Abstract
BACKGROUND
The Woven EndoBridge (WEB) device was granted premarket approval in the United States following results of the Woven EndoBridge Intrasaccular Therapy (WEB-IT) study. WEB-IT reported excellent adequate angiographic occlusion of treated aneurysms with a high safety profile. These results were achieved, however, in the context of a prospective study with strict inclusion criteria and rigorous training support.
OBJECTIVE
To review early as-practiced clinical experience with the WEB device in the United States.
METHODS
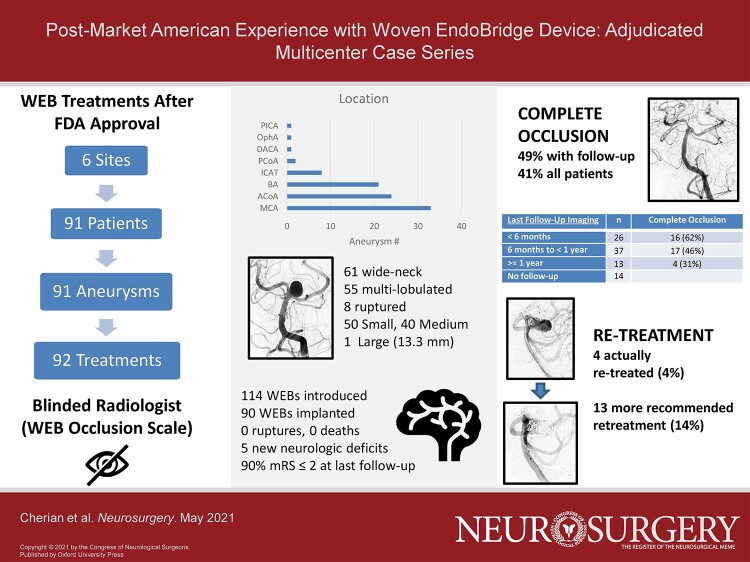
Retrospective review across 6 institutions identified 91 patients undergoing 92 treatment sessions for WEB device placement in treatment of 91 intracranial aneurysms. Details regarding demographics, aneurysm characteristics, treatment considerations, clinical outcomes, and aneurysm occlusion were obtained and analyzed in a multicenter database. Angiograms from the index procedure and follow-up studies were reviewed by a blinded and independent adjudicator.
RESULTS
The middle cerebral, anterior communicating, and basilar artery complexes were the commonly treated locations. Eight patients presented with ruptured aneurysms. A mean of 1.2 devices were introduced per case. Technical failure without deployment of a WEB device occurred in 2% (2/92) of sessions. Complete aneurysm occlusion for patients with imaging follow-up was 49% (mean follow-up of 8 mo). Four aneurysms were retreated. 90% of patients had modified Rankin Scale ≤ 2 at last clinical follow-up with no mortalities.
CONCLUSION
Immediate postmarket experience with the WEB device, newly introduced at American centers, confirms safe procedural use, but long-term efficacy remains unclear. Early challenges include accurate sizing and device selection.
Keywords: WEB, Intrasaccular flow disruption, Brain aneurysm
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- FDA
Food and Drug Administration
- IT
Intrasaccular Therapy
- SL
single-layer
- SLS
single-layer sphere
- WEB
Woven EndoBridge
- WNBA
wide-neck bifurcation aneurysm
The Woven EndoBridge (WEB) device (MicroVention, Aliso Viejo, California) is the first intrasaccular device for intracranial aneurysm treatment in the United States. It was granted Food and Drug Administration (FDA) approval following results of the Woven EndoBridge Intrasaccular Therapy (WEB-IT) study.1 WEB-IT demonstrated complete occlusion in 53.8% of treated wide-neck bifurcation aneurysms (WNBAs) at 12-month follow-up with high safety. These results were achieved, however, in the context of a prospective investigational device study with strict inclusion criteria and rigorous training support. This study seeks to ascertain early clinical results in the United States following market introduction.
METHODS
Population
Institutional Review Board approval (IRB #H-33 883) with a waiver for individual consent was obtained. The 6 enrolled institutions report endovascularly treating at least 100 aneurysms annually. One institution enrolled two patients in the WEB-IT (MicroVention) trial. Cases of patients undergoing WEB device treatment were identified. Details regarding patients, aneurysms, treatments, and clinical outcomes were summarized in a multicenter database. Complications during treatment and in follow-up were identified.
