Abstract
Adipose tissue distribution in the human body is highly heterogeneous, and the relative mass of different depots is differentially associated with metabolic disease risk. Distinct functions of adipose depots are mediated by their content of specialized adipocyte subtypes, best exemplified by thermogenic adipocytes found in specific depots. Single-cell transcriptome profiling has been used to define the cellular composition of many tissues and organs, but the large size, buoyancy, and fragility of adipocytes have rendered it challenging to apply these techniques to understand the full complexity of adipocyte subtypes in different depots. Discussed here are strategies that have been recently developed for investigating adipocyte heterogeneity, including single-cell RNA-sequencing profiling of the stromal vascular fraction to identify diverse adipocyte progenitors, and single-nuclei profiling to characterize mature adipocytes. These efforts are yielding a more complete characterization of adipocyte subtypes in different depots, insights into the mechanisms of their development, and perturbations associated with different physiological states such as obesity. A better understanding of the adipocyte subtypes that compose different depots will help explain metabolic disease phenotypes associated with adipose tissue distribution and suggest new strategies for improving metabolic health.
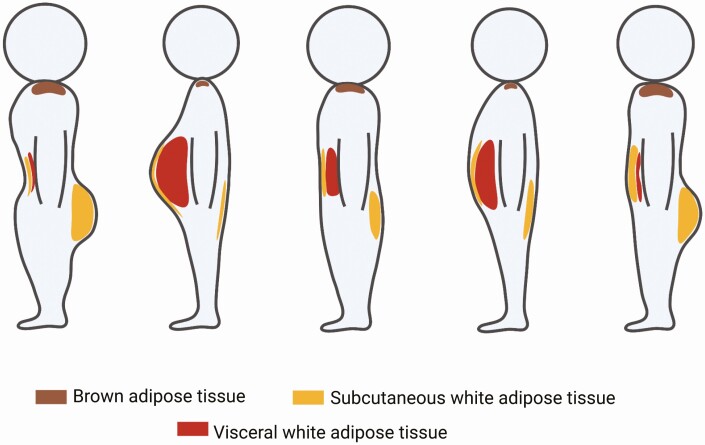
Body weight and height are 2 routinely measured descriptors of an individual’s shape and size. The body mass index (BMI) is a standard composite metric based on weight and height used for assessing degree of overweight and obesity. Obesity is quantitatively defined as a condition where a person has a BMI of 30 or higher [1]. Yet, these metrics do not sufficiently capture the full complexity of human body shape, and BMI is not an accurate predictor of metabolic disease risks. In fact, individuals with identical BMI may have drastically different body shapes (Fig. 1). For example, athletes may have a high BMI because of large muscle mass but maintain excellent metabolic health. Conversely, inactive, low-BMI individuals with large waist circumferences are at higher risk for developing metabolic diseases. The current BMI criteria are inadequate in assessing metabolic health risk, as exemplified by the acknowledgement that Asian populations have different associations between BMI, percentage of body fat, and health risks compared with European populations [2].
Figure 1.
Heterogeneous adipose tissue distribution contributes to diversity in body shape. Individuals with identical BMI may have drastically different body shapes because of different adipose depot volumes in different parts of the body. In the extremes, “apple-shaped” individuals have adipose tissue concentrated in the abdomen (second left) and “pear-shaped” individuals have relatively high gluteal and femoral adipose tissue volume (left most). In other cases, individuals may have relatively even adipose distribution.
One potential reason for the discrepant associations between BMI and cardiometabolic disease risk is that this metric does not capture differences in adipose tissue distribution that characterize human body shapes. A “pear-shaped” or “hourglass-shaped” body type describes an individual with relatively high adipose tissue volume in the gluteal and femoral regions; “apple-shaped” individuals have significant adipose accumulation in the abdomen. These body shapes are better captured by the ratio of waist-to-hip circumferences, which correlate well with the ratio of intra-abdominal to subcutaneous fat content measured by tomography [3], and is a more effective measure for metabolic disease risk [4, 5].
Importantly, the relative distribution of fat between depots is plastic and can vary with physiological conditions. For example, redistribution from peripheral to the central body region is seen in women undergoing menopausal transition, and is associated with the development of type 2 diabetes [6]. Conversely, a shift in relative adiposity from visceral to subcutaneous depots is seen with rosiglitazone usage, which coincides with improved glucose tolerance [7]. The findings of dynamic changes between adipose depots in response to physiological and pharmacological conditions suggest that modifying the relative distribution of adipose depots may be a therapeutic strategy for improving metabolic health. The approach requires understanding of the cellular composition and mechanisms underlying depot-specific adipose tissue development and expansion.
