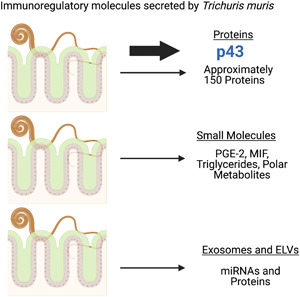
Key words: Immunoregulation, Trichuris, whipworm
Trichuris, whipworm nematode infections are prevalent in humans, domestic livestock and mammals. All share an epithelial dwelling niche and similar life cycle with the chronic infections that follow implying that immune evasion mechanisms are operating. Nematode excretory secretory (ES) products have been shown to be a rich source of immunomodulatory molecules for many species. The Trichuris muris model is a natural parasite of mice and has been used extensively to study host–parasite interactions and provides a tractable platform for investigation of the immunoregulatory capacity of whipworm ES. The present review details progress in identification of the composition of T. muris ES, immunomodulatory components and their potential mechanisms of action. The adult T. muris secretome is dominated by one protein with modulatory capacity although remains to be completely characterized. In addition, the secretome contains multiple other proteins and small molecules that have immunomodulatory potential, certainly by comparison to other Trichuris species. Moreover, T. muris-derived exosomes/exosome-like vesicles contain both protein and multiple miRNAs providing an alternate delivery process for molecules with the potential to modulate host immunity.
Introduction
The mouse model of human disease
Trichuris is a genus comprising intestinal nematodes including the human whipworm parasite, Trichuris trichiura, that is currently believed to infect approximately 465 million people worldwide causing considerable morbidity and economic hardship (Hotez et al., 2014; Pullan et al., 2014). It was estimated to be responsible for the loss of 0.64 million disability adjusted life years from 1990 to 2010 and was ranked 10th in the neglected tropical disease rankings (Hotez et al., 2014) with long lived, chronic infections the norm. It is mostly children who suffer pathology with Trichuris dysentery syndrome, growth retardation, cognitive problems and finger clubbing commonly reported (Cooper et al., 1991; MacDonald et al., 1991; Nokes and Bundy, 1992). Trichuris trichiura has been shown to be relatively resistant to drug treatment and reinfection after worm clearance is common (Bundy et al., 1987) necessitating development of new chemotherapeutics or vaccine development, both of which have proved slow. This results in a situation in which populations in endemic areas have low level worm burdens as they are constantly exposed to infectious eggs; they do however, generate partially acquired immunity with age (Bundy et al., 1988). Chronic human helminth infection has been associated with immunoregulation (van Riet et al., 2007; Smits et al., 2010; Alcantara-Neves et al., 2017) although it is important to recognize that these infections cause a considerable collective damage to endemic communities (Cooper and Bundy, 1988).
Our laboratory and many others have used the murine parasite Trichuris muris, a natural parasite of mice which is closely related to the human parasite to study host whipworm interactions (Cliffe and Grencis, 2004). In this scenario, many variables such as infection levels, host genetics and nutrition, can be controlled. A single low dose infection or more realistically repeated low level i.e. trickle infections (Bancroft et al., 2001; Glover et al., 2019) of T. muris can be used to reflect the infection levels and dynamics seen in the wild. Parasites reach patency around 33 days after infection and adult worms survive for extended periods with some adults dying from senescence from 100 days onwards.
The majority of inbred laboratory mouse strains expel a high dose infection, whereas immune-deficient mouse strains will take high dose infections through to chronicity showing that host protective immunity can operate (Cliffe and Grencis, 2004; Klementowicz et al., 2012; Grencis et al., 2014). Moreover, transient immunosuppression given to immunocompetent strains of mouse during the second week of infection will also allow progression to patency (Lee and Wakelin, 1983). Low dose and trickle infections lead to chronicity without external immunosuppression and all without obvious negative effects on the health of the host. Taken together with the observations that chronic infection is associated with qualitative and quantitative differences in the immune response generated when compared to resistant mice suggest that the parasite has immunomodulatory activity (Klementowicz et al., 2012; Grencis et al., 2014; Colombo and Grencis, 2020). This is further emphasized by blocking the regulatory cytokine, interleukin (IL)-10 during the chronic phases of infection, which leads to excessive intestinal pathology sometimes with fatal consequences (Grencis et al., 2014). An earlier study examined the effect of abbreviated infections or chronic infection upon the capacity of the host to clear a subsequent infection. These data strongly indicated that exposure to later stages of the infection prior to a challenge was less effective at inducing protection and thus potentially immunomodulatory (Else et al., 1989). Research from our laboratory using models of chemical contact hypersensitivity that depend upon type 1 or type 2 cytokine responses showed that a chronic T. muris infection modulated challenge responses to the sensitizers in the ear, a remote non-mucosal site distant from the caecum where the parasite resides (Grencis et al., 2014). It was also apparent that modulation operated only against type 1 sensitization and not type 2 sensitization influencing local cytokine responses and ear pathology. This depression in sensitization was associated with a reduction in movement of class-II positive cutaneous dendritic cells from the skin and elevated IL-10 levels in the ear draining lymph node (Grencis et al., 2014). This would suggest that the immunoregulation induced by T. muris infection operating systemically reflects that generated during chronic T. muris intestinal infection, i.e. a regulated Th1 response. It has also been shown that a chronic T. muris infection can modulate the brain inflammatory response in stroke (Denes et al., 2010). Systemic effects of chronic T. muris infection were subsequently observed by Chenery et al. (2016) where haematopoetic responses in the bone marrow were influenced by intestinal infection driven cytokines. Broadhurst et al. (2010) showed that T. trichiura infection could induce remission in an individual with ulcerative colitis and associated increase in IL-22+ CD4 cells and decrease in IL-17+ CD4 cells supporting an immunomodulatory role for human whipworm infection. How T. muris or T. trichiura achieves such immunomodulation remains to be completely defined but, by analogy with other parasitic nematodes (Hewitson et al., 2009) is likely to involve the excretions and secretions (ES) of the worm especially from the later larval and adult stages of the parasite.