Endovascular Procedure
All procedures were performed using either transfemoral or transradial access. WEB devices were deployed through VIA microcatheters (MicroVention, Aliso Viejo, California). Although multiple devices may have been trialed for a given case, only one device was implanted. The WEB single-layer (SL) device was implanted in all cases except in two cases where the single-layer sphere (SLS) was used. Adjunctive techniques of balloon remodeling, coiling, and intracranial stenting were utilized in 5 cases.
In general, patients were prescribed aspirin and clopidogrel prior to WEB placement. Most patients were maintained on combined antiplatelet therapy for at least 1 mo following treatment. Platelet function testing was done before treatment in 80% of cases. Antiplatelet therapy was titrated at the discretion of each institution. In 2 of the 8 patients presenting with ruptured aneurysms, aspirin monotherapy was used prior to WEB deployment. No antiplatelet therapy was used in the remaining six.
Core Lab
Angiographic images were graded by an independent and blinded radiologist with over a decade of experience in interventional neuroradiology. Centers were instructed to provide treatment projections prior to device deployment, immediate postdeployment views, and last available follow-up images. Follow-up imaging studies were graded using the WEB occlusion scale (WOS) to calculate the rate of complete occlusion (WOS A or B).2
RESULTS
Cohort
Multicenter registry analysis identified 91 patients across 6 institutions with attempted treatment of 91 aneurysms using the WEB device (MicroVention) between 2019 and 2020 (see Table 1). 79% of patients were female and 25% harbored multiple aneurysms. Aneurysm location most often involved the middle cerebral, anterior communicating, and basilar artery complexes (see Table 2). Five aneurysms treated in this cohort would have been excluded by location in the WEB-IT study. Eight patients (9%) presented with ruptured aneurysms. Three patients had a history of aneurysm rupture at another site. One patient had a distant history of a ruptured aneurysm managed with coiling with significant recurrence.
TABLE 1.
Patient Demographics
| Age at treatment | n |
|---|---|
| 20-40 | 3 |
| 40-60 | 30 |
| 60-80 | 55 |
| >80 | 3 |
| Sex | |
| Male | 19 |
| Female | 72 |
| Presentation | |
| SAH | 8 |
| SAH at another site | 2 |
| Aneurysm recurrence | 1 |
| Compressive neuropathy | 1 |
| Other ICH or Stroke | 11 |
| Incidental | 70 |
| Stated Indication for Treatment | |
| Size | 47 |
| Location | 34 |
| Shape | 26 |
| Age | 11 |
| Ruptured | 8 |
| Multiplicity | 6 |
| Symptomatic | 6 |
| Growth | 6 |
| Family history | 5 |
| Prior hemorrhage | 2 |
| Risk factors | 1 |
| Recurrence | 1 |
TABLE 2.
Aneurysm Characteristics
| Location | N | |
|---|---|---|
| Middle cerebral artery | 33 | 36% |
| Anterior communicating artery | 24 | 26% |
| Basilar artery | 21 | 23% |
| Internal carotid artery terminus | 8 | 9% |
| Posterior communicating artery | 2 | 2% |
| Ophthalmic artery | 1 | 1% |
| Posterior inferior cerebellar artery | 1 | 1% |
| Distal anterior cerebral artery | 1 | 1% |
| Multilobulated | ||
| Yes | 55 | 60% |
| Maximum Measured Diameter | ||
| Small (<7 mm) | 50 | 55% |
| Medium (7 to 12 mm) | 40 | 44% |
| Large (13 to 24 mm) | 1 | 1% |
Maximum measured diameters ranged from 3.6 to 13.3 mm with a mean of 6.9 mm. All aneurysms except one were small or medium with a maximum measured diameter <13 mm. 67% (61/91) of targeted aneurysms were wide-neck intracranial aneurysms with a neck size ≥4 mm or a dome-to-neck ratio <2. 60% of aneurysms were multi-lobulated.