Adipocyte Heterogeneity in Adipose Depots
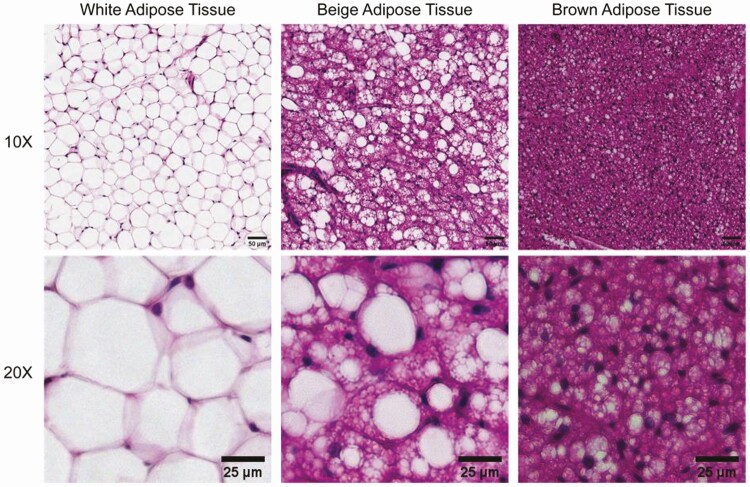
The differences in metabolic disease risks associated with adipose tissue distribution has prompted examination of adipose tissue from different anatomical regions, both in mice and in humans. The most remarkable difference between adipose depots is between brown adipose tissue of mice, which is localized in the interscapular region, and white inguinal adipose tissue (Fig. 2). White adipose tissue contains adipocytes that are much larger in size, have a single large lipid droplet, and are primarily involved in energy storage during the fed state and energy release during fasting. In contrast, brown adipose tissue adipocytes are smaller in size, contain multiple lipid droplets, and their primary role is thermogenesis through lipid oxidation and uncoupled respiration. A third adipocyte subtype, the beige/brite adipocyte, arises in predominantly white depots in response to cold, and its morphology is intermediate between white and brown adipocytes in both cell size and content of large and small lipid droplets (Fig. 2). In humans, most adipose depots appear to be composed of white adipocytes, but beige/brite adipocytes are found in the supraclavicular and paravertebral regions as well as in the perivascular adipose depots [8].
Figure 2.
Morphological features of brown, beige, and white adipocytes in adipose tissues. White adipocytes in white adipose tissue, exemplified by mouse gonadal fat (left) are larger and contain a single lipid droplet per cell. Brown adipocytes in mouse interscapular brown adipose tissue (right), are smaller in size and contain numerous lipid droplets. Beige adipocytes in inguinal fat of cold-exposed mice (middle), are intermediate in size and have characteristics of both white and brown adipocytes, having a large droplet and multiple small droplets in the same cell, or multiple small droplets. Bottom row displays a higher magnification image of each tissue type. Scale bar: 50 μm (top row); 25 μm (bottom row).
Despite morphological similarity, metabolomic and proteomic approaches have shown that different human white adipose depots are functionally different [9, 10]. These differences are also seen by bulk RNA-sequencing (RNA-seq), which produces an aggregate profile of all cells in the tissue. Because adipose tissue contains a large percentage of cells other than adipocytes, such as endothelial and immune cells, these results cannot establish whether differences in expression profiles between depots are attributable to adipocytes. Recent advances in adipose tissue single-cell RNA-seq profiling (scRNA-seq) can allow a deeper understanding of individual adipocytes within depots, and have the potential to reveal distinct subtypes of adipocytes, their abundance, and whether they contribute to body shape and metabolic health.
