Key words: Germ-free mice, microbial diversity hypothesis, microbiota, Trichuris muris
Parasitic worms are amongst the most common pathogens to infect humans and have a long-established history of inflicting disease in their hosts. There is a large body of evidence that states intestine-dwelling helminths ensure their survival by influencing the host immune response against them. In recent years, it has become apparent that the large and diverse microbial communities that exist in the gastrointestinal (GI) tract of the host and within the parasite itself have a pivotal role in worm survival and persistence. Using a variety of mouse models (including laboratory, germ-free and rewilded mice), there have been new insights into how bacteria and worms interact with each other; this includes the discovery that Trichuris is unable to hatch and/or infect their host in the absence of bacteria, and that these worms contain a Trichuris-specific gut microbiota. These interactions are determined in part by the capacity of the host, gut microbiota and worms to communicate via metabolites such as butyrate, which are microbially derived and have known immunoregulatory properties. By exploring the contribution of gut bacteria to worm infections and the intricate relationship that exists between them, an exciting and emerging field in whipworm parasitology is established.
Introduction
The accepted view of the gastrointestinal (GI) tract is an open tube from the mouth to the anus that has evolved to control and interact with the external environment. The host GI tract is the largest immunological organ in the body; involving mechanisms to identify, control and eliminate dangerous pathogens present, while at the same time minimizing inappropriate inflammatory responses against benign antigens such as commensal bacteria and diet. In addition, the mammalian host GI tract also provides a favourable ecosystem with a stable temperature of approximately 37°C and a continual influx of nutrients that permit the growth of a complex microbiota. While numerically bacteria are the largest inhabitants of the GI tract, the GI tract has a number of diverse macro- and microbiotic communities which include viruses, fungi, protozoans and of particular interest for this review intestinal-dwelling parasitic nematodes.
Almost every mucosal surface on a mammal's body is heavily colonized with a variety of bacteria. Colonization begins at birth and initiates a variety of complex processes that lead to the establishment of a truly symbiotic relationship between the microbiota and host. We are only beginning to understand the numerous roles the microbiota plays in human health and the development, progression and resolution of disease. The gut microbiota (bacteria that inhabit the GI tract) has been the focus of intense research for the last few decades and is known to contribute to immunity, host metabolism and behaviour. The microbiota is known to mediate these functions by both direct (cell-to-cell) and indirect (bacterial-derived by-products) mechanisms which can involve interactions with the mammalian host immune cells during development (Elahi et al., 2013; Gensollen et al., 2016), hormone and behaviour signalling in the endocrine system (Lyte and Bailey, 1997; Diaz Heijtz et al., 2011), influencing disease progression of autoimmune disease such as inflammatory bowel disease (IBD), (Halfvarson et al., 2017) or type 1 diabetes (Markle et al., 2013), as well as affecting drug metabolism and efficacy (Zimmermann et al., 2019). From the time of birth, there is a known bidirectional interplay between the mammalian host and microbiota that is influenced by the composition, in terms of both diversity and abundance, of the gut microbiota which impacts host health (Dominguez-Bello et al., 2010).
While gut microbiota coevolves with the host immune system to influence host health, key factors that influence this process include enhanced sanitation and antibiotic usage, which are different in low/middle-income countries compared to high-income countries. Epidemiological evidence indicates that in high-income countries, there is low to no incidence of enteric parasite infections, but there has been a steady rise in autoimmune and allergic-related conditions, like asthma, eczema, diabetes and IBD in humans (Bach, 2002). Improved diagnostic tools to identify the incidence and prevalence of disease have likely contributed to the rise in autoimmune and allergic-related conditions observed over time; however, it is also well documented that impeding parasite exposure (and the subsequent effects of parasitic infections) in early life results in a disruption of the normal immune development and leads to a greater predisposition to autoimmune and allergic diseases in life (Strachan, 1989; Bach, 2002). This theory is called the ‘hygiene hypothesis’ (Strachan, 1989; Bach, 2002), and has been further refined to the ‘microbial diversity’ hypothesis (also known as the ‘old friends’ hypothesis or ‘microflora hypothesis’) which proposes that enhanced sanitation and hygiene in high-income countries has resulted in a reduction of microbial exposure and/or gut microbiota composition in early life, that alters immune development and causes a predisposition to allergic diseases (Wold, 1998; Rook, 2009; Scudellari, 2017). Therefore, the means by which the gut microbiota can impact human health is highly complex and varied, and there is growing evidence of epidemiological, microbiological and immunological data that support these hypotheses. However, there tends to be a focus on research that examines the bidirectional relationship between the microbiota and host. The presence of enteric multicellular parasites adds an additional level of complexity, and increases our understanding of the pervasive importance of the gut microbiota, and how these relationships impact mammalian host health and disease. Here we will focus on the interaction between the gut microbiota and enteric parasites, specifically whipworm infections by Trichuris and its dependency on the gut microbiota for propagation, persistent infection and survival in the mammalian gut.
The gut microbiota
The gut microbiota is a highly dynamic community of >1000 different bacterial species primarily comprised of phyla belonging to Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria and Verrucomicrobia (Arumugam et al., 2011). In return for the host providing a protective, nutrient-rich niche for the gut microbiota to inhabit, some of these bacteria perform essential functions for host health including the synthesis of vitamins (such as vitamin K, Gustafsson, 1959), the degradation of complex nutrients (Bäckhed et al., 2005), limiting pathogen invasion (Stecher et al., 2005) and contributing to immune system development (Stappenbeck et al., 2002; Flint et al., 2012). During the first three years of life, there is a succession of colonization events, for humans, this is marked by events such as a diet change from milk to solid foods that promotes the development of a stable gut microbial composition that is able to contribute to host health and readily adapt to lifestyle changes over a life time (Wu et al., 2011). Antibiotic usage is the most robust and quick-acting factor that can alter microbial diversity, closely followed by microbial changes induced by changes to the host diet (Jakobsson et al., 2010; David et al., 2013). An imbalance in the microbial community (referred to as a state of dysbiosis) can have a profound impact on increasing host susceptibility to disease and it is typically associated with an increase of facultative anaerobic bacteria (Shin et al., 2015; Rizzatti et al., 2017).