Treatment and Short-Term Follow-up
Patients underwent 92 endovascular treatment sessions with the WEB device for attempted treatment of 91 aneurysms (Figure, Supplemental Digital Content). Mean and median age at time of treatment were 63 and 65 yr. Aspirin and clopidogrel were given before 72% of cases. A GIIb/IIIa inhibitor was administered during 2 cases requiring stent implantation.
Transfemoral access was used exclusively in 74% of cases. In the remaining cases, transradial access was used with one case requiring eventual conversion to a transfemoral approach. Triaxial support with a long sheath was used in 74% of cases. 114 WEB devices were introduced in total across these treatment sessions with a mean of 1.2 devices trialed per case. Four patients required intracranial stenting after WEB device deployment with one of these cases also utilizing balloon microcatheter inflation. The fifth patient also required balloon microcatheter inflation during WEB deployment.
WEB device deployment was abandoned in 2 cases. In the first case done for treatment of an anterior communicating artery aneurysm, 2 introduced devices failed to protect the origins of the A2 branches, and no device was implanted. This patient would ultimately be successfully treated at a second treatment session with a third differently sized WEB device. In the second case, the VIA 21 microcatheter could not be delivered into the distal aneurysm neck and kicked out during attempted deployment of the SL 4-3 WEB device. The aneurysm was subsequently treated with primary coiling. This case was excluded from further analysis of WEB efficacy.
69% of patients were discharged by postprocedure day one and 93% of patients were discharged home (see Table 3). Two minor access complications of groin site bleeding were seen in addition to one major transfemoral access complication necessitating open vascular endarterectomy. Five procedures (5%) were complicated by new or worsened neurologic deficits in the postprocedure period with eventual near resolution of symptoms in three cases. One of these five was taken back to the endovascular suite and found to have an M2 occlusion. This was successfully managed with intracranial stenting following GIIb/IIIa inhibitor load.
TABLE 3.
Clinical and Angiographic Follow-up
| Postprocedure day of discharge | ||
| 0 day | 2 | 2% |
| 1 day | 62 | 67% |
| 2 day | 12 | 13% |
| 3 to 7 | 8 | 9% |
| 7 + | 8 | 9% |
| Discharge location | ||
| Home | 86 | 93% |
| Skilled nursing facility | 3 | 3% |
| Rehabilitation facility | 3 | 3% |
| Final mRS | ||
| 0 | 64 | 70% |
| 1 | 10 | 11% |
| 2 | 2 | 2% |
| 3 | 4 | 4% |
| 4 | 2 | 2% |
| 5 | 0 | 0% |
| 6 | 0 | 0% |
| Unknown | 10 | 11% |
| Blinded web occlusion | ||
| A (complete occlusion) | 19 | 21% |
| B (complete occlusion with device recess filling) | 18 | 20% |
| C (neck remnant) | 23 | 26% |
| D (aneurysm residual) | 16 | 18% |
| No follow-up or nondiagnostic | 14 | 16% |
Angiographic and Clinical Follow-up
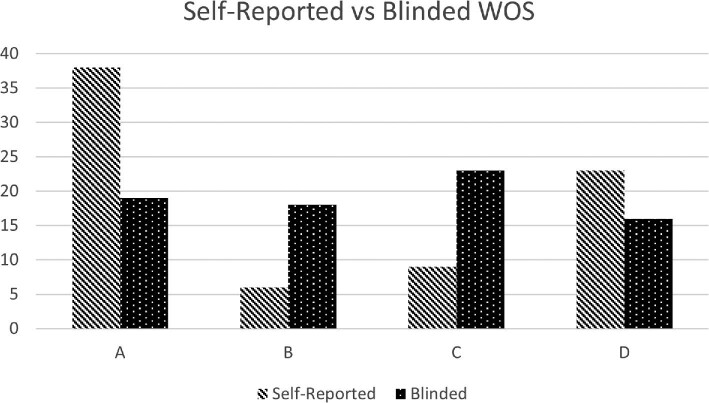
76 of the 90 (84%) WEB-treated aneurysms had available vascular imaging follow-up performed over a total of 95 imaging studies, 80 of which were formal angiograms. 49% (37/76) demonstrated complete occlusion at last available follow-up by independent blinded assessment. A total of 23 aneurysms demonstrated neck remnants and 16 demonstrated frank aneurysm residual. Mean imaging follow-up was 8 mo, with 50 patients having at least 6 mo of follow-up (see Table 4). When including the one aneurysm for which intrasaccular treatment could not be performed and those without follow-up, the complete occlusion rate was 41% (37/91). A comparison of operator reported occlusion vs blinded assessment is seen in Figure 1.
