Abstract
Background
Previous studies comparing total and reverse shoulder arthroplasty (TSA/RSA) are subject to surgeon selection bias. This study objective is to compare the outcomes and cost of outpatient TSA/RSA to inpatient TSA/RSA.
Methods
108,889 elective inpatient and outpatient TSA/RSA from Medicare claims data (2016–2018). 90-day readmission and total 90-day costs were compared following propensity score matching.
Results
Younger and healthier patients are receiving outpatient TSA/RSA. Outpatient TSA/RSA was associated with fewer 90-day readmissions (OR 0.48 CI 0.38–0.59, p < 0.001) and lower 90-day costs (−20.1% CI -19.1%; −21.1%, p < 0.001).
Conclusions
Outpatient TSA/RSA surgery offers lower complication rates and total costs.
Level of evidence
III.
Keywords: Total shoulder arthroplasty, Reverse shoulder arthroplasty, Outpatient, Ambulatory surgery center, Cost analysis, Readmission
1. Introduction
With an aging population, demand for total and reverse shoulder arthroplasty (TSA/RSA) is growing rapidly.1 Traditionally performed in the inpatient setting, TSA and RSA are increasingly offered as an outpatient procedure for selected patients. Multiple studies have demonstrated that outpatient (compared to inpatient) TSA/RSA results in a significant cost reduction along with similar to improved complication rates and improved patient satisfaction.2, 3, 4, 5, 6, 7 However, under current reimbursement guidelines from the Centers for Medicare and Medicaid Services (CMS), TSA and RSA are still considered inpatient procedures as they are included in the so-called “inpatient-only” list.8
A lack of data on adequate patient selection algorithms represents another barrier to wider implementation as not all patients are suitable candidates for outpatient TSA or RSA. Older and more comorbid patients likely carry elevated risks associated with surgery which merits postoperative observation in an inpatient setting. One recent study has even suggested an age of >70 years to be a contraindication for outpatient TSA/RSA.9 However, existing studies comparing inpatient to outpatient TSA/RSA lack generalizability and are subject to substantial selection bias as they mainly include single-surgeon or single-institutional data, are limited by small sample sizes and lack proper comorbidity matching.
Using recent national Medicare claims data we therefore aimed to compare inpatient to outpatient TSA/RSA in terms of 1) patient characteristics (to identify potential surgeon decision-making) and 2) costs and complications (to assess whether outpatient TSA/RSA is as economic and safe as previous literature would suggest).2 Finally, 3) we sought to identify incremental costs associated with various comorbidities in inpatient and outpatient TSA/RSA and specific patient subgroups more likely to benefit from outpatient surgery.
Medicare claims data were used to allow generalizability and sufficient power, and propensity score matching was applied to address selection bias.
2. Methods
2.1. Data source, study design, and study sample
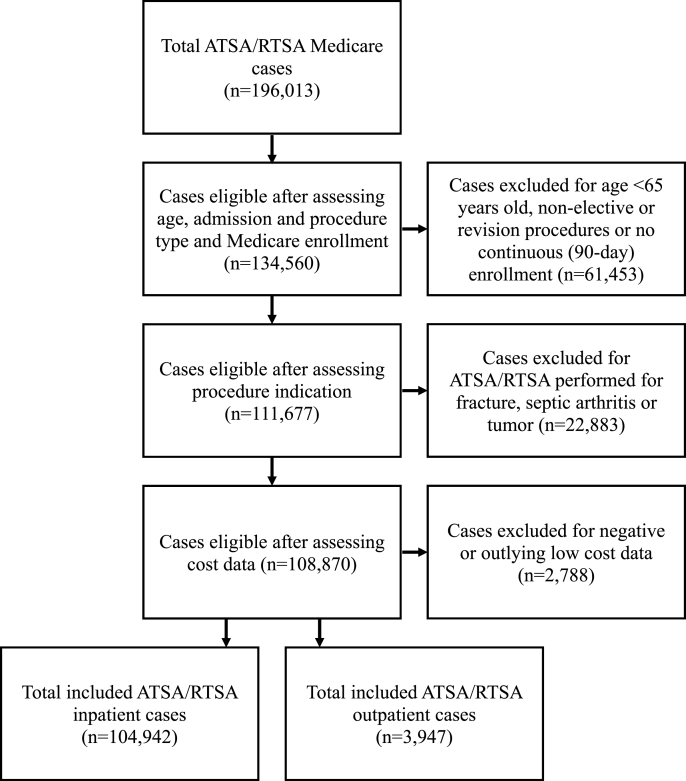
Data for this retrospective cohort study was extracted from the CMS Limited Data Set.10 We identified patients who received TSA or RSA surgery in the inpatient or outpatient setting between 2016 and 2018. Cases were defined using international classification of diseases, 10th revision (ICD-10) and current procedural terminology (CPT) codes (Supplementary Table 1). The following exclusion criteria were applied: non-elective procedures, revision procedures, patients under the age of 65 (to ensure Medicare eligibility based on age), diagnosis of fracture, tumor, or septic arthritis, duplicate or incomplete claims, or missing demographic information. Patients were only included if they had continuous enrollment following surgery for 90 days in order to determine 90-day readmissions and costs. Patients with cost data whose values were reported as ‘negative’, 0, or 3 standard deviations below the mean were also removed as they represent outliers (Fig. 1).
Fig. 1.
Medicare dataset flowchart of included and excluded ATSA/RTSA cases performed between 2016 and 2018.
2.2. Study variables
The main effect of interest was TSA/RSA in either the inpatient or outpatient setting. Outpatient surgeries were identified as those with a length of stay (LOS) of 0 days, taking place at a hospital in an outpatient setting or ambulatory surgical center.