Here, we aim to review immunomodulation by Trichuris infection with a focus on known and potential immunomodulatory proteins in T. muris ES, regulatory RNAs and exosome-like vesicles (ELVs).
Excretory secretory (ES) proteins and other molecules
The ES of multicellular parasites has been the source of target molecules for disruption of the host/parasite interaction, the hunt for vaccine candidates and a potent source of immunomodulatory proteins. A study in 2018 (Eichenberger et al., 2018) provided a detailed characterization of T. muris adult ES (day 35 post infection), the T. muris secretome. Using two biological replicates 148 proteins were confidently identified corresponding to 34.1% of the total predicted secreted proteins from the T. muris genome as based on the presence of a signal peptide. GO pfam analysis suggested a dominance of trypsin peptidases, thioredoxin-like and tetracopeptide repeat molecules and in terms of putative molecular function, a dominance of protein binding, metal ion binding and nucleic acid binding molecules. Proteases are highly represented and a number of serine proteases and whey acidic protein (WAP) domain proteins were also identified. Of particular interest were the SCP/TAPS or CAP-domain proteins which although have been shown to be abundant in other soil transmitted helminths had not been well characterized in clade I nematodes and have been suggested to play multiple roles including potential immunomodulatory function (Cantacessi and Gasser, 2012). Substantial research on the ES of Trichuris suis has also been undertaken that has shown multiple and varied immunomodulatory activities in vitro for total ES and fractions of T. suis ES. This included the suppression of proinflammatory cytokine secretion by activated bone marrow-derived macrophages (BMDMs) and bone marrow-derived dendritic cells, the up-regulation of nitric oxide and arginase and the induction of IL-10 from BMDMs. ES was also shown to suppress the proliferation of CD4+ T cells in vitro. Three proteins from ES were identified for further study in recombinant form (a triosephosphate isomerase MW 27 kDa, a nucleoside diphosphate kinase MW 26 kDa and a small nuclear ribonucleoprotein MW 8 kDa) which exhibited modulatory effects on BMDM in vitro. Readers are referred to Leroux et al. (2018) for more details. A study by Laan et al. (2017) demonstrated that T. suis ES contained compounds that downregulated inflammatory cytokine production from LPS stimulated human dendritic cells. The active component was identified as prostaglandin E2 (PGE-2), the secretion of which was found to be cyclooxygenase independent. A study by Bancroft and Grencis (unpublished) showed that T. muris also secreted a similar molecule, putatively PGE-2, from the L3 stage onwards. Addition of aspirin and indomethacin affected worm motility suggesting that T. muris secretion of PGE-2 was similar to that seen in the studies with T. suis (Laan et al., 2017). Rhoads et al. (2000) suggested a chymotrypsin/elastase inhibitor secreted by T. suis may act as an immunomodulatory mediator. Adult T. muris was also found to have a potential orthologue of l-dopachrome-methyl ester tautomerase (i.e. macrophage inhibition factor, MIF) although little is known as to any possible immunomodulatory activity (Pennock et al., 1998). A study by Santos et al. (2013) also detected a homologue of MIF in T. trichiura extracts using a proteomic approach. This study also identified fructose bisphosphate aldolase and heat shock protein 70 as potential immunomodulatory mediators. Whether any of these molecules play a role in vivo, remains to be determined.