TABLE 4.
Patients With Complete Occlusion Grouped by Last Follow-up Imaging Period
| Last follow-up imaging period | n | Complete occlusion |
|---|---|---|
| <6 mo | 26 | 16 (62%) |
| 6 mo to < 1 yr | 37 | 17 (46%) |
| ≥1 yr | 13 | 4 (31%) |
| No follow-up or nondiagnostic | 14 |
FIGURE 1.
Bar graph comparing WEB Occlusion Scale (WOS) (MicroVention) results as scored by the performing institution and by blinded independent adjudicator for cases with imaging follow-up. A and B indicate complete occlusion. A = complete occlusion; B = complete occlusion with device recess filling; C = aneurysm neck remnant; D = aneurysm filling beyond aneurysm neck.
One anterior communicating, one middle cerebral, and two basilar apex aneurysms were retreated (4.4%, 4/91). In the first, residual filling on follow-up of a WEB-treated anterior communicating artery aneurysm was treated with Y-stent coiling. In the second, significant middle cerebral artery aneurysm recurrence seen on 6 mo follow-up was treated with coiling and flow diverter placement. In the third, a small area of residual aneurysm filling immediately following WEB device placement underwent retreatment with coiling after subsequent follow-up demonstrated significant aneurysm regrowth. In the fourth, a second WEB device was implanted after the initially deployed WEB device had compacted within the dome of the aneurysm on 1-yr follow-up. Excluding these four cases, aneurysm retreatment was recommended in 13 additional cases (14%) on the opinion of the independent blinded radiologist reviewing imaging follow-up.
In the 5 treated aneurysms at locations that would have been excluded in WEB-IT, follow-up was available in 4. Complete occlusion was seen on follow-up in one aneurysm involving the posterior inferior cerebellar artery. Residual neck filling was noted in treated ophthalmic and distal anterior cerebral artery aneurysms. Frank aneurysm residual was seen in a posterior communicating artery aneurysm.
Clinical follow-up was available on 86 of the 91 patients with mean follow-up exceeding 5 mo. There were no delayed aneurysmal ruptures. On last clinic follow-up, 89% had an modified Rankin Scale (mRS) of 0, and 96% of patients had mRS ≤ 2. Of the remaining 3 patients with a mRS of 3 on last clinic follow-up, 2 had presented with Hunt-Hess grade 5 subarachnoid hemorrhages. There were no mortalities.
Case Example 1
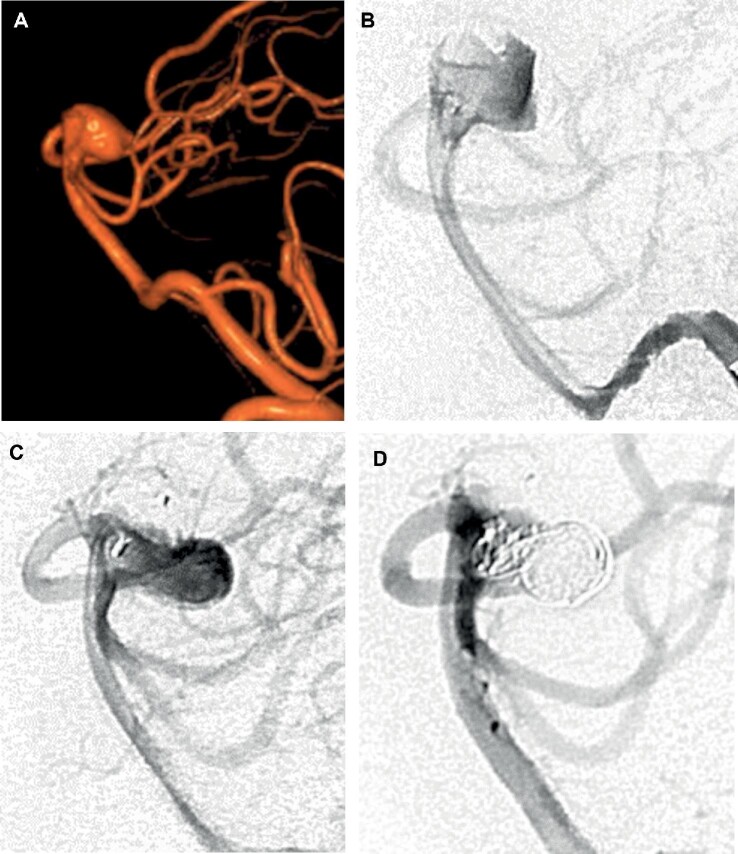
A 62-yr-old woman presented with double vision and was found to have an incidental 10-mm basilar apex aneurysm (Figure 2). She was offered elective aneurysm treatment and was started on aspirin 325 mg and clopidogrel 75 mg daily. WEB SL 10-4 device was initially selected but when deployed was found to be too large, covering the origins of the posterior cerebral and superior cerebellar arteries. WEB SL 9-4 device was then successfully deployed and found to be well-sized before being detached.