Technological advancements in the ability to decipher the composition and function of the microbiota, including targeted culturing techniques for recalcitrant strains, culture-independent techniques including 16S rRNA amplicon sequencing and whole-genome sequencing coupled with metabolomics and computational analysis have transformed our understanding of how important the gut microbiota is in host health (Browne et al., 2016). Bacterial composition of the microbiota, in particular microbial diversity, is crucial, as it increases the likelihood of containing species that are capable of degrading complex carbohydrates into microbial fermentation products being present in the GI tract. The gut microbiota is dominated by obligate anaerobes, specifically the phyla Firmicutes (Gram-positive) and Bacteroides (Gram-negative). Members of these phyla are characterized by encoding a large variety of enzymes that hydrolyse complex dietary and host carbohydrates that provide benefit to the host (Flint et al., 2012; El Kaoutari et al., 2013). For example, short-chain fatty acids (SCFAs) are small organic carboxylic acids (with chain lengths up to six carbons), of which acetate (C2), propionate (C3) and butyrate (C4) are the most abundant metabolites produced from anaerobic fermentation of dietary fibre and resistant starch. There is growing evidence that SCFA function as metabolic moieties in crosstalk between the host and gut microbiota to exert immunomodulatory activity upon the host. Namely these metabolites have also been identified as an energy supply for colonocytes, regulation of T regulatory (Treg) cell populations in the mucosal immune system and a potential role for treatment of autoimmune diseases (Cummings et al., 1987; Furusawa et al., 2013; Gill et al., 2018).
Germ-free animal models and Trichuris
Germ-free animals do not contain any detectable microorganisms, and are commonly used as a reductionist approach to investigating the relationship between the host and single (or known multiple-gnotobiotic) bacterial species (Smith et al., 2007; Flint et al., 2012). Recently these approaches have been used to triangulate the relationships between the host, its microbiota and intestinal multicellular organisms (such as helminths).
In the late 19th century, Louis Pasteur was the first to postulate that bacteria residing in the GI tract formed a relationship with the host that was critical for survival (Pasteur, 1885). This theory was perceived to be true, until a decade later it was disproved by raising germ-free guinea-pigs, and demonstrating life was possible without bacteria (Nuttal and Thierfelder, 1895). However, throughout the early 20th century, scientists continued to pursue the understanding of the relationship between the host and the bacteria that inhabit the GI tract. Using a variety of animal models and human subjects, researchers found that bacteria must be confined to mucosal surfaces for health (including areas such as the intestinal lumen, nasal cavity and aspects of the reproductive system), and a breach of this containment resulted in severe sepsis and/or death (Cushing and Livingood, 1900; Reyniers, 1959). Today, germ-free mice are the most common germ-free model used due to the number of immune-comprised models available, and their ability to breed frequently with large litters in a single cage. Germ-free mice are born and raised in sterile flexible-film isolators maintained under positive pressure to the external environment, and receive sterile chow, bedding, water and HEPA-filtered sterile air to limit exposure to live microbial antigens (Smith et al., 2007). Interaction and manipulation of the gut microbiota by enteric worms as a potent mechanism to regulate the host immune system and worm persistence are emerging as a topic of study where the use of germ-free models can provide us insight into this intricate and complex relationship. For intestinal parasitic infections, such as Trichuris, there is a critical dependency on a living host for propagation and survival, making germ-free models a means to provide mechanistic insight into the relationship between bacteria and worm infection (Hayes et al., 2010; Leung et al., 2018; White et al., 2018).
Helminth-microbe interactions
Microbial cues to induce Trichuris muris infection
Trichuris trichiura is one of the most prevalent soil-transmitted parasites, and infects approximately 500 million people worldwide (World Health Organization, 2020). Although whipworm infections rarely cause mortality in humans, individuals with a high worm burden can suffer from severe morbidities including diarrhoea/abdominal pain, malnutrition and infected children often have impaired growth/physical development that impacts their health status for life (World Health Organization, 2020). Bacterial diversity and density vary along the GI tract, with the densest microbial population residing within the caecum and colon of both mice and humans, reaching upwards of 100 trillion bacteria. Intestinal whipworms also inhabit this microbial-dense niche, and it is highly likely that these worms and the surrounding microbiota interact with each other (either directly or indirectly), as well as their mammalian host. An overview of how Trichuris interacts with the mammalian host, in particular the host immune system during acute and/or chronic infection, and the details of the Trichuris life cycle is presented in this special issue by Mair et al. (2021).
To decipher how bacteria in the human GI tract interacts with T. trichiura, researchers use mouse models as the host, and infect mice with the murine-specific T. muris whipworm. Trichuris muris infection follows the same strict oral-faecal route of infection, and worms embed themselves in mouse caecum and colon tissue, which closely mimics human whipworm infection and pathology (Klementowicz et al., 2012). As with T. trichiura, T. muris remain within the large intestine (which includes the caecum and colon) for their lifespan, and this has resulted in a complex relationship between the parasite and mouse microbiota, where it is known that the parasite manipulates and exploits the gut bacteria to induce persistent infection (White et al., 2018). It is now apparent that T. muris is dependent on cues from both the host and gut microbiota to initiate infection and generate pathology in the host. Embryonated eggs incubated with gut explants containing caecal bacteria, or bacterial cultures of E. coli (Hayes et al., 2010) and/or Staphylococcus aureus (Koyama, 2013) were shown to provide important microbial cues that enable successful worm larvae (stage one, L1) hatching to occur at the start of infection. Hayes et al. (2010) also found that the intestinal bacteria formed aggregates around egg poles, and that this interaction was mediated by adhesion organelles on the surface of bacterial cell membranes, namely type 1 fimbria. Hatching of embryonated eggs from the closely related whipworm T. suis (pig trichuriasis) did not occur using the same bacterial species (both Gram-negative Escherichia coli strains and Gram-positive Lactobacilli, Streptococci and Enterococci strains) that induce T. muris hatching (Vejzagić et al., 2015), suggesting that host–worm–microbiota specific interactions exist. Antibiotic treatment prior to T. muris infection resulted in a significant depletion of gut microbiota that reduced hatching rates, further demonstrating a dependence of Trichuris on the microbiota to initiate infection (Hayes et al., 2010).