Single-cell Level Approaches to Identify Adipocyte Progenitors and Subtypes
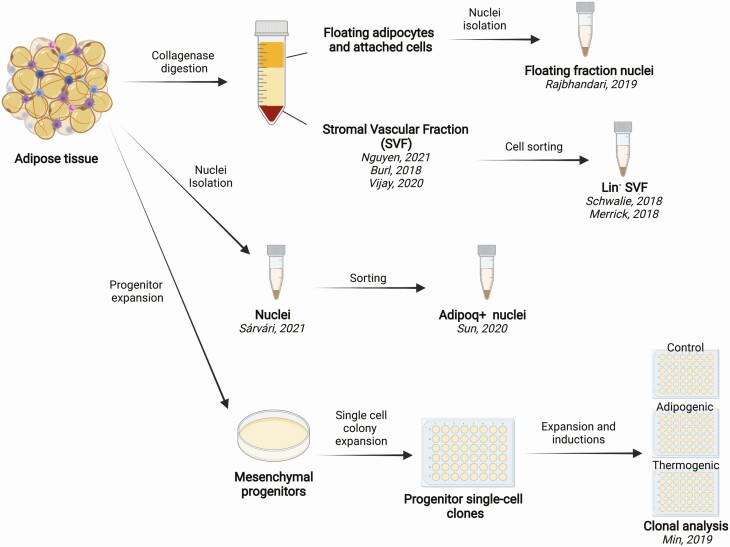
scRNA-seq permits assessment of cellular heterogeneity in multiple tissue types, and it has led to the discovery of new cell types and subtypes [11]. However, the application of single-cell technologies to studying adipose heterogeneity is limited because of the physical properties of mature adipocytes. A typical scRNA-seq protocol involves dissociation of a tissue or cultured cells into a single-cell suspension before construction of sequencing libraries. Mature adipocytes are large, highly buoyant, and adhere to each other and to other cells via an extensive extracellular matrix. Preparing single adipocytes requires harsh collagenase digestion, which can rupture or damage the large, lipid-filled cells. Cell loss during dissociation can be cell size dependent, further biasing the results. Moreover, the size of adipocytes and their buoyancy precludes the use of current droplet-based single-cell separation approaches [12]. Facing these limitations, a number of alternative strategies have been employed to investigate adipocyte heterogeneity at the single-cell level (Fig. 3).
Figure 3.
Multiple single-cell profiling approaches in assessing adipocyte heterogeneity. Because adipocytes in adipose tissue are difficult to isolate, different approaches have been used for assessing adipocyte heterogeneity at the single-cell level. Collagenase digestion separates adipose tissue into a floating cell layer and a stromal vascular fraction (SVF), which pellets after centrifugation. Cells in SVF are amenable to cell sorting and single-cell profiling, as exemplified in previous work [13-17]. The single-nuclei profiling approach has been applied to the collagenase-digested floating cell layer [18], as well as directly on adipose tissue [19, 20]. As an alternative to direct profiling of primary adipocytes, clonally expanded adipose tissue-derived progenitor cells were profiled before and after differentiation and thermogenic induction [21].
In some studies, the focus has been on profiling cells in adipose tissue that are devoid of large quantities of lipid. These cells are obtained after collagenase digestion of adipose tissue and centrifugation to pellet the stromal vascular fraction (SVF), separating them from lipid-laden, floating cells. The SVF contains multiple cell types, including immune, endothelial, and progenitor cells. As detailed later, some studies have profiled the entire SVF from mouse or human depots, whereas others have applied selection methods to enrich for adipocyte progenitors. A limitation of this approach is that heterogeneity arising later in the adipogenic differentiation process, as the maturing adipocytes accumulate lipid, is not captured in SVF profiles.
To directly assess heterogeneity of mature adipocytes, some of the studies reviewed below profile RNA within single nuclei isolated from adipose tissues (snRNA-seq). Limitations of this approach are that, because only the nuclear transcripts are measured, gene isoform information and mitochondrial RNA are not captured. Also, nuclear mRNA quantity may not accurately reflect total mRNA cellular levels. Despite these limitations, snRNA-seq enables profiling of this otherwise inaccessible cell type to reveal existing heterogeneity.
One of the advantages emerging from single-cell profiling is the ability to infer the trajectory leading to specific cell types from profiles of cells at different stages of differentiation. This has been best achieved in tissues with high turnover rates, such as the intestinal epithelium [22]. However, adipocytes have a notoriously slow turnover rate, with only ~10% of adult human adipocytes turnover annually [23]. This slow turnover makes it unfeasible to capture, in an accessible sample of tissue, the number of primary cells at each step of differentiation necessary to establish the full human adipogenesis trajectory. As an alternative to single-cell profiling of tissue, our laboratory and others have taken a clonal analysis approach to investigate adipocyte development and heterogeneity [24-26]. This approach profiles clones expanded from single progenitor cells, before and after adipose differentiation. The approach has the caveat of biasing for highly proliferating progenitor clones and of eliminating developmental cues that may be present in intact tissue. Yet, it provides a means for closely monitoring the adipogenesis process and the option to test multiple experimental conditions.