FIGURE 2.
Case example 1. 62-yr-old woman with incidental basilar apex aneurysm. A, AP projection prior to treatment. B, Deployed WEB SL 10-4 device is too large with covering of posterior cerebral and superior cerebellar artery origins. C, WEB SL 9-4 device successfully deployed and detached. D, 6-mo angiographic follow-up demonstrating complete aneurysm obliteration.
She was discharged home at her neurologic baseline on postprocedure day 3 after requiring management of hypertension, vomiting, and headache. She remained well on clinic follow-up at 1 mo and her clopidogrel was stopped. A total of 6-mo angiographic follow-up demonstrated complete aneurysm obliteration.
Case Example 2
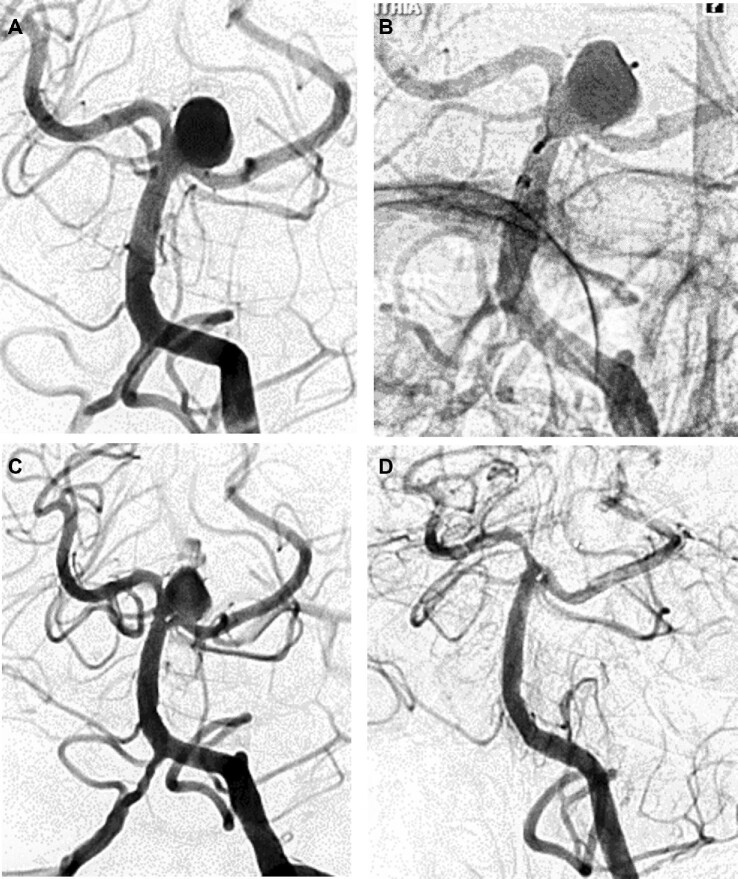
A 55-yr-old woman was found to have an incidental multilobulated wide-neck basilar apex aneurysm during headache workup (Figure 3). Moderate tortuosity of the basilar artery was noted. Elective treatment with the WEB device was offered. WEB SL 9-5 device was initially trialed but found to be too tall with parent vessel encroachment. Ultimately, SL 8-3 was implanted. Immediate postdeployment angiography demonstrated a small area of residual aneurysm filling. This residual area of filling grew significantly on 3-mo angiographic follow-up. She then underwent uneventful coiling of the residual area.