The ES of T. muris is unusual as although it secretes a mixture of proteins throughout its life in adults 90+% of total secreted protein is a single poly-cysteine and histidine tailed protein termed p43 (Bancroft et al., 2019). This protein was shown to have a novel sequence and protein structure but following sub-structure analysis using Probis (Konc and Janezic, 2012) revealed domains with high homology to TSP-1 repeats and IL-13 Rα-2. It did not show high levels of homology with the cytokine interferon (IFN)-γ which had been suggested from previous studies (Grencis and Entwistle, 1997). Structurally p43 is a compact molecule containing 36 cysteines all disulphide bonded, implying a very stable conformation consistent with the harsh environment into which it is secreted, the caecum and proximal colon. It also contains a natural poly-histidine tail which has the potential to bind a number of different divalent metal cations. The observation that domains of the molecule shared homology with IL-13 Rα-2, a receptor which binds with extremely high affinity to IL-13 neutralizing IL-13 function (Lupardus et al., 2010; Karmele et al., 2019) raised the possibility that p43 may share a similar function. This would potentially benefit the parasite as IL-13 is known to be critical to expulsion of T. muris (Bancroft et al., 1998). Indeed, p43 was shown to bind to IL-13 and also glycosaminoglycans (GAGs) putatively through the TSP-1 repeat region with nanomolar affinities. Moreover, IL-13-driven immune responses could be downregulated by p43 both in vitro and in vivo. p43 is expressed in all larval stages of T. muris (Bancroft et al., 2019) with the largest quantity of secreted p43 from adult parasites consistent with high levels of production of a poly-cysteine and histidine-tailed protein by other species of whipworm e.g. T. suis (Leroux et al., 2018). Chronic infection by whipworms is associated with extensive remodelling of the extracellular matrix (Foth et al., 2014; Dawson et al., 2020), which is decorated with multiple GAGs. Following chronic T. muris infection increases in expression of heparan sulphate, fibronectin and collagen I are observed in the infected caecum (Thompson, Sutherland and Grencis, unpublished observations). It is hypothesized that p43 is secreted into the area adjacent to intestinal epithelial cells (IECs) occupied by the parasite and tethers to locally expressed GAGs with high affinity. It is notable that the parasite constantly burrows providing access to both apical and basal epithelium and therefore has access to IL-13 on epithelial cells. Binding analysis is supportive of sequential GAG–IL-13 interactions and the possibility of tethered p43 interacting with IL-13. It is noteworthy that the major effector mechanisms that remove T. muris from the intestine, increased epithelial turnover and upregulation of mucins, are driven by IL-13 acting locally in the intestinal niche (Cliffe et al., 2005; Hasnain et al., 2011). Excitingly, preliminary data reveal that the homologue to p43 in T. trichiura also binds to GAGs and human IL-13 and inhibits human IL-13 function in vitro (Bancroft et al., unpublished data).
Intriguingly, the interaction of p43 with a model GAG, heparan sulphate, was shown to be highly zinc dependent. The reasons for this are unknown. Zinc is an important metal ion co-factor essential for multiple biological processes. Preliminary research in our laboratory has shown that T. muris adult ES contains elevated levels of zinc (Bancroft and Grencis, unpublished). Interestingly, a study investigating the effects of helminth infection showed that it played a clear role in driving serum levels of zinc and iron in infected human populations independent of diet (Lee et al., 2019). The mechanisms underlying these observations are likely to be complex and involve parasite, host immunity and the intestinal microbiota as it is known that zinc and iron can influence immune function (Wessels et al., 2017) and the microbiota (Knezevic et al., 2020). It is tempting to suggest that the dependency of p43 (and the T. trichiura homologue) binding activity on zinc indicates that regulation of zinc during infection may be critical in the relationship between host immunity, parasite and microbiota. A role for the poly-histidine tail found in p43 and its homologues in zinc binding remains to be determined.
During natural infection, T. muris is acquired by repeated low dose infections. Using a trickle infection approach in the laboratory, Glover et al. (2019) showed that there was a steady build up in worm burden coincident with a dominant Th1 response until partial protective immunity was acquired due to the development of a stronger Th2 response. This suggests that adult parasites are present before effective IL-13-mediated immunity is acquired and thus copious p43 is secreted into the intestinal niche, which would bind IL-13 from the developing Th2 response, effectively delaying host protection.
It is also noteworthy that following a variety of infection regimes little p43 specific antibody is generated and a p43-specific T cell response is largely absent (Bancroft et al., 2019). Again, this suggests compartmentalization of p43 to the intestinal niche, which precludes access to the normal antigen processing pathways. However, purified p43 is potently immunogenic when used together with adjuvant to conventionally immunize mice (Bancroft et al., 2019). Indeed, to date purified native p43 is the only single Trichuris protein that has been shown to be capable of providing almost total protection against challenge infection equivalent to the protection of total adult ES. Importantly, it protects against low dose challenge infection, the type of infection that is more reflective of natural challenge which drives a Th1 response that depresses Th2-mediated protective immunity (Shears et al., 2018b; Bancroft et al., 2019). Although p43 has clear immunomodulatory activity our understanding of potential other important functions for this protein in both parasite biology and host interaction remain to be defined (Fig. 1).
Fig. 1.
Modulation of protective immunity to Trichuris muris. A role for p43 secreted by adult worms through interaction with GAGs and IL-13. (A) Space-filled model of p43 highlighting TSP-1 and IL-13 Rα-2 homology (red). (B) Space-filled model of p43 docked with IL-13. (C) Diagrammatic representation of caecal epithelium overlaid by thick mucus shown in light green. A single adult worm is shown with anterior end embedded in the epithelium and posterior bulbous end free within the lumen. Chronic infection is IFN-γ driven and regulated by CD4+ Th cells producing IL-10. Secretion of copious p43 by adult worms is found within the worm niche and binds to extracellular matrix e.g. GAGs with high affinity. Repeated T. muris challenge infections following invasion of L1 larvae, as seen in trickle infections, begin to drive the generation of a Th2 response and the production of IL-13. GAG bound p43 will bind IL-13 with high affinity and prevent IL-13 effector function. The net result is a delay and reduced efficacy in host protective immunity, potentially enhancing parasite survival. Models of p43 courtesy of C. Levy.