Outcomes of interest included complications and cost of care as determined by Medicare payments. Complications included 1) all-cause readmission within 90 days, 2) 90-day readmission due to surgical site infection (SSI), pulmonary embolism (PE), deep vein thrombosis (DVT), urinary tract infection (UTI), pneumonia, myocardial infarction (MI), acute kidney injury (AKI), cerebrovascular accident (CVA), and blood transfusion, 3) Mortality, and 4) discharge to a skilled nursing facility (SNF). Reasons for readmission were identified using ICD-10 codes (Supplementary Table 2). Cost outcomes were further specified into 1) Medicare payments related to surgery and hospitalization (‘Hospitalization cost’). 2) 90-day post-discharge cost not including cost of surgery (’90-day post-discharge cost’), 3) Medicare claims the day of surgery as well as during the 90-day post-discharge period (‘Total 90-day cost’).
Other study variables were baseline patient demographics (age, race [White, Black, or Other], and gender) and comorbidities (these were defined using ICD-10 codes [Supplementary Table 3] and included the Deyo-Charlson comorbidity index, smoking, obesity (using a cutoff of 35 kg/m2), and type 2 diabetes mellitus [DM] with and without insulin dependence). The latter was singled out from the Deyo-Charlson comorbidity index given its hypothesized clinical relevance in patient selection.
2.3. Statistical analysis
For our first study question, understanding current patient selection criteria, univariable analysis was used to assess the relationship between the patient characteristics and the location of surgery (Table 1). Given our sample size where these differences easily reach statistical significance, we report both p-values and standardized differences in univariable comparisons. A standardized difference of 0.1 (or 10%) was used to indicate a meaningful difference between groups.
Table 1.
Univariate analysis of unmatched patient demographics and comorbidities.
| Variables | Inpatient (N) | Inpatient (%) | Outpatient (N) | Outpatient (%) | P-Value | SDD (%) |
|---|---|---|---|---|---|---|
| Age (years) | <0.001 | 22.5 | ||||
| 65-70 | 26,685 | 25.4 | 1270 | 32.2 | ||
| 70-75 | 31,180 | 29.7 | 1259 | 31.9 | ||
| 75-80 | 26,440 | 25.2 | 919 | 23.3 | ||
| 80-85 | 14,543 | 13.9 | 355 | 9.0 | ||
| 85+ | 6094 | 5.8 | 144 | 3.7 | ||
| Sex | <0.001 | 18.0 | ||||
| Male | 44,484 | 42.4 | 2025 | 51.3 | ||
| Female | 60,458 | 57.6 | 1922 | 48.7 | ||
| Race | 0.003 | 6.4 | ||||
| White | 99,181 | 94.5 | 3694 | 93.6 | ||
| Black | 2684 | 2.6 | 100 | 2.5 | ||
| Other | 3077 | 2.9 | 153 | 3.9 | ||
| Charlson Deyo Index | <0.001 | 23.3 | ||||
| 0 | 54,808 | 52.2 | 2400 | 60.8 | ||
| 1 | 28,301 | 27.0 | 1042 | 26.4 | ||
| 2 | 12,201 | 11.6 | 310 | 7.9 | ||
| >2 | 9632 | 9.2 | 195 | 4.9 | ||
| Year of surgery | <0.001 | 14.0 | ||||
| 2016 | 35,290 | 33.6 | 1132 | 28.7 | ||
| 2017 | 38,498 | 36.7 | 1435 | 36.4 | ||
| 2018 | 31,154 | 29.7 | 1380 | 35.0 | ||
| Comorbidities | ||||||
| Smoking | 4408 | 4.2 | 189 | 4.8 | 0.071 | 2.8 |
| Obesity | 18,230 | 17.4 | 501 | 12.7 | <0.001 | 13.1 |
| Hypertension | 64,851 | 61.8 | 2338 | 59.2 | 0.001 | 5.2 |
| Myocardial Infarction | 4953 | 4.7 | 170 | 4.3 | 0.229 | 2.0 |
| Congestive Heart Failure | 5523 | 5.3 | 117 | 3.0 | <0.001 | 11.6 |
| Peripheral Vascular Disease | 3809 | 3.6 | 100 | 2.5 | <0.001 | 6.4 |
| Cerebrovascular Disease | 1530 | 1.5 | 39 | 1.0 | 0.015 | 4.3 |
| Dementia | 1325 | 1.3 | 20 | 0.5 | <0.001 | 8.1 |
| COPD | 18,721 | 17.8 | 546 | 13.8 | <0.001 | 11.0 |
| Rheumatoid Disease | 5935 | 5.7 | 155 | 3.9 | <0.001 | 8.1 |
| Mild Liver Disease | 944 | 0.9 | 20 | 0.5 | 0.010 | 4.7 |
| Severe Liver Disease | 49 | 0.1 | 0 | 0.0 | 0.175 | 3.1 |
| Renal Disease | 9391 | 9.0 | 196 | 5.0 | <0.001 | 15.7 |
| End Stage Renal Disease | 237 | 0.2 | 3 | 0.1 | 0.049 | 3.9 |
| Diabetes without Complications | 17,083 | 16.3 | 591 | 15.0 | 0.029 | 3.6 |
| Diabetes with Complications | 5083 | 4.8 | 107 | 2.7 | <0.001 | 11.2 |
| Diabetes Insulin Dependent | 3226 | 3.1 | 73 | 1.9 | <0.001 | 7.9 |
| Diabetes Non-Insulin Dependent | 18,153 | 17.3 | 612 | 15.5 | 0.003 | 4.8 |
SDD, Standardized difference; COPD, Chronic obstructive pulmonary disease.
For the main analysis comparing outcomes of cost and complications between the inpatient and outpatient setting, a 1:3 propensity