FIGURE 3.
Case example 2. 55-yr-old woman with incidental basilar apex aneurysm. A, Lateral projection of 3-dimensional reconstruction prior to treatment. B, WEB SL 8-3 successfully implanted, but small area of residual aneurysm filling seen. C, 3-mo angiographic follow-up demonstrates significant growth in residual filling. D, Growing residual treated with coiling.
DISCUSSION
WEB and WNBAs
The WEB device (MicroVention) is a self-expanding braided mesh intended for intrasaccular aneurysm implantation with subsequent disruption of blood flow into the aneurysm.3 Ideally, the fine mesh covers the aneurysm ostium and facilitates parent vessel reconstruction. The 2 models of the device (SL or SLS) differ in shape and size configurations. Given its shape and ability to withstand compaction when laterally compressed, the WEB device is conceptually attractive for management of WNBAs.4 WNBAs have proven difficult to treat with conventional techniques. A recent meta-analysis of WNBAs in the literature with core lab adjustment demonstrated complete aneurysm occlusion in 40% following primary coiling or stent coiling.5
WEB-IT was an adjudicated, prospective, multicenter single-arm premarket study that enrolled 150 adults with WNBAs.1 At 12-mo angiographic follow-up, 53.8% of treated aneurysms with follow-up had complete occlusion by the WEB occlusion scale.1,2 Only one primary safety event (0.7%) occurred during the study. As the first United States premarket approval trial of an intrasaccular aneurysm device, the WEB-IT study represented the first American clinical experience with the WEB device. Significant training support was provided, including hand-on experience using a lifelike simulator and in some cases patient-specific replicated models. Key exclusion criteria in WEB-IT included vascular tortuosity or unfavorable morphology.
An analysis of 3-yr results of WEB treatment in the combined population of 2 prospective European case series (WEBCAST and WEBCAST-2) was recently published.3 Inclusion of cases treated by the WEB device was performed autonomously in each center. Aneurysm occlusion was evaluated using a 3-grade scale: complete occlusion, neck remnant, and aneurysm remnant. Complete occlusion at the 3-yr follow-up was reported as 51%. The methodology of this analysis and its reported outcomes have been criticized, particularly regarding exclusion of retreatments in calculating device efficacy.6
Present Study: Postmarket United States WEB Experience
The present work represents clinical experience with the WEB device in the United States after market approval. Overall, safety of the device during the procedure was replicated. There were no intraoperative ruptures and only one technical failure to deliver the WEB device. Given 2 cases of postoperative stroke with persisting deficits, the primary safety endpoint as defined by WEB-IT would be 2.2% with no mortalities.
Independent and blinded adjudicated assessment of aneurysm occlusion was disappointing with a complete occlusion rate of 49% at mean follow-up of 8 mo. Complete occlusion was 42% (21/50) in patients with at least 6 mo follow-up and 31% (4/13) in patients with at least 1-yr follow-up. In predicting occlusion durability, WEB-IT demonstrated worsened grade occlusion in 12%, stable occlusion in 79%, and improved occlusion in 9% between 6- and 12-mo angiographic follow-up.1 The 49% complete occlusion rate is therefore unlikely to improve significantly, especially when considering that 21% of aneurysms had frank residual aneurysm filling on last follow-up.
Self-reported occlusion was higher than that seen with blinded adjudication (58% v 49% complete occlusion). This is unsurprising given previous comparisons between self-reported and core-laboratory adjudicated angiographic outcomes.7 However, this factor may be intensified by the subjective interpretation of the WEB device recess vs residual neck filling.
WEB-IT classified inability to deploy a WEB device and adjunctive implants as effectiveness failures. In this series, this class of effectiveness failure occurred in 6 treatments. When adjusting for this stricter assessment, complete occlusion was lower at 43% (34/79). If patients without follow-up are included, the complete occlusion was 37% (34/92). These rates approach obliteration rates seen with conventional endovascular techniques.
Though the retreatment rate as performed was low, 22% (17/76) either had retreatment or were recommended retreatment by blinded imaging assessment.