Metabolomic profiling has also been carried out on T. muris egg/L1 extracts and adult ES. A study comparing the metabolomes and lipidomes of T. muris eggs and Nippostrongylus brasiliensis L3 using liquid chromatography-mass spectrometry (LC-MS) showed that many of the major polar metabolites identified have potential anti-inflammatory properties whereas the lipidomic analysis revealed a high level of triglycerides. There was little overlap between these parasites which is perhaps not surprising as they ultimately occupy different niches and have markedly different life cycles (Yeshi et al., 2020). Wangchuk et al. (2019) used targeted gas chromatography-mass spectrometry and LC-MS of adult ES and identified known polar and non-polar metabolites together with fatty acids including short chain fatty acids (SCFAs). A number of these compounds have been shown to have anti-inflammatory activity in a variety of assays although their activity in vivo is unknown. SCFAs that are known to be produced by a number of commensal bacteria and have immunomodulatory effects were also identified although their origin is still a matter of debate. Propionate was the major SCFA detected from T. muris although butyrate was also found (Wangchuk et al., 2019). Certainly butyrate, which is known to influence colonocyte growth and T regulatory cells, is unlikely to be T. muris derived as this species does not contain the genes required to synthesize butyrate or any butyrate transporter orthologues (Foth et al., 2014). The source may be related to the fact that T. muris has its own intestinal microbiota (White et al., 2018).
Exosomes and exosome-like vesicles
In 2014, Buck et al. (2014) showed that exosomes secreted by Heligmosomoides polygyrus, which reside in the murine small intestine could transfer small RNAs and nematode proteins to mammalian cells to modulate innate immunity. Tritten et al. (2017) isolated ELVs/particles the size of exosomes from adult T. muris over an 18–48 h period of in vitro culture and from them identified 14 T. muris miRNA candidates with high confidence and 73 putative Trichuris proteins. Eichenberger et al. (2018) identified 364 T. muris proteins in ELVs from adult worms, the most common being trypsin domain-protein, sperm-coating protein extracellular proteins, a poly-cysteine and histidine-tailed protein, a glyceraldehyde-3-phosphate dehydrogenase and a TB2/DP1HVA22 domain containing protein. A T. muris tetraspanin supportive of ELV formation was also identified. Only 13.7% of the identified T. muris proteins had a transmembrane domain and 33% had a signal peptide. Of the 56 miRNAs identified, 34 had homologues to other nematodes and 22 were novel. Shears et al. (2018a) identified 125 proteins from adult T. muris ELVs and again confirmed a number of potential T. muris-derived exosome associated proteins many of which did not contain a classical signal peptide. Moreover, the ELVs were able to induce a degree of protection when used for immunization, without adjuvant.
Eichenberger et al. (2018) showed that T. muris ELVs could be internalized by mouse colonic organoids raising the possibility of the ELVs communicating with intestinal epithelial cells. Duque-Correa et al. (2020) developed a novel caecaloid model micro-injected with T. muris adult-derived ELVs which enabled study of the host pathogen interaction in vitro. Their study argues that the caecum is a unique and different niche to the colon and so provides additional and novel data to that provided in Eichenberger et al. (2018). RNA-seq data showed a significant downregulation of viral response associated genes by caecal IECs. A downregulation of IFN-stimulated genes suggested that a direct effect on the caecal epithelium may at least partly explain the anti-inflammatory effects of whipworm infection. This was the first demonstration of an involvement of type I IFNs in T. muris infection and Duque-Correra postulated that this immunosuppression may allow ELV cargo entry to the host immune system. White et al. (2020) compared small RNAs in ELVs from T. muris and Heligmosomoides bakeri and found they were quite distinct from each other and concluded that this did not depend on their method of preparation but reflected their different intestinal niches and different functions within their hosts. Layton et al. (2020) reviewed regulatory RNAs: miRNAs, siRNAs and piRNAs and advocated that miRNAs played a wider role than mere regulation of transcription of the host but also were involved in communication between different taxonomic kingdoms including microbiota.
Genomic analysis of potential novel immunomodulatory genes
In 2011 the draft genome of Trichinella spiralis was published (Mitreva et al., 2011) providing valuable information on a clade I parasitic nematode for the first time. Furthermore, in 2014, the publication of the genome of three whipworm species: T. muris, T. trichiura (Foth et al., 2014) and T. suis (Jex et al., 2014) expanded the available information on clade I nematodes and the potential identification of many genes that could encode immunomodulatory molecules. Collectively, these papers are a wealth of information for studies on host–parasite interactions in whipworm infection. The T. spiralis study compared parasitic and non-parasitic genes by evaluating the T. spiralis and Caenorhabditis elegans genome and also focused on potential vaccine candidates from secreted proteins. The T. suis study provided transcriptomic data from different life cycle stages together with the description of small non-coding RNAs. Multiple parasite genes and miRNAs were identified as having potential immunomodulatory activity and the reader is referred to Jex et al. (2014) for more details. Trichuris suis has been used in trials to treat humans for a range of clinical conditions, most notably inflammatory bowel disease (IBD) (Summers et al., 2005), see Hayes and Grencis (2021) supporting an immunomodulatory role for T. suis infection.