More recently, a new method has been developed to capture both contextual and gene expression profiling information in a single experiment. This spatial transcriptomic approach combines expression profiling with position information on sliced frozen tissue, using an array of barcoded reverse transcription primers [27]. Although the approach does not guarantee measurements at single-cell resolution, it does not require single-cell isolation and may be amendable for investigating adipose heterogeneity. It remains to be evaluated whether the protocol for this technique is amenable for adipocytes with its large size and high lipid content.
Current Insights Derived From Studies of the SVF
The SVF from mouse inguinal adipose tissue was among the first to be profiled with single-cell technology [13-16]. These studies revealed the multiple cell types that comprise adipose tissue, including numerous subtypes of immune cells, endothelial cells, and multiple adipocyte progenitor subpopulations. Although the age of mice profiled and the cell sorting strategies differed, all studies observed distinct stem-like (Cd34+, Dpp4+) cells as well as committed adipocyte precursors (Pparg+), within the progenitor population. These studies also revealed the existence of subsets of progenitor cells that can exert antiadipogenic effects [13, 28], and regulate thermogenesis [29].
Nguyen et al. identified a novel age-dependent, nonadipogenic population when comparing SVF of young (10 weeks) and aged (48 weeks, 72 weeks) mice [16]. Cells in this population express preadipocyte markers (Cd34, Pdgfra, and Pref-1) and inflammatory markers (Lgals1, Cd36, and NFkB1), suggesting an adipose progenitor origin. These aging-induced cells inhibited proliferation and differentiation of neighboring adipose precursors. Further understanding of the features of different adipocyte progenitor subtypes, and of the mechanisms by which they impair differentiation will be necessary to understand their normal physiological function and whether they play a role in metabolic disease.
An extensive profiling study of human adipose SVF from multiple depots of 14 obese individuals showed that progenitors clustered primarily by depot of origin and subsequently by degree of adipose differentiation [30]. Notably, among the visceral-depot progenitors, a subpopulation carries a unique mesothelial signature (MSLN, ITLN1) and higher expression of mitochondrial genes, whereas other subpopulations represented progenitors present across multiple different depots. The mesothelial signature is consistent with earlier reports of visceral adipose’ mesothelial origin [31]. The authors speculate that the high mitochondrial expression in this progenitor population might reflect the potential to give rise to beige adipocytes within the visceral adipose depot, yet the adipogenic differentiation capacity of this subpopulation remains to be investigated.
Current Insights Derived From snRNA-seq
Upon cold exposure or thermogenic stimulation, some white adipose tissue depots acquire a beige phenotype. To characterize the beige adipocyte expression profile and developmental trajectory, Rajbhandari et al. [18, 32] used the snRNA-seq approach. They isolated the nuclei of floating adipocytes from the subcutaneous inguinal white adipose tissue of mice housed at room temperature, after 4-day cold exposure, or after stimulation with the beta-adrenergic agonist CL-316243. Their results revealed multiple adipocyte subtypes, differing in genes with known roles in adipose tissue functions. They also observed an adipocyte subpopulation expressing transcripts associated with lipolysis (Adrb3, Acsl2, Lipe, Pnpla2). Transcripts associated with thermogenesis (Ucp1, Ppargc1a, Cidea, Dio2) were preferentially induced in this adipocyte subpopulation [18]. These results are consistent with the existence of a specific adipocyte subtype that is more lipolytic and gives rise to “beige” adipocytes in response to thermogenic stimuli.
Sun et al. [29] used snRNA-seq to profile mouse interscapular brown adipose tissue after 4-day cold exposure, 120-day thermoneutrality, or room temperature conditions. They identified a subpopulation of nuclei from mouse inguinal and visceral white adipose depots that increased with higher environmental temperature, expressing Cyp2e1. This population was also detected in human deep-neck adipose tissue, within both unilocular and multilocular adipocytes. Knockdown of Aldh1a1, which was tightly coexpressed with Cyp2e1, resulted in upregulation of Ucp1 and other thermogenic genes in the tissue, suggesting that changes in metabolism within specific adipocyte subtypes can affect vicinal adipocytes and overall depot function.
To investigate the dynamics of adipose tissue remodeling during the development of obesity, Sárvári et al. [19] profiled the nuclei of the epididymal white adipose tissue from mice fed a low-fat or high-fat diet for 18 weeks. They identified subpopulations with transcripts suggestive of a high capacity for de novo lipogenesis (Acaca, Elovl6, Igf2r, and Pparg), and others specialized in lipid uptake (Abcg1, Apoe, and Cd36). Intriguingly, stressed, lipid-scavenging adipocytes also have high expression of genes involved in hypoxia and autophagy (Gadd45g, Hif1a, Rab7). The 3 subtypes are differentially represented in the experimental systems, with high-fat diet fed mice having higher proportion of stressed lipid-scavenging adipocytes and near absence of lipogenic adipocytes.