Challenges with WEB
Sizing is a significant technical issue. 21% of cases required introduction of at least 2 WEB devices and in 3 cases, three WEB devices were trialed in a single session. Trialing of multiple devices could incur significant marginal costs. After WEB implantation, adequate lateral compression of the device is needed to prevent device compaction.4,8 Undersizing may partly explain the poorer rates of complete occlusion seen in this series. Sizing failure, however, may not improve with experience as only certain sizes are available. An experienced European center reported an annual sizing failure rate of 25% throughout a 7-yr period.8
More difficult aneurysms might also decrease WEB efficacy. The aneurysms in this cohort are likely more representative of real-world practice than cases prospectively selected for WEB-IT. 60% of aneurysms were multilobulated in shape. 5.4% of cases required upfront rescue stenting or balloon-assistance. In one case, the WEB device could not be delivered. These results suggest added complexity from aneurysm shape and parent vessel tortuosity. Anatomic complexity would be expected to increase difficulty in sizing accurately, delivering the device, and deploying the device in the intended orientation. The challenge of matching device shape and size with a targeted aneurysm may be aided in the future by 3-dimensional modeling techniques.9,10
Dual-antiplatelet use at the time of intervention was higher than in WEB-IT (85% v 69%). This may reflect operator uncertainty and may evolve with increased experience. Although current FDA labeling11 states that the safety and effectiveness of the WEB device has not been established for ruptured aneurysms, some American centers have already reported its safe use in treating ruptured aneurysms.12 The number of ruptured aneurysms in this series is too small to clarify this issue, though there were no intraoperative ruptures during treatment of eight ruptured aneurysms. One of these cases done initially without antiplatelet therapy, was complicated by need for rescue stenting and GIIb/IIIa inhibitor loading.
Future Directions
Upon reviewing cases in the series, one subjective impression was that aneurysms projecting at an angle away from the parent vessel were more likely to occlude. Others have noted a strong correlation between WEB compaction and aneurysm nonocclusion with high arterial inflow.13 Formal quantification of aneurysm morphology and flow dynamics analyzed against aneurysm occlusion with the WEB device could be tested in future work.
Five patients in this series were treated at locations not indicated for WEB treatment, and 33% of treated aneurysms were not WNBAs. This validates prior predictions on the expansion of device usage following initial approval beyond originally studied indications.14 This postmarket experience suggests that the efficacy of the WEB device remains to be proven. A randomized trial comparing conventional endovascular techniques and microsurgery against WEB treatment would aid greatly in establishing device efficacy.6,15
CONCLUSION
Immediate postmarket clinical experience with the WEB device (MicroVention), newly introduced at American centers, confirms safe procedural use, but disappointing angiographic efficacy. Early challenges include accurate sizing and device selection. A randomized trial is needed.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors acknowledge the Endovascular Research Group.
Contributor Information
Jacob Cherian, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Neurosurgery, Emory University, Atlanta, Georgia, USA.
Stephen R Chen, Department of Radiology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ajit Puri, Department of Radiology, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Kunal Vakharia, Department of Neurological Surgery, Jacobs School of Medicine at Biomedical Sciences, Buffalo, New York, USA.
Elad Levy, Department of Neurological Surgery, Jacobs School of Medicine at Biomedical Sciences, Buffalo, New York, USA.
Sheila Eshraghi, Department of Neurosurgery, Emory University, Atlanta, Georgia, USA.
Brian M Howard, Department of Neurosurgery, Emory University, Atlanta, Georgia, USA.
Frank C Tong, Department of Neurosurgery, Emory University, Atlanta, Georgia, USA.
C Michael Cawley, Department of Neurosurgery, Emory University, Atlanta, Georgia, USA.
Bradley Gross, Department of Neurological Surgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Matthew D Alexander, Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, Utah, USA.
Ramesh Grandhi, Department of Neurosurgery, University of Utah, Salt Lake City, Utah, USA.
Visish M Srinivasan, Department of Neurological Surgery, Barrow Neurological Institute, Phoenix, Arizona, USA.
Jan-Karl Burkhardt, Department of Neurosurgery, Penn Medicine, Philadelphia, Pennsylvania, USA.