The interaction of Trichuris muris and the gut microbiota
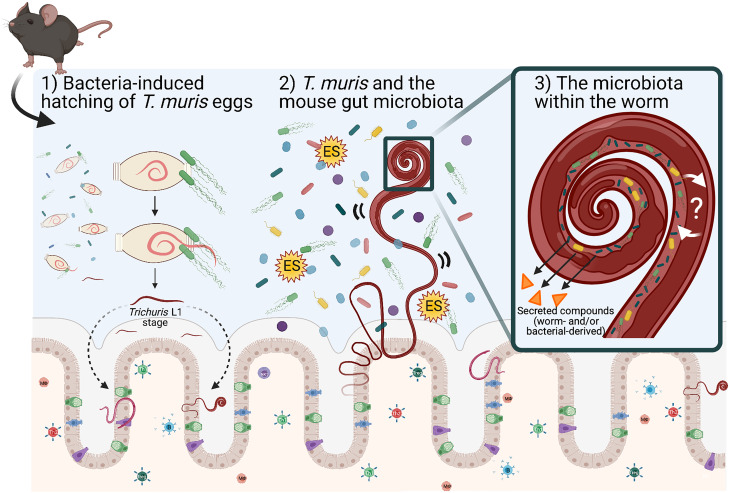
Many enteric parasites have a tropism for the large intestine, which is the same mucosal niche as the majority of bacteria in the gut microbiota. Within this niche, Trichuris and the gut microbiota are likely to interact with each other through either direct or indirect mechanisms, and it is this interaction and relationship that can influence each other's growth and survival in the mammalian GI tract. Trichuris muris infection protects immunodeficient mice (Nod2−/−) from intestinal abnormalities associated with this IBD-mouse model by enhancing the growth of protective bacterial populations like the Clostridiales, and in turn inhibiting pro-inflammatory species (Ramanan et al., 2016). What mechanisms are responsible for inducing changes in the mouse gut microbiota composition (in terms of abundance and diversity) and how they subsequently induce anti-inflammatory responses in the host are largely unknown. We propose mechanisms for altering the gut microbiota may include the physical presence of whipworms in the lumen (worms embedded in intestinal epithelial cells may disrupt surface cell adhesions for bacteria and possible competition for space), secreted worm-derived compounds, competition for nutrients against members of the gut microbiota, and/or worm-induced changes in the host during infection that alter the local GI immune (Th1 or Th2) environment that may also affect the survival of members within the gut microbiota (Fig. 1).
Fig. 1.
Schematic representing known and proposed mechanisms of how T. muris interacts with the large intestinal microbiota. (1) Upon entry into the large intestine, worm eggs interact with bacteria to promote hatching and initiate infection of the large intestine. (2) Once embedded in host epithelium, T. muris is proposed to influence the composition of the mouse gut microbiota by direct and indirect mechanisms (competition for space and/or nutrients for growth, by the secretion of metabolites including Trichuris ES and bacterial-derived compounds). (3) T. muris has also evolved to have its own worm gut microbiota, which is derived from the mouse gut microbiota. The identity and function of worm-selective pressures on their gut microbiota, and how the worm-specific bacteria interact with the worm host are unknown. Schematic shows the gut epithelial barrier with epithelial cells (beige), Goblet cells (green), Tuft cells (blue) and enteroendocrine cells (purple) covered in thick mucus (grey). Image created with BioRender.com.
In the absence of all microorganisms, as observed in germ-free mice, a genetically susceptible host becomes resistant to T. muris infection using either embryonated eggs or in vitro hatched L1 larvae (White et al., 2018). On the other end of the spectrum, laboratory mice rewilded into the environment develop a more diverse microbiota which renders them more susceptible to T. muris infection with greater parasite burdens and worm biomass than their laboratory-dwelling counterparts (Leung et al., 2018).
Another study of rewilded mice found that the gut microbiota of T. muris-infected rewilded mice promoted the expansion of Firmicutes, Bacteroidetes, Proteobacteria phyla, and this compositional change was associated with a reduction in colonization resistance against other invading species (specifically members from the Enterobacteriaceae and Rikenellaceace families) when compared to rewilded uninfected mice (Bär et al., 2020). These findings provide further evidence of how important the microbiota is to whipworm infection, and how delicate the balance that exists between microbial diversity (in terms of both composition and abundance) and the severity and persistence for successful worm infection.
Using a well-established model of chronic worm infection, when laboratory wild-type mice are given a single low dose of embryonated eggs (20–30 T. muris eggs) upon transition from larvae to adult worms (day 28 post infection), the gut microbiota undergo compositional changes (Houlden et al., 2015; Schachter et al., 2020). Specifically, bacterial taxa like Firmicutes, Mucispirillum and Proteobacteria increase as a result of infection; whilst Parabacteroides and Prevotella decrease in abundance (Holm et al., 2015; Houlden et al., 2015; Schachter et al., 2020). The observed changes in microbial composition from infection appear to be parasite-dependent and transient in nature. In addition, this altered gut environment makes the host less favourable for re-infection with Trichuris (White et al., 2018), but whether or not it makes the microbiota more permissive to other infections, like Salmonella (as observed with infection of the small intestinal parasite Heligmosomoides polygyrus bakerii), has yet to be studied (Brosschot et al., 2021). However, three months post infection when no mature whipworms are detectable, the gut microbiota regains bacterial diversity equivalent to their uninfected counterparts (Holm et al., 2015; Houlden et al., 2015).