Current Insights Derived From Clonal Approaches
We and other laboratories have taken a clonal analysis approach to investigate human adipocyte heterogeneity [24-26]. Xue et al. and Shinoda et al. [25, 26] used immortalized preadipocytes from human supraclavicular tissue and found they differentiated into distinct adipocyte subtypes, including one with characteristics of thermogenic cells. We leveraged a methodology that generates large numbers of human adipocyte progenitors [33] to clonally expand 52 human primary progenitor cells from abdominal subcutaneous adipose tissue. Clones were split into replicate wells and subjected to 15-day adipose differentiation, 12-day adipose differentiation followed by 3-day thermogenesis stimulation, or maintained in the undifferentiated state. Gene expression profiles obtained from samples in the adipose differentiated state segregated into 4 cell subtypes [34], one of which displayed high responsiveness to thermogenic stimulation and uniquely expressed the human thermogenic adipocyte marker LINC00473 [35]. This adipocyte subtype was further characterized by differences in genes controlling iron metabolism, consistent with cells being primed for development of iron-dependent thermogenic activity, and was enriched in KCNK3, which was found enriched in thermogenic adipocytes by Shinoda et al. [26]. Profiles of the other adipocyte subtypes also revealed functional specialization, including differences in adiponectin and leptin production. The clonal analysis results are in agreement with results obtained directly from profiling of adipose tissues, which reveal a high level of heterogeneity indicative of important functional differences between adipocytes.
Conclusions
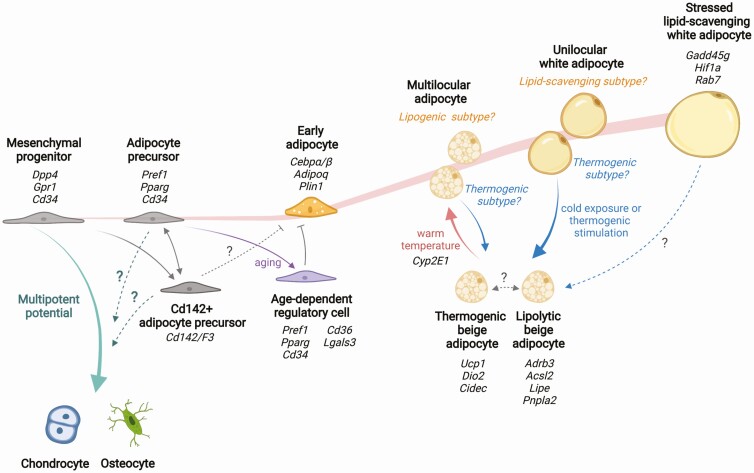
The application of single-cell profiling technology to adipose tissue research has produced large, complex datasets from diverse clinical samples and preclinical models. Many genes that distinguish cell subpopulations are not associated with known cellular mechanisms, pointing to the many gaps in our knowledge of adipocyte and adipose tissue functions. Nevertheless, this work has produced consistent new findings, including cells that can inhibit adipose differentiation [13, 16], adipocyte subpopulations with higher lipolytic or hypertrophic potential [18, 19], and adipocytes primed for thermogenic activity [34]. The integration of current knowledge suggests a potential model of adipocyte development trajectory and cellular interactions that contribute to the formation of distinct adipose depots (Fig. 4).
Figure 4.
Model of adipose development based on single-cell transcriptomics. New adipocytes are developed from multipotent mesenchymal progenitors. Once committed to adipocyte fate, cells accumulate lipid droplets and expand in size. Mature white adipocytes contain single large lipid droplet (unilocular), whereas developing adipocytes and beige adipocytes have multiple smaller lipid droplets per cell (multilocular). Gene signatures of adipocytes in different developmental states are identified from single-cell RNA-sequencing of the progenitors and single nuclei RNA-sequencing of the mature adipocytes. The developmental stages of the reported adipocyte subtypes are inferred based on knowledge of adipocyte development. The full trajectory and the determinants for cell states or fate transitions remain to be identified.
Additional research characterizing the properties of diverse cell subpopulations and state transitions are critical, and require the development of robust experimental systems to test mechanistic hypotheses. Ultimately, as a comprehensive view of the adipose development landscape emerges, we anticipate being able to more accurately assess the metabolic state of an obese individual, and with knowledge of factors determining adipocyte states, we aspire to design personalized interventions effective in improving metabolic health.
