, Chen L. Autophagy upregulation ameliorates cell injury in Sequestosome 1 knockout podocytes in vitro. Biochem Biophys Res Commun. 2017;490(2):98-103. [DOI] [PubMed] [Google Scholar]
- 87. Li W, Du M, Wang Q, et al. . FoxO1 promotes mitophagy in the podocytes of diabetic male mice via the PINK1/Parkin pathway. Endocrinology. 2017;158(7):2155-2167. [DOI] [PubMed] [Google Scholar]
- 88. Rogacka D, Audzeyenka I, Piwkowska A. Regulation of podocytes function by AMP-activated protein kinase. Arch Biochem Biophys. 2020;692:108541. [DOI] [PubMed] [Google Scholar]
- 89. Emma F, Montini G, Parikh SM, Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol. 2016;12(5):267-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maassen JA, ‘T Hart LM, Van Essen E, et al. . Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103-S109. [DOI] [PubMed] [Google Scholar]
- 91. Jansen JJ, Maassen JA, van der Woude FJ, et al. . Mutation in mitochondrial tRNA(Leu(UUR)) gene associated with progressive kidney disease. J Am Soc Nephrol. 1997;8(7):1118-1124. [DOI] [PubMed] [Google Scholar]
- 92. Hall AM, Vilasi A, Garcia-Perez I, et al. . The urinary proteome and metabonome differ from normal in adults with mitochondrial disease. Kidney Int. 2015;87(3):610-622. [DOI] [PubMed] [Google Scholar]
- 93. Sidhom E-H, Kim C, Kost-Alimova M, et al. . Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J Clin Invest. 2021;131(5):e141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Widmeier E, Yu S, Nag A, et al. . ADCK4 deficiency destabilizes the coenzyme Q complex, which is rescued by 2,4-dihydroxybenzoic acid treatment. J Am Soc Nephrol. 2020;31(6):1191-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ge M, Molina J, Ducasa GM, et al. . APOL1 risk variants affect podocyte lipid homeostasis and energy production in focal segmental glomerulosclerosis. Hum Mol Genet. 2021;30(3-4):182-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mishra A, Ayasolla K, Kumar V, et al. . Modulation of apolipoprotein L1-microRNA-193a axis prevents podocyte dedifferentiation in high-glucose milieu. Am J Physiol Renal Physiol. 2018;314(5):F832-F843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zheng W, Guo J, Liu ZS. Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective. Clin Epigenetics 2021;13(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stoccoro A, Coppedè F. Mitochondrial DNA methylation and human diseases. Int J Mol Sci. 2021;22(9):4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zheng LD, Linarelli LE, Liu L, et al. . Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin Epigenetics. 2015;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de Oliveira AA, de Oliveira TF, Bobadilla LL, et al. . Sustained kidney biochemical derangement in treated experimental diabetes: a clue to metabolic memory. Sci Rep. 2017;7:40544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deng Q, Wen R, Liu S, et al. . Increased long noncoding RNA maternally expressed gene 3 contributes to podocyte injury induced by high glucose through regulation of mitochondrial fission. Cell Death Dis. 2020;11(9):814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J Am Soc Nephrol. 2018;29(4):1223-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liang Y, Liu H, Zhu J, et al. . Inhibition of p53/miR-34a/SIRT1 axis ameliorates podocyte injury in diabetic nephropathy. Biochem Biophys Res Commun. 2021;559:48-55. [DOI] [PubMed] [Google Scholar]
- 105. Dong W, Zhang H, Zhao C, Luo Y, Chen Y, Silencing of miR-150-5p ameliorates diabetic nephropathy by targeting SIRT1/p53/AMPK pathway. Front Physiol. 2021;12:624989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Long J, Badal SS, Ye Z, et al. . Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126(11):4205-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee YH, Kim SH, Kang JM, et al. . Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol. 2019;317(4):F767-F780. [DOI] [PubMed] [Google Scholar]
- 108. Liu X, Xu C, Xu L, et al. . Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism. 2020;111:154334. [DOI] [PubMed] [Google Scholar]
- 109. Zhang Q, He L, Dong Y, et al. . Sitagliptin ameliorates renal tubular injury in diabetic kidney disease via STAT3-dependent mitochondrial homeostasis through SDF-1α/CXCR4 pathway. FASEB J. 2020;34(6):7500-7519. [DOI] [PubMed] [Google Scholar]
- 110. Cassis P, Locatelli M, Cerullo D, et al. . SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 2018;3(15):e98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Croteau D, Luptak I, Chambers JM, et al. . Effects of sodium-glucose linked transporter 2 inhibition with ertugliflozin on mitochondrial function, energetics, and metabolic gene expression in the presence and absence of diabetes mellitus in mice. J Am Heart Assoc. 2021;10(13):e019995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wei PZ, Szeto CC. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta. 2019;496:108-116. [DOI] [PubMed] [Google Scholar]
- 113. Hou Y, Li S, Wu M, et al. . Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310(6):F547-F559. [DOI] [PubMed] [Google Scholar]
- 114. Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes. 2017;66(4):791-800. [DOI] [PubMed] [Google Scholar]
- 115. Guder WG, Schmolke M, Wirthensohn G. Carbohydrate and lipid metabolism of the renal tubule in diabetes mellitus. Eur J Clin Chem Clin Biochem. 1992;30(10):669-674. [PubMed] [Google Scholar]
- 116. Secker PF, Beneke S, Schlichenmaier N, et al. . Canagliflozin mediated dual inhibition of mitochondrial glutamate dehydrogenase and complex I: an off-target adverse effect. Cell Death Dis. 2018;9(2):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.