We know that the absence of a microbiota (germ-free mice) prevents Trichuris hatching and infection, whilst the presence of a highly diverse microbiota actually enhances host susceptibility to infection. However, more research is needed to understand how microbial diversity influences worm persistence and survival in the gut, and what role the microbiota may have in manipulating the host immune system response against the invading worms. It is well documented that susceptibility to worm infection is largely dictated by the host immune system response against parasitic infection; CD4+ T helper cell 2 (Th2) responses mount a targeted immune response which leads to rapid worm expulsion, whereas if the host mounts a CD4+ T helper cell 1 response against whipworm infection, the host experiences chronic infections with low worm burdens (Else et al., 1992). This is in contrast to other helminth infections where parasite-derived ES products activate tuft cells to initiate Th2 responses, and specifically tuft cell hyperplasia in the gut during chronic infections (Gerbe et al., 2016). Germ-free data from enteric roundworm infection using either antibiotic-treated of L3 H. polygyrus larvae or with eggs hatched using an auxotrophic E. coli strain (HA107 that cannot replicate in mice without exogenous supplementation of microbial amino acids) found that worms had impaired fitness as noted by reduced fecundity, and the germ-free hosts had mounted a more robust type 2 humoral responses in the absence of gut bacteria (Rausch et al., 2018; Russell et al., 2020) implicating a possible function of the microbiota in buffering host immune responses to worm infections. Mouse models with varying degrees of microbial diversity (from rewilded to single species colonization of germ-free mice) highlight how critical the composition of the gut microbiota is for Trichuris infection. Manipulation of the gut microbiota in mice to contain genetically altered bacteria may shed light on how these bacteria interact with Trichuris and what functions different genera of bacteria have that contribute to successful whipworm infection.
Reoccurring Trichuriasis and the gut microbiota
A limited number of field studies have been performed investigating the relationship between intestinal helminth infections and the host microbiota; although there are conflicting reports. A field study examining the microbiota composition of Ecuadorian children found T. trichuria infection did not induce significant changes in the gut microbiota composition (Cooper et al., 2013), whereas individuals infected with intestinal helminths (including Trichuris) within the Malaysian indigenous (Orang Asli) populations were found to have higher microbial diversity than their negative counterparts (Lee et al., 2014). It is possible that the conflicting data in human field studies, and their comparison to mouse models are due to the fact that infected individuals are rarely exposed to a single, low-dose helminth infection but rather continuous re-occurring infection by the same or multiple parasites. A recent study in Tanzania, was one of the first to identify a reduction in the abundance of gut bacteria: Bacteroidetes, Proteobacteria and Actinobacteria associated with a cohort of women and children solely infected with T. trichuria (Chen et al., 2021); which is similar to the microbial changes reported in mouse studies (Holm et al., 2015; Houlden et al., 2015). Parallels between human and mouse studies were further addressed by identifying bacterial taxa that increased in both T. trichuria-only infected Indonesian individuals and T. muris-infected mice, these genera included Bacteroides, Collinsella, Subdoligranulum, Escherichia/Shigella, Prevetoella and Streptococcus (Rosa et al., 2021). There has also been the development of a mouse model to partially address the discrepancy in human and mouse data that involves a trickle model of T. muris infection, where mice are routinely (weekly) infected with low doses of embryonated eggs, thereby closely mimicking the low-dose chronic human infection by T. trichiura (Glover et al., 2019). This simulated model of natural infection with repeated exposure to T. muris caused a decrease in microbial diversity early on during infection that rapidly resolved over time to control levels upon repeated exposure (Glover et al., 2019). Interestingly, this tickle model was associated with the development of partial protective immunity supporting the concept of a complex interplay between host immunity, parasite and microbiota. Overall, laboratory experiments and field studies indicate that primary infection by Trichuris influences the microbiota composition, but the true nature of the relationship between the microbiota and intestinal parasites remains largely unknown.
Trichuris muris’ intimate relationship with bacteria
While multiple studies suggest that the large metazoan parasite Trichuris has evolved to respond to microbial cues in the gut to initiate hatching (and infection) of the host, mature whipworms have also been shown to harbour their own unique gut microbiota by 16S rRNA gene denaturing gel electrophoresis and fluorescence in situ hybridization (White et al., 2018). Irrespective of the host microbiota composition, mature whipworms acquire a distinct microbiota selected from members residing in the host gut during infection (White et al., 2018). The worm microbiota is largely dominated by bacterial species belonging to the phylum Proteobacteria (White et al., 2018), which begs the questions as to how does the worm select these bacteria, why Proteobacteria and what role do these particular bacteria provide for worms? Currently, worm-specific selective pressures on bacteria are unknown, and whether the bacteria are selected based on their function in the gut microbiota, their ability to adapt to new environments (such as the worm gut) or if they are exploited as an energy source (food) still need to be answered. The fact whipworms evolve and generate their own microbiota adds complexity to the host–parasite–microbiota relationship and raises new questions in understanding each of the mutualistic relationships present in the gut. Future work may include using a defined model system of a single microbial species in a germ-free host with the nematode Trichuris to address these outstanding questions, simply because of the many advantages this system has to offer in terms of experimental tractability for exploring the relationship between Trichuris and the microbiota.
Metabolites as moieties for cross-talk
Trichuris has developed effective strategies against the host immune system by exploiting its immunoregulatory properties to permit chronic infection; identification of potential candidates in this cross-talk is likely to be metabolites produced by (directly or indirectly) the worm during infection. In chronic infection, T. muris worms reach maturity at day 33 post infection, at which point mucosal immune and pathological changes in response to infection have occurred, and the microbial population and the worm have adapted to each other. Faecal metabolite analysis at day 41 of chronic infection identified increased concentrations of amino acids including phenylalanine, serine, threonine, leucine, ornithine and glycine, with a decrease in the abundance of vitamin D2/D3 derivatives, fatty acid and glycerophospholipid metabolites, compounds involved in heme degradation and metabolites involved in the biosynthesis of tryptophan, lysine, cystine and arginine amino acids when compared to naïve samples (Houlden et al., 2015). This altered metabolic profile can be interpreted as a hallmark of the skewed gut microbiota due to chronic infection and the ability of the microbiota to differentially harvest resources from the host diet. What is currently unclear is the origin of these observed metabolite changes and the relative contributions of the host, parasite and microbiota.