The T. muris reference genome (Foth et al., 2014) was the first study to show the pairwise genomes of a major human STH and its murine counterpart. It described both sex and life cycle stage specific genes. It also contained detailed RNA-seq data on host gene expression in chronic low dose T. muris infection when immune-mediated regulation is believed to operate. Trichuris muris in the mouse has an unusual niche in common with other Trichuris spp. with an anterior end forming syncytial tunnels within the intestinal epithelium and occupying between 1000 and 2000 epithelial cells. The large posterior end of the worm hangs free within the caecal lumen. Whipworms also possess atypical structures for nematodes, the bacillary band and the stichosome (Sheffield, 1963) which together occupy the bulk of the anterior half of later larval stages and adult parasites. The stichosome is believed to perform a secretory function associated with digestion whereby stichocytes are ducted into the worm intestine (Lee and Wright, 1978). The bacillary band has been suggested to be involved in both absorption of nutrients and secretion (Tilney et al., 2005; Hansen et al., 2016; Lopes-Torres et al., 2020). Looking at the patterns of parasite gene expression in the anterior region of adult T. muris, Foth et al. (2014) identified a dominance of chymotrypsin A-like serine proteases and protease inhibitors. These inhibitors had high similarity to a mammalian secretory leucocyte peptidase inhibitor (SLPI). They were encoded for by 63 genes, which is far higher than in any other nematode species so far examined and a high percentage of these genes suggested they encoded secreted proteins based on the presence of a signal peptide. It was suggested that these proteins had potential immunomodulatory function, degrading intestinal mucins which provide a physical barrier to parasite entry into epithelial cells (Foth et al., 2014). Certainly, T. muris ES contains serine proteases that can degrade the intestinal mucin Muc2 (Hasnain et al., 2012). The SLPI-like proteins mostly contained a WAP domain and the T. muris genome contained 44 genes which encoded between 1 and 9 WAP domains (Foth et al., 2014). WAP domains of unknown function in mammalian SLPIs and elafins have immunomodulatory, anti-inflammatory and anti-microbial properties (Williams et al., 2006; Scott et al., 2011; Wilkinson et al., 2011). They are produced by epithelial cells and have a role in modulating inflammation and wound healing. The direct role of T. muris-produced WAP proteins has not been assessed but they have been postulated to play a role in regulating mucosal immunity (Wilkinson et al., 2011). Interestingly, one WAP protein from T. muris has been used in vaccination studies with some efficacy (Briggs et al., 2018). The reference genome for T. muris provided a basis for comparison with the human pathogen T. trichiura highlighting a number of important similarities supporting a commonality of potential function in human infections (Foth et al., 2014).
Concluding remarks
Trichuris muris has been used to inform on the complex interaction between the host, parasite and the rich microbial ecosystem that forms part of its niche. It has also provided valuable information on the host immune system per se. Low level infection with T. muris has been shown, in immunocompetent mice to result in changes in host pathology and a remodelling of the epithelial matrix. However, considering this parasite breaches the epithelial barrier exposing the host to the caecal microbiota and potential pathobionts, T. muris must regulate its environmental niche as many other helminths do. But, it is also evident that the parasite exerts effects beyond its niche. Indeed, infection has been shown to affect the host at sites as far away as the brain implying a systemic regulation. The ES is at the host–parasite interface and is a rich source of molecules and ELVs which have been suggested to have immunoregulatory activity. Publication of the genome of T. muris and closely related species has also given scope to uncover previously undiscovered immunoregulatory molecules. What is unusual about T. muris (and by extrapolation about other Trichuris spp. and clade I nematodes (Giorello et al., 2017)), is the dominance of ES from adult parasites quantitatively by a single novel protein, in the case of T. muris, p43. This protein has been shown to depress IL-13 function, both in vitro and in vivo. Excitingly, recent unpublished study shows this also occurs with the homologue of p43 from T. trichiura showing potential implications for regulation of clinical outcome in IL-13-mediated pathologies. It is however, also clear that there is a wealth of other Trichuris-derived molecules including proteins, small molecules and nucleic acids found in the ES that the parasites potentially utilize to modulate host responses that remain to be characterized and potentially exploited.
Author contributions
AJB conducted the literature search, designed and wrote the review and figure. RKG provided feedback and comments on the manuscript.
Financial support
The work in RKG's laboratory is funded by Wellcome Trust investigator award (Z10661/Z/18/Z). The Wellcome Trust Centre for Cell Matrix Research, University of Manchester is also supported by centre funding from the Wellcome Trust (088785/Z/09/Z).