Jeremiah N Johnson, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA.
Peter Kan, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA; Department of Neurosurgery, University of Texas Medical Branch, Galveston, Texas, USA.
Supplemental Digital Content. Figure. Study flowchart. Flowchart outlining the relationship between centers, patients, aneurysms, treatments, and deployed WEB devices (MicroVention).
REFERENCES
- 1. Arthur AS, Molyneux A, Coon AL et al. The safety and effectiveness of the woven endobridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: final 12-month results of the pivotal WEB intrasaccular therapy (WEB-IT) study [published online ahead of print: April 16, 2019] J NeuroIntervent Surg. doi:10.1136/neurintsurg-2019-014815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fiorella D, Arthur A, Byrne J et al. Interobserver variability in the assessment of aneurysm occlusion with the WEB aneurysm embolization system. J NeuroIntervent Surg. 2015;7(8):591-595. [DOI] [PubMed] [Google Scholar]
- 3. Pierot L, Szikora I, Barreau X et al. Aneurysm treatment with WEB in the cumulative population of two prospective, multicenter series: 3-year follow-up [published online ahead of print: June 12, 2020]. J NeuroIntervent Surg doi:10.1136/neurintsurg-2020-016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal N, Hoit D, DiNitto J et al. How to WEB: a practical review of methodology for the use of the woven endobridge [published online ahead of print: January 31, 2020].J NeuroIntervent Surg. doi:10.1136/neurintsurg-2019-015506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiorella D, Arthur AS, Chiacchierini R, Emery E, Molyneux A, Pierot L. How safe and effective are existing treatments for wide-necked bifurcation aneurysms? Literature-based objective performance criteria for safety and effectiveness. J NeuroIntervent Surg. 2017;9(12):1197-1201. [DOI] [PubMed] [Google Scholar]
- 6. Chapot R, Mosimann PJ, Darsaut TE, Raymond J. Retreatments must be included in the evaluation of device performance [published online ahead of print: September 14, 2020]. J NeuroIntervent Surg. doi:10.1136/neurintsurg-2020-016619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rezek I, Lingineni RK, Sneade M, Molyneux AJ, Fox AJ, Kallmes DF. Differences in the angiographic evaluation of coiled cerebral aneurysms between a core laboratory reader and operators: results of the cerecyte coil trial. AJNR Am J Neuroradiol. 2014;35(1):124-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Rooij WJ, van Rooij SBT, Kortman HG, Peluso JP. The woven endobridge finally coming home across the atlantic: what to expect? AJNR Am J Neuroradiol. 2018;39(11):1964-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jang L, Alvarado J, Pepona M et al. Three-dimensional bioprinting of aneurysm-bearing tissue structure for endovascular deployment of embolization coils [published online ahead of print: September 25, 2020]. Biofabrication. doi:10.1088/1758-5090/abbb9b. [DOI] [PubMed] [Google Scholar]
- 10. Yan EG, Rennert RC, Levy DM, Levy ML. Three-Dimensional modeling of complex pediatric intracranial aneurysmal malformations with a virtual reality system [published online ahead of print: September 2, 2020]. doi:10.1097/SIH.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 11. Premarket Approval (PMA). Accessed April 28, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P170032. Accessed April 28, 2021. [Google Scholar]
- 12. Al Saiegh F, Hasan D, Mouchtouris N et al. Treatment of acutely ruptured cerebral aneurysms with the woven endobridge device: experience Post-FDA approval. [published online ahead of print May 1, 2020]. doi:10.1093/neuros/nyaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caroff J, Mihalea C, Da Ros V et al. A computational fluid dynamics (CFD) study of WEB-treated aneurysms: can CFD predict WEB “compression” during follow-up? J Neuroradiol. 2017;44(4):262-268. [DOI] [PubMed] [Google Scholar]
- 14. Fahed R, Darsaut TE, Raymond J. The introduction of innovations in neurovascular care: patient selection and randomized allocation. World Neurosurg. 2018;118:e99-e104. [DOI] [PubMed] [Google Scholar]
- 15. Raymond J, Januel A-C, Iancu D et al. The RISE trial: a randomized trial on intra-saccular endobridge devices. Interv Neuroradiol. 2020;26(1):61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.