A reduction in luminal SCFA butyrate occurs during infection, and in turn host cells downregulate the expression of butyrate transporters, suggesting a transition to utilising other readily available energy sources available in the gut (White, 2016). Bacteria are the major source of SCFA in the gut, specifically the abundance and function of butyrate producers including taxa within the Firmicutes phylum like Clostridiales clusters IV and XIVa, and Ruminococcaceae (Louis et al., 2010), as well as members of Bacteroidetes, Actinobacteria and Proteobacteria that produce and utilize butyrate for the synthesis of essential amino acids are able to influence host health. Many of these bacteria are reduced in number during worm infection, and contribute to the alterations of microbial-derived metabolites like butyrate (Houlden et al., 2015). Trichuris is unable to synthesize butyrate, and supplementation of butyrate during chronic infection had minimal impact on worm burden or disease severity (Colombo and Grencis, unpublished data). Interpretation of this data may suggest that the worm presence and infection in the large intestine induces a mucosal inflammatory response that makes the gut less favourable for the growth of butyrate-producing bacteria like Clostridia and Ruminococcacae that accounts for the reduction in butyrate-regulated FoxP3+ CD4+ Treg cells in the colonic lamina propria (Houlden et al., 2015).
Trichuris-specific metabolites
Trichuris muris eggs are devoid of bacteria (White et al., 2018); therefore, at this life cycle stage, all identified metabolites are likely to be worm origin (rather than microbial). Using mass spectrometry, T. muris-egg specific metabolites were identified to belong to amino acid metabolism, carbohydrate metabolism and nucleotide metabolism (Yeshi et al., 2020). Within 90 min of entering the gut, the first-stage larvae are released from the egg and begin to borough into the epithelium; at which point the worms begin to grow and mature and release proteins into the niche called excretory–secretory (ES) products (Bancroft et al., 2019). In contrast to other parasitic metabolites, such as those produced by Nippostrongylus brasiliensis infection; Trichuris ES-enriched fractions contain an abundance of fatty acids including SCFA, amino acids and enriched organic compounds (Wangchuk et al., 2019). Specifically, lactic acid, oleic acid, succinate acid, the SCFAs: propionate, butyrate and acetate; alanine, glutamine, sugars: fructose, galactose, glucosamine and glucose-6-phosphate; and adenine and uridine (nucleobases/nucleosides) were found to be increased (Wangchuk et al., 2019). However, T. muris lacks known gene homologs for the synthesis of these metabolites, for example, there are no gene clusters identified to generate de novo synthesis of SCFA butyrate in the worm genome (White, 2016). Therefore, the identified metabolites by Wangchuk et al. (2019) are more likely to be a combination of Trichuris-derived metabolites and metabolic compounds generated by their own gut microbiota, which adult worms acquire from the host microbiota.
In chronic infection, the immune system generates a ‘tolerogenic-like’ immune response against the worms, which is likely to be mediated from a number of Trichuris-specific anti-inflammatory metabolites increased in ES-fractions including glucosamine, uridine and butyrate (Wangchuk et al., 2019). High levels of succinate and lactic acid were found in the ES-fraction, and the immunoregulatory effect of butyrate, succinate and lactic acid may have a role in contributing to anaerobic metabolism in the caecum, which would reflect a possible beneficial trait of the worm gut microbiota (Wangchuk et al., 2019). Extracellular vesicles (EV) also present in the ES faction of T. muris are internalized by the host cells and been found to contain a multitude of proteins including those involved in proteolysis, mRNAs and microRNAs (Eichenberger et al., 2018). Recently, using mouse caecaloids (organoids made from the caecum), it has been shown that T. muris-specific EVs downregulate responses to nucleic acid recognition and type 1 interferon immune-mediated responses (Duque-Corre et al., 2020). How worm-specific EVs interact with the microbiota is unclear (Eichenberger et al., 2018). Not only does the identification of T. muris-secreted metabolites during infection provide a tool for understanding how whipworms interact with both the host and bacterial environment, but also may lead to the design of novel approaches to controlling chronic worm infections.
Treatments
A variety of anthelmintic drugs are used to treat Trichuris infections in humans and livestock. For T. muris infection anthelmintic drugs include, but are not limited to the administration of albendazole, mebendazole, levamisole, pyrantel pamoate and ivermectin that are also commonly used to treat T. trichiura infections (Keiser et al., 2012). How these drugs impact the gut microbiota in the host and worm remains largely elusive. Houlden et al. (2015) found mebendazole treatment on naïve mice did not influence the composition of the gut microbiota, however many of the other drugs have yet to be studied. A reduction in microbial species diversity was observed in antibiotic-treated mice prior to infection that led to both reduced worm burden in the mice, and a modulated T-cell-dependent immune response mounted against existing worms (Hayes et al., 2010; Houlden et al., 2015). Transient antibiotic treatment of mice prior to a high-dose T. muris infection led to enhanced susceptibility to infection (Scott et al., 2018) and it was proposed that this was dependent upon the generation of dysregulated macrophages promoting type 1 immune responses, again supporting a complex interplay between host and microbiota influencing infection outcome. As an alternative to drug therapy, repeated administration of live beneficial bacteria like the probiotic strain Lactobacillus rhamnosus (isolate JB-1) caused an acceleration of worm expulsion in mice, mediated by an IL-10/Goblet cell pathway induced in the murine host (McClemens et al., 2013). Manipulation of the host microbiota with probiotics to reduce worm burden further highlights the intricate relationship between the gut microbiota and whipworms during infection.