Acknowledgments
Funding: Work in the Corvera laboratory related to the topic of this review is funded by grants R01 DK123028 and DK089101.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Centers for Disease Control and Prevention. Defining adult overweight & obesity. April 28, 2021. https://www.cdc.gov/obesity/adult/defining.html. Accessed November 2, 2021.
- 2.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation; 8-11 December 2008. Geneva: World Health Organization. 2011. [Google Scholar]
- 3. Ashwell M, Cole TJ, Dixon AK. Obesity: new insight into the anthropometric classification of fat distribution shown by computed tomography. Br Med J (Clin Res Ed). 1985;290(6483):1692-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vazquez G, Duval S, Jacobs DR Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115-128. [DOI] [PubMed] [Google Scholar]
- 5. Emdin CA, Khera AV, Natarajan P, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317(6):626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mtintsilana A, Micklesfield LK, Chorell E, Olsson T, Goedecke JH. Fat redistribution and accumulation of visceral adipose tissue predicts type 2 diabetes risk in middle-aged black South African women: a 13-year longitudinal study. Nutr Diabetes. 2019;9(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Punthakee Z, Alméras N, Després JP, et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet Med. 2014;31(9):1086-1092. [DOI] [PubMed] [Google Scholar]
- 8. Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11(4):253-256. [DOI] [PubMed] [Google Scholar]
- 9. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697-738. [DOI] [PubMed] [Google Scholar]
- 10. Raajendiran A, Krisp C, Souza DP, et al. Proteome analysis of human adipocytes identifies depot-specific heterogeneity at metabolic control points. Am J Physiol Endocrinol Metab. 2021;320(6):E1068-E1084. [DOI] [PubMed] [Google Scholar]
- 11. Camp JG, Platt R, Treutlein B. Mapping human cell phenotypes to genotypes with single-cell genomics. Science. 2019;365(6460):1401-1405. [DOI] [PubMed] [Google Scholar]
- 12. Chen G, Ning B, Shi T. Single-cell RNA-seq technologies and related computational data analysis. Front Genet. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwalie PC, Dong H, Zachara M, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559(7712):103-108. [DOI] [PubMed] [Google Scholar]
- 14. Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, Granneman JG. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 2018;28(2):300-309.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merrick D, Sakers A, Irgebay Z, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364(6438):eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen HP, Lin F, Yi D, et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev Cell. 2021;56(10):1437-1451.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vijay J, Gauthier MF, Biswell RL, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2(1):97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajbhandari P, Arneson D, Hart SK, et al. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. Elife. 2019;8:e49501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sárvári AK, Van Hauwaert EL, Markussen LK, et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 2021;33(2):437-453.e5. [DOI] [PubMed] [Google Scholar]
- 20. Sun W, Dong H, Balaz M, et al. Single-nucleus RNA-Seq reveals a new type of brown adipocyte regulating thermogenesis. bioRxiv. 2020;587:98-102. [Google Scholar]
- 21. Min SY, Kady J, Nam M, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22(3):312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fawkner-Corbett D, Antanaviciute A, Parikh K, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell. 2021;184(3):810-826.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783-787. [DOI] [PubMed] [Google Scholar]
- 24. Min SY, Kady J, Nam M, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22(3):312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xue R, Lynes MD, Dreyfuss JM, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med. 2015;21(7):760-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinoda K, Luijten IH, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21(4):389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ståhl PL, Salmén F, Vickovic S, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353(6294):78-82. [DOI] [PubMed] [Google Scholar]
- 28. Hepler C, Shan B, Zhang Q, et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife. 2018;7:e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun W, Dong H, Balaz M, et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587(7832):98-102. [DOI] [PubMed] [Google Scholar]
- 30. Vijay J, Gauthier MF, Biswell RL, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2(1):97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16(4):367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raajendiran A, Ooi G, Bayliss J, et al. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep. 2019;27(5):1528-1540.e7. [DOI] [PubMed] [Google Scholar]
- 33. Rojas-Rodriguez R, Lujan-Hernandez J, Min SY, et al. Generation of functional human adipose tissue in mice from primed progenitor cells. Tissue Eng Part A. 2019;25(11-12):842-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Min SY, Desai A, Yang Z, et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc Natl Acad Sci U S A. 2019;116(36):17970-17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tran KV, Brown EL, DeSouza T, et al. Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473. Nat Metab. 2020;2(5):397-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.