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Alcantara-Neves NM, Viega RV, Ponte JC, da Cunha SS, Simões SM, Cruz ÁA, Yazdanbakhsh M, Matos SM, Silva TM, Figueiredo CA, Pontes-de-Carvalho LC, Rodrigues LC, Fiaccone RL, Cooper PJ and Barreto ML (2017) Dissociation between skin test reactivity and anti-aero allergen IgE: determinants among urban Brazilian children. PLoS ONE 12, 1–13. 10.1371/journal.pone.0174089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft AJ, McKenzie AN and Grencis RK (1998) A critical role for IL-13 in resistance to intestinal nematode infection. Journal of Immunology 160, 3453–3461. [PubMed] [Google Scholar]
- Bancroft AJ, Else KJ, Humphreys NE and Grencis RK (2001) The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. International Journal of Parasitology 31, 1627–1637. [DOI] [PubMed] [Google Scholar]
- Bancroft AJ, Levy CW, Jowitt TA, Hayes KS, Thompson S, McKenzie EA, Ball MD, Dubaissi E, France AP, Bellina B, Sharpe C, Mironov A, Brown SL, Cook PC, MacDonald AS, Thornton DJ and Grencis RK (2019) The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nature Communications 10, 2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs N, Wei J, Versteeg L, Zhan B, Keegan B, Damania A, Pollet J, Hayes KS, Beaumier C, Seid CA, Leong J, Grencis RK, Bottazzi ME, Sastry KJ and Hotez PJ (2018) Trichuris muris whey acidic protein induces type 2 protective immunity against whipworm. PLoS Pathogens 14, e1007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH and Loke P (2010) IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Science Translational Medicine 2, 1–11. 10.1126/scitranslmed.3001500 [DOI] [PubMed] [Google Scholar]
- Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, Ceroni A, Babayan SA, Blaxter M, Ivens A and Maizels RM (2014) Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nature Communications 5, 5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy DA, Cooper ES, Thompson DE, Didier JM, Anderson RM and Simmons I (1987) Predisposition to Trichuris trichiura infection in humans. Epidemiology and Infection 98, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy DA, Cooper ES, Thompson DE, Didier JM and Simmons I (1988) Effect of age and initial infection intensity on the rate of reinfection with Trichuris trichiura after treatment. Parasitology 97(Pt 3), 469–476. [DOI] [PubMed] [Google Scholar]
- Cantacessi C and Gasser RB (2012) SCP/TAPS proteins in helminths – where to from now? Molecular and Cellular Probes 26, 54–59. [DOI] [PubMed] [Google Scholar]
- Chenery AL, Antignano F, Hughes MR, Burrows K, McNagny K and Zaph C (2016) Chronic Trichuris muris infection alters hematopoiesis and causes IFN-g expressing T-cell accumulation in the bone marrow. European Journal of Immunology 46, 2587–2596. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ and Grencis RK (2004) The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Advances in Parasitology 57, 255–307. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C and Grencis RK (2005) Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science (New York, N.Y.) 308, 1463–1465. [DOI] [PubMed] [Google Scholar]
- Colombo SAP and Grencis RK (2020) Immunity to soil-transmitted helminths: evidence from the field and laboratory models. Frontiers in Immunology 11, 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ES and Bundy DA (1988) Trichuris is not trivial. Parasitology Today 4, 301–306. [DOI] [PubMed] [Google Scholar]
- Cooper ES, Spencer J, Whyte-Alleng CA, Cromwell O, Whitney P, Venugopal S, Bundy DA, Haynes B and MacDonald TT (1991) Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet (London, England) 338, 1104–1107. [DOI] [PubMed] [Google Scholar]
- Dawson HD, Chen C, Li RW, Bell LN, Shea-Donohue T, Kringel H, Beshah E, Hill DE and Urban JF Jr. (2020) Molecular and metabolomic changes in the proximal colon of pigs infected with Trichuris suis. Scientific Reports 10, 12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R and Rothwell N (2010) Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. Journal of Neuroscience 30, 10086–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa MA, Schreiber F, Rodgers FH, Goulding D, Forrest S, White R, Buck A, Grencis RK and Berriman M (2020) Development of caecaloids to study host-pathogen interactions: new insights into immunoregulatory functions of Trichuris muris extracellular vesicles in the caecum. International Journal of Parasitology 50, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger RM, Talukder MH, Field MA, Wangchuk P, Giacomin P, Loukas A and Sotillo J (2018) Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host-parasite communication. Journal of Extracellular Vesicles 7, 1428004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else KJ, Wakelin D and Roach TI (1989) Host predisposition to trichuriasis: the mouse – T. muris model. Parasitology 98(Pt 2), 275–282. [DOI] [PubMed] [Google Scholar]