In humans, there is limited and conflicting information regarding the impact of anthelmintic treatment on the gut microbiota. The majority of infected individuals have worm burdens below clinical thresholds, and that can remain in their host for years contributing to poor nutrition and impaired growth and development, particularly in children (Callender et al., 1992; Nokes et al., 1992). It is unclear if the enhanced burden of chronic whipworms or the altered gut microbiota associated with these infections is the cause or effect of these health concerns in individuals, and if employing long-term administration of prebiotic and/or probiotic treatments to alter the gut microbiota would be a step forward in controlling parasitic infections.
Conclusions
The importance of the microbiota during helminth infection is becoming increasingly recognized, and is also the start of an exciting and crucial area of parasitological research. Microbial changes have been reported for multiple intestinal helminths including infections with Ascaris lumbricoides (Ramírez-Carrillo et al., 2020), H. polygyrus (Walk et al., 2010; Kreisinger et al., 2015), Necator americanus (hookworm; Cantacessi et al., 2014) and of interest for this review T. trichiura (Lee et al., 2014; Chen et al., 2021) and T. muris (Holm et al., 2015; Houlden et al., 2015; Schachter et al., 2020).
Using novel tools such as germ-free models to decipher the intricate relationship that the microbiota has with whipworms opens new perspectives on how these helminths function and infect their hosts. In addition, field studies of re-wilding mice, or potentially wild, naturally occurring infection mouse models provide evidence into the complexity and dependence of the gut microbiota and T. muris, and possible insight into T. trichuria infections. Helminth metabolites are also likely to be candidates for regulating the immune system, positively contributing to microbial richness and diversity in individuals who suffer from chronic inflammatory diseases, or in turn as potential candidates for anthelminthic drugs (as discussed in Garcia-Bustos et al., 2019 for Caenorhabditis elegans). Harnessing how enteric parasites, such as whipworms, interact and modulate the gut microbiota will open new avenues for exploring anthelminthic treatments and addressing the growing concern of the ‘microbial diversity’ hypothesis regarding the dramatic increase in the incidence of allergic and autoimmune diseases in developed countries. Human trials using Trichuris eggs (specifically T. suis) show promise as a probiotic agent, and has been used in trials as a treatment for multiple sclerosis (Fleming et al., 2011, 2019; Yordanova et al., 2021; Williams et al., 2017). More recently, clinical intervention trials using T. suis eggs against psoriasis and IBD are ongoing in North America, and it will be interesting to see how Trichuris can reduce symptoms mediated by an altered immune response and potentially by changes in gut microbial composition (Prosberg and Petersen, 2018–2021). Understanding how Trichuris interacts with the gut microbiota during infection is of particular interest, as it is the organ that harbours the largest and most diverse microbial community within our bodies. In conclusion, we anticipate there will be substantial growth in this field and more research examining how to harness this information to develop new helminth therapies that control the spread and infection in humans.
Author contributions
M.A.E.L. conducted the literature search, designed and wrote the review and figure. R.K.G. and I.S.R. provided feedback and comments on the manuscript.
Financial support
M.A.E.L. and R.K.G. are funded by Wellcome Trust investigator award (Z10661/Z/18/Z awarded to RKG). The Wellcome Trust Centre for Cell Matrix Research, University of Manchester is also supported by centre funding from the Wellcome Trust (203128/Z/16/Z).
Ethical standards
Not applicable.
Conflict of interest
None.
References
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende D, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD and Bork P (2011) Enterotypes of the human gut microbiome. Nature 73, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. New England Journal of Medicine 347, 911–920. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA and Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science (New York, N.Y.) 307, 1915–1920. [DOI] [PubMed] [Google Scholar]
- Bancroft A, Levy C, Jowitt T, Hayes K, Thompson S, Mckenzie E, Ball MD, Dubaissi E, France AP, Bellina B, Sharpe C, Mironov A, Brown SL, Cook PC, S MacDonald A, Thornton DJ and Grencis RK (2019) The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nature Communications 10, 2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär J, Leung J, Hansen C, Loke P, Hall A, Conour L and Graham A (2020) Strong effects of lab-to-field environmental transitions on the bacterial intestinal microbiota of Mus musculus are modulated by Trichuris muris infection. FEMS Microbiology Ecology 96, fiaa167. [DOI] [PubMed] [Google Scholar]
- Brosschot TP, Lawrence KM, Moeller BE, Kennedy MHE, FitzPatrick RD, Gauthier CM, Shin D, Gatti DM, Conway KME and Reynolds LA (2021) Impaired host resistance to Salmonella during helminth co-infection is restored by anthelmintic treatment prior to bacterial challenge. PLoS Neglected Tropical Diseases 15, e0009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D and Lawley TD (2016) Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender J, Grantham-Mcgregor S, Walker S and Cooper E (1992) Trichuris infection and mental development in children. The Lancet 339, 181. [DOI] [PubMed] [Google Scholar]
- Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, Nolan MJ, Mitreva M, Krause L and Loukas A (2014) Impact of experimental hookworm infection on the human gut microbiota. The Journal of Infectious Diseases 210, 1431–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Mozzicafreddo M, Pierella E, Carletti V, Piersanti A, Ali SM, Ame SM, Wang C and Miceli C (2021) Dissection of the gut microbiota in mothers and children with chronic Trichuris trichiura infection in Pemba Island, Tanzania. Parasites & Vectors 14, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P, Walker A, Reyes J, Chico M, Salter S, Vaca M and Parkhill J (2013) Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS ONE 8, e76573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Pomare E, Branch W, Naylor C and Macfarlane G (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H and Livingood L (1900) Experimental and surgical notes upon the bacteriology of the upper portion of the alimentary canal, with observations on the establishment there of an amicrobic state as a preliminary to operative procedures on the stomach and small intestine. John's Hopkins Hospital Reports 9, 543–549. [Google Scholar]
