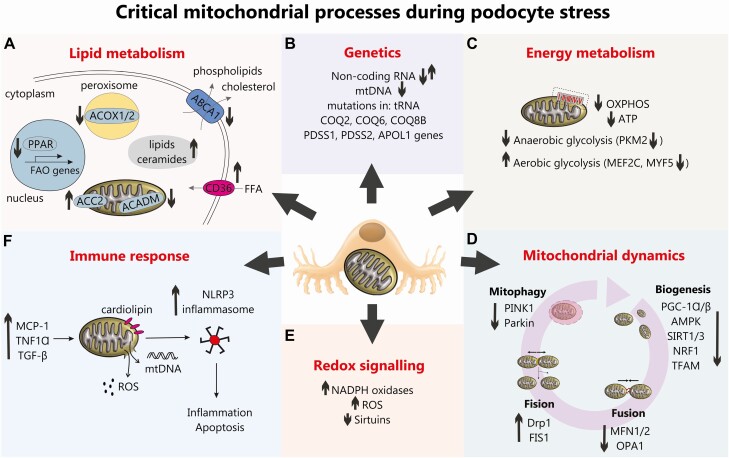
Figure 1.
An overview of critical mitochondrial processes during podocyte stress. (A) Lipid metabolism. An upregulated CD36 in DN promotes free fatty acids (FFA) uptake, whereas downregulated ABCA1 hampers extracellular transport of phospholipids and cholesterol. Dysregulated lipid metabolism in podocytes in DN is also associated with the decreased fatty acids β-oxidation (FAO), which results from the reduced expression of peroxisome proliferator-activated receptor (PPAR), peroxisomal acyl-CoA oxidase (ACOX1/2) and mitochondrial acyl-CoA dehydrogenase (ACADM), as well as the increased ACC2 activity. All this results in accumulation of lipids and ceramides in cytoplasm. (B) Genetics. Mutations in mitochondrial genes COQ2, COQ6, COS8B, PDSS1, PDSS2, and APOL1 have all been associated with DN and noncoding RNAs (eg, miRNA-21 and long-noncoding RNAs Tug1 and Meg3) have been shown to regulate podocyte bioenergetics and fusion/fission in DN. (C) Energy metabolism. A reduction in oxidative phosphorylation (OXPHOS) and ATP production has been demonstrated in DN in addition to podocyte “glycolytic switch.” (D) Mitochondrial dynamics. A reduction in podocyte mitochondrial biogenesis pathways are observed in DN alongside a reduction in mitochondrial fusion, an increase in podocyte mitochondrial fission (22, 70) and a reduction in podocyte mitophagy (81). (E) Altered podocyte redox signaling in DN is evidenced by an increase in NADPH oxidases (32), an increase in reactive oxygen species (34), a reduction in sirtuins which can lead to a reduction in FOXO1 (62). (F) Increased immune signaling (eg, increased MCP-1, tumor necrosis factor-α and TGF-β) in DN can damage podocyte mitochondria and cause an increase in ROS production and release of mtDNA which along with oxidized cardiolipin accumulated in mitochondrial outer membrane, can trigger NLRP3-inflammasome assembly leading to inflammation and apoptosis.