- Foth BJ, Tsai IJ, Reid AJ, Bancroft AJ, Nichol S, Tracey A, Holroyd N, Cotton JA, Stanley EJ, Zarowiecki M, Liu JZ, Huckvale T, Cooper PJ, Grencis RK and Berriman M (2014) Whipworm genome and dual-species transcriptome analyses provide molecular insights into an intimate host-parasite interaction. Nature Genetics 46, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorello AN, Kennedy MW, Butti MJ, Radman NE, Corsico B and Franchini GR (2017) Identification and characterization of the major pseudocoelomic proteins of the giant kidney worm, Dioctophyme renale. Parasites and Vectors 10, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Colombo SAP, Thornton DJ and Grencis RK (2019) Trickle infection and immunity to Trichuris muris. PLoS Pathogens 15, e1007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grencis RK and Entwistle GM (1997) Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact? Parasitology 115(Suppl), S101–S106. [DOI] [PubMed] [Google Scholar]
- Grencis RK, Humphreys NE and Bancroft AJ (2014) Immunity to gastrointestinal nematodes: mechanisms and myths. Immunological Reviews 260, 183–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TV, Hansen M, Nejsum P, Mejer H, Denwood M and Thamsborg SM (2016) Glucose absorption by the bacillary band of Trichuris muris. PLoS Neglected Tropical Diseases 10, e0004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Thornton DJ and Grencis RK (2011) Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunology 33, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, McGuckin MA, Grencis RK and Thornton DJ (2012) Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Neglected Tropical Diseases 6, e1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KS and Grencis RK (2021) Trichuris muris and comorbidities-within a mouse model context. Parasitology. doi: 10.1017/S0031182021000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson JP, Grainger JR and Maizels RM (2009) Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Molecular and Biochemical Parasitology 167, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fevre EM, Furst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ and Naghavi M (2014) The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Neglected Tropical Diseases 8, e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jex AR, Nejsum P, Schwarz EM, Hu L, Young ND, Hall RS, Korhonen PK, Liao S, Thamsborg S, Xia J, Xu P, Wang S, Scheerlinck JP, Hofmann A, Sternberg PW, Wang J and Gasser RB (2014) Genome and transcriptome of the porcine whipworm Trichuris suis. Nature Genetics 46, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmele EP, Pasricha TS, Ramalingam TR, Thompson RW Gieseck RL III, Knilans KJ, Hegen M, Farmer M, Jin F, Kleinman A, Hinds DA, 23andMe Research Team, Pereira A, de Queiroz Prado R, Bing N, Tchistiakova L, Kasaian MT, Wynn TA and Vannella KM (2019) Anti-IL-13Ralpha2 therapy promotes recovery in a murine model of inflammatory bowel disease. Mucosal Immunology 12, 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klementowicz JE, Travis MA and Grencis RK (2012) Trichuris muris: a model of gastrointestinal parasite infection. Seminars in Immunopathology 34, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic J, Starchl C, Tmava Berisha A and Amrein K (2020) Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients 12, 1–16. 10.3390/nu12061769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konc J and Janezic D (2012) ProBiS-2012: web server and web services for detection of structurally similar binding sites in proteins. Nucleic Acids Research 40(Web Server issue), W214–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan LC, Williams AR, Stavenhagen K, Giera M, Kooij G, Vlasakov I, Kalay H, Kringel H, Nejsum P, Thamsborg SM, Wuhrer M, Dijkstra CD, Cummings RD and van Die I (2017) The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB Journal 31, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton E, Fairhurst AM, Griffiths-Jones S, Grencis RK and Roberts IS (2020) Regulatory RNAs: a universal language for inter-domain communication. International Journal of Molecular Science 21, 1–18. 10.3390/ijms21238919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD and Wakelin D (1983) Cortisone-induced immunotolerance to nematode infection in CBA/Ca mice. II. A model for human chronic Trichuriasis. Immunology 48, 571–577. [PMC free article] [PubMed] [Google Scholar]
- Lee TD and Wright KA (1978) The morphology of the attachment and probable feeding site of the nematode Trichuris muris (Schrank, 1788) Hall, 1916. Canadian Journal of Zoology 56, 1889–1905. [DOI] [PubMed] [Google Scholar]
- Lee SC, Tang MS, Easton AV, Devlin JC, Chua LL, Cho I, Moy FM, Khang TF, Lim YAL and Loke P (2019) Linking the effects of helminth infection, diet and the gut microbiota with human whole-blood signatures. PLoS Pathogens 15, e1008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux LP, Nasr M, Valanparambil R, Tam M, Rosa BA, Siciliani E, Hill DE, Zarlenga DS, Jaramillo M, Weinstock JV, Geary TG, Stevenson MM, Urban JF Jr., Mitreva M and Jardim A (2018) Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Scientific Reports 8, 15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Torres EJ, Girard-Dias W, de Souza W and Miranda K (2020) On the structural organization of the bacillary band of Trichuris muris under cryopreparation protocols and three-dimensional electron microscopy. Journal of Structural Biology 212, 107611. [DOI] [PubMed] [Google Scholar]
- Lupardus PJ, Birnbaum ME and Garcia KC (2010) Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure (London, England: 1993) 18, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Choy MY, Spencer J, Richman PI, Diss T, Hanchard B, Venugopal S, Bundy DA and Cooper ES (1991) Histopathology and immunohistochemistry of the caecum in children with the Trichuris dysentery syndrome. Journal of Clinical Pathology 44, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitreva M, Jasmer DP, Zarlenga DS, Wang Z, Abubucker S, Martin J, Taylor CM, Yin Y, Fulton L, Minx P, Yang SP, Warren WC, Fulton RS, Bhonagiri V, Zhang X, Hallsworth-Pepin K, Clifton SW, McCarter JP, Appleton J, Mardis ER and Wilson RK (2011) The draft genome of the parasitic nematode Trichinella spiralis. Nature Genetics 43, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokes C and Bundy DA (1992) Trichuris trichiura infection and mental development in children. Lancet (London, England) 339, 500. [DOI] [PubMed] [Google Scholar]
- Pennock JL, Behnke JM, Bickle QD, Devaney E, Grencis RK, Isaac RE, Joshua GW, Selkirk ME, Zhang Y and Meyer DJ (1998) Rapid purification and characterization of L-dopachrome-methyl ester tautomerase (macrophage-migration-inhibitory factor) from Trichinella spiralis, Trichuris muris and Brugia pahangi. Biochemical Journal 335(Pt 3), 495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan RL, Smith JL, Jasrasaria R and Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors 7, 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads ML, Fetterer RH, Hill DE and Urban JF Jr. (2000) Trichuris suis: a secretory chymotrypsin/elastase inhibitor with potential as an immunomodulator. Experimental Parasitology 95, 36–44. [DOI] [PubMed] [Google Scholar]
- Santos LN, Gallo MB, Silva ES, Figueiredo CAV, Cooper PJ, Barreto ML, Loureiro S, Pontes-De-Carvalho LC and Alcantara-Neves NM (2013) A proteomic approach to identify proteins from Trichuris trichiura extract with immunomodulatory effects. Parasite Immunology 35, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Weldon S and Taggart CC (2011) SLPI and elafin: multifunctional antiproteases of the WFDC family. Biochemical Society Transactions 39, 1437–1440. [DOI] [PubMed] [Google Scholar]
- Shears RK, Bancroft AJ, Hughes GW, Grencis RK and Thornton DJ (2018a) Extracellular vesicles induce protective immunity against Trichuris muris. Parasite Immunology 40, e12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears RK, Bancroft AJ, Sharpe C, Grencis RK and Thornton DJ (2018b) Vaccination against whipworm: identification of potential immunogenic proteins in Trichuris muris excretory/secretory material. Scientific Reports 8, 4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield HG (1963) Electron microscopy of the bacillary band and stichosome of Trichuris muris and T. vulpis. Journal of Parasitology 49, 998–1009. [PubMed] [Google Scholar]
- Smits HH, Everts B, Hartgers FC and Yazdanbakhsh M (2010) Chronic helminth infections protect against allergic diseases by active regulatory processes. Current Allergy and Asthma Reports 10, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RW, Elliott DE, Urban JF Jr., Thompson R and Weinstock JV (2005) Trichuris suis therapy in Crohn's disease. Gut 54, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Guild GM, Vranich KA and Artis D (2005) Adaptation of a nematode parasite to living within the mammalian epithelium. Journal Experimental Zoology, Part A: Comparative Experimental Biology 303, 927–945. [DOI] [PubMed] [Google Scholar]
- Tritten L, Tam M, Vargas M, Jardim A, Stevenson MM, Keiser J and Geary TG (2017) Excretory/secretory products from the gastrointestinal nematode Trichuris muris. Experimental Parasitology 178, 30–36. [DOI] [PubMed] [Google Scholar]
- van Riet E, Hartgers FC and Yazdanbakhsh M (2007) Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212, 475–490. [DOI] [PubMed] [Google Scholar]
- Wangchuk P, Kouremenos K, Eichenberger RM, Pearson M, Susianto A, Wishart DS, McConville MJ and Loukas A (2019) Metabolomic profiling of the excretory-secretory products of hookworm and whipworm. Metabolomics 15, 101. [DOI] [PubMed] [Google Scholar]
- Wessels I, Maywald M and Rink L (2017) Zinc as a gatekeeper of immune function. Nutrients 9, 1–44. 10.3390/nu9121286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EC, Houlden A, Bancroft AJ, Hayes KS, Goldrick M, Grencis RK and Roberts IS (2018) Manipulation of host and parasite microbiotas: survival strategies during chronic nematode infection. Science Advances 4, 1–10. 10.1126/sciadv.aap7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Kumar S, Chow FW, Robertson E, Hayes KS, Grencis RK, Duque-Correa MA and Buck AH (2020) Extracellular vesicles from Heligmosomoides bakeri and Trichuris muris contain distinct microRNA families and small RNAs that could underpin different functions in the host. International Journal of Parasitology 50, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TS, Roghanian A, Simpson AJ and Sallenave JM (2011) WAP domain proteins as modulators of mucosal immunity. Biochemical Society Transactions 39, 1409–1415. [DOI] [PubMed] [Google Scholar]
- Williams SE, Brown TI, Roghanian A and Sallenave JM (2006) SLPI and elafin: one glove, many fingers. Clinical Sciences (London) 110, 21–35. [DOI] [PubMed] [Google Scholar]
- Yeshi K, Creek DJ, Anderson D, Ritmejeryte E, Becker L, Loukas A and Wangchuk P (2020) Metabolomes and lipidomes of the infective stages of the gastrointestinal nematodes, Nippostrongylus brasiliensis and Trichuris muris. Metabolites 10. 10.3390/metabo10110446. [DOI] [PMC free article] [PubMed] [Google Scholar]