- David L, Maurice C, Carmody R, Gootenberg D, Button J, Wolfe LA, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ and Turnbaugh PJ (2013) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H and Pettersson S (2011) Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences 108, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N and Knight R (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the USA 107, 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa M, Schreiber F, Rodgers F, Goulding D, Forrest S, White R, Forrest S, White R, Buck A, Grencis RK and Berriman M (2020) Development of caecaloids to study host–pathogen interactions: new insights into immunoregulatory functions of Trichuris muris extracellular vesicles in the caecum. International Journal for Parasitology 50, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger R, Talukder M, Field M, Wangchuk P, Giacomin P, Loukas A and Sotillo J (2018) Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication. Journal of Extracellular Vesicles 7, 1428004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF and Way SS (2013) Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A, Armougom F, Gordon J, Raoult D and Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nature Reviews Microbiology 11, 497–504. [DOI] [PubMed] [Google Scholar]
- Else KJ, Hültner L and Grencis RK (1992) Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology 75, 232–237. [PMC free article] [PubMed] [Google Scholar]
- Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD, Field AS, Boland J and Fabry Z (2011) Probiotic helminth administration in relapsing–remitting multiple sclerosis: a phase 1 study. Multiple Sclerosis Journal 17, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J, Hernandez G, Hartman L, Maksimovic J, Nace S, Lawler B, Risa T, Cook T, Agni R, Reichelderfer M, Luzzio C, Rolak L, Field A and Fabry Z (2019) Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: results of the HINT 2 clinical trial. Multiple Sclerosis Journal 25, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint HJ, Scott KP, Louis P and Duncan SH (2012) The role of the gut microbiota in nutrition and health. Nature Reviews. Gastroenterology & Hepatology 9, 577–589. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K and Ohno H (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos JF, Sleebs BE and Gasser RB (2019) An appraisal of natural products active against parasitic nematodes of animals. Parasites & Vectors 12, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, Iyer S, Kasper D and Blumberg R (2016) How colonization by microbiota in early life shapes the immune system. Science (New York, N.Y.) 352, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM and Jay P (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P, van Zelm M, Muir J and Gibson P (2018) Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Alimentary Pharmacology & Therapeutics 48, 15–34. [DOI] [PubMed] [Google Scholar]
- Glover M, Colombo S, Thornton D and Grencis R (2019) Trickle infection and immunity to Trichuris muris. PLoS Pathogens 15, e1007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B (1959) Vitamin K deficiency in germfree rats. Annals of the New York Academy of Sciences 78, 166–174. [DOI] [PubMed] [Google Scholar]
- Halfvarson J, Brislawn C, Lamendella R, Vázquez-Baeza Y, Walters W, Bramer L, D'Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R and Jansson JK (2017) Dynamics of the human gut microbiome in inflammatory bowel disease. Nature Microbiology 2, 17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K, Bancroft A, Goldrick M, Portsmouth C, Roberts I and Grencis R (2010) Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science (New York, N.Y.) 328, 1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm J, Sorobetea D, Kiilerich P, Ramayo-Caldas Y, Estellé J, Ma T, Madsen L, Kristiansen K and Svensson-Frej M (2015) Chronic Trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of Lactobacilli. PLoS ONE 10, e0125495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden A, Hayes K, Bancroft A, Worthington J, Wang P, Grencis R and Roberts I (2015) Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: effects reversed by pathogen clearance. PLoS ONE 10, e0125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson H, Jernberg C, Andersson A, Sjölund-Karlsson M, Jansson J and Engstrand L (2010) Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 5, e9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Tritten L, Adelfio R and Vargas M (2012) Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasites & Vectors 5, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klementowicz J, Travis M and Grencis R (2012) Trichuris muris: a model of gastrointestinal parasite infection. Seminars in Immunopathology 34, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K (2013) Evidence for bacteria-independent hatching of Trichuris muris eggs. Parasitology Research 112, 1537–1542. [DOI] [PubMed] [Google Scholar]
- Kreisinger J, Bastien G, Hauffe HC, Marchesi J and Perkins SE (2015) Interactions between multiple helminths and the gut microbiota in wild rodents. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 20140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Tang MS, Lim YA, Choy SH, Kurtz ZD, Cox LM, Gundra UM, Cho I, Bonneau R, Blaser MJ, Chua KH and Loke P (2014) Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Neglected Tropical Diseases 8, e2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JM, Budischak SA, Chung The H, Hansen C, Bowcutt R, Neill R, Shellman M, Loke P and Graham AL (2018) Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biology 16, e2004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Young P, Holtrop G and Flint H (2010) Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environmental Microbiology 12, 304–314. [DOI] [PubMed] [Google Scholar]
- Lyte M and Bailey M (1997) Neuroendocrine–bacterial interactions in a neurotoxin-induced model of trauma. Journal of Surgical Research 70, 195–201. [DOI] [PubMed] [Google Scholar]
- Mair I, Else KJ and Forman R (2021) Trichuris muris as a tool for holistic discovery research: from translational research to environmental bio-tagging. Parasitology, 1–47. doi: 10.1017/S003118202100069X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ and Danska JS (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (New York, N.Y.) 339, 1084–1088. [DOI] [PubMed] [Google Scholar]
- McClemens J, Kim JJ, Wang H, Mao Y, Collins M, Kunze W, Bienenstock J, Forsythe P and Khan WI (2013) Lactobacillus rhamnosus ingestion promotes innate host defense in an enteric parasitic infection. Clinical and Vaccine Immunology 20, 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES and Bundy DA (1992) Parasitic helminth infection and cognitive function in school children. Proceedings of the Royal Society of London. Series B: Biological Sciences 247, 77–81. [DOI] [PubMed] [Google Scholar]
- Nuttal GHF and Thierfelder H (1985) Tierisches leben ohne bakterien im verdauungskanal. Hoppe Seyler's Zeitschrift Physiologische Chemie 21, 109–112. [Google Scholar]
- Pasteur L (1885) Observations relatives à la note de M. Duclaux. Comptes rendus de l'Académie des Sciences 100, 68–69. [Google Scholar]
- Prosberg PV and Petersen AM (2018. –2021) Probiotic treatment of ulcerative colitis with Trichuris suis ova (TSO); (PROCTO). NCT Identifier: NCT03565939. Sponsored by ParaTech A/S.
- Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YA, Loke P and Cadwell K (2016) Helminth infection promotes colonization resistance via type 2 immunity. Science (New York, N.Y.) 352, 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Carrillo E, Gaona O, Nieto J, Sánchez-Quinto A, Cerqueda-García D, Falcón LI, Rojas-Ramos OA and González-Santoyo I (2020) Disturbance in human gut microbiota networks by parasites and its implications in the incidence of depression. Scientific Reports 10, 3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch S, Midha A, Kuhring M, Affinass N, Radonic A, Kühl AA, Bleich A, Renard BY and Hartmann S (2018) Parasitic nematodes exert antimicrobial activity and benefit from microbiota-driven support for host immune regulation. Frontiers in Immunology 9, 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyniers JA (1959) The pure-culture concept and gnotobiotics. Annals of the New York Academy of Sciences 78, 3–16. [Google Scholar]
- Rizzatti G, Lopetuso L, Gibiino G, Binda C and Gasbarrini A (2017) Proteobacteria: a common factor in human diseases. Biomed Research International 2017, 1–7. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G (2009) Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa BA, Snowden C, Martin J, Fischer K, Kupritz J, Beshah E, Supali T, Gankpala L, Fischer PU, Urban JF Jr. and Mitreva M (2021) Whipworm-associated intestinal microbiome members consistent across both human and mouse hosts. Frontiers in Cellular and Infection Microbiology 11, 637570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G, Faubert C, Verdu E and King I (2020) The gut microbiota limits Th2 immunity to Heligmosomoides polygyrus bakeri infection. Biorxiv, 2020.01.30.927111. doi: 10.1101/2020.01.30.927111. [DOI] [Google Scholar]
- Schachter J, Alvarinho de Oliveira D, da Silva CM, de Barros Alencar ACM, Duarte M, da Silva MMP, Ignácio ACPR and Lopes-Torres EJ (2020) Whipworm infection promotes bacterial invasion, intestinal microbiota imbalance, and cellular immunomodulation. Infection and Immunology 88, e00642-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Andrusaite A, Andersen P, Lawson M, Alcon-Giner C, Leclaire C, Caim S, Le Gall G, Shaw T, Connolly JPR, Roe AJ, Wessel H, Bravo-Blas A, Thomson CA, Kästele V, Wang P, Peterson DA, Bancroft A, Li X, Grencis R, Mowat AM, Hall LJ, Travis MA, Milling SWF and Mann ER (2018) Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Science Translational Medicine 10, eaao4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudellari M (2017) News feature: cleaning up the hygiene hypothesis. Proceedings of the National Academy of Sciences 114, 1433–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N, Whon T and Bae J (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends in Biotechnology 33, 496–503. [DOI] [PubMed] [Google Scholar]
- Smith K, McCoy K and Macpherson A (2007) Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology 19, 59–69. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T, Hooper L and Gordon J (2002) Nonlinear partial differential equations and applications: developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proceedings of the National Academy of Sciences 99, 15451–15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Macpherson A, Hapfelmeier S, Kremer M, Stallmach T and Hardt W (2005) Comparison of Salmonella enterica Serovar typhimurium colitis in germfree mice and mice pretreated with streptomycin. Infection and Immunity 73, 3228–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan P (1989) Hay fever, hygiene, and household size. British Medical Journal 299, 1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejzagić N, Adelfio R, Keiser J, Kringel H, Thamsborg S and Kapel C (2015) Bacteria-induced egg hatching differs for Trichuris muris and Trichuris suis. Parasites & Vectors 8, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST, Blum AM, Ewing SA, Weinstock JV and Young VB (2010) Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflammatory Bowel Diseases 11, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P, Kouremenos K, Eichenberger RM, Pearson M, Susianto A, Wishart DS, McConville MJ and Loukas A (2019) Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 15, 101. [DOI] [PubMed] [Google Scholar]
- White E (2016) Infection by the Gastrointestinal Parasite Trichuris muris: Defining the Microbiota of the Parasite and the Host (PhD), 1–197. University of Manchester. [Google Scholar]
- White E, Houlden A, Bancroft A, Hayes K, Goldrick M, Grencis R and Roberts I (2018) Manipulation of host and parasite microbiotas: survival strategies during chronic nematode infection. Science Advances 4, eaap7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Dige A, Rasmussen TK, Hvas CL, Dahlerup JF, Iversen L, Stensvold CR, Agnholt J and Nejsum P (2017) Immune responses and parasitological observations induced during probiotic treatment with medicinal Trichuris suis ova in a healthy volunteer. Immunology Letters 188, 32–37. [DOI] [PubMed] [Google Scholar]
- Wold AE (1998) The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53(46 Suppl), 20–55. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020) Soil-Transmitted Helminth Infections. WHO Fact Sheets. Geneva, Switzerland: World Health Organization. Available at https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupt K, Baldassano R, Nessel L, Li H, Bushman FD and Lewis JD (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, N.Y.) 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshi K, Creek D, Anderson D, Ritmejerytė E, Becker L, Loukas A and Wangchuk P (2020) Metabolomes and lipidomes of the infective stages of the gastrointestinal nematodes, Nippostrongylus brasiliensis and Trichuris muris. Metabolites 10, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova IA, Ebner F, Schulz AR, Steinfelder S, Rosche B, Bolze A, Paul F, Mei HE and Hartmann S (2021) The worm-specific immune response in multiple sclerosis patients receiving controlled Trichuris suis ova immunotherapy. Life 11, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R and Goodman A (2019) Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science (New York, N.Y.) 363, eaat9931. [DOI] [PMC free article] [PubMed] [Google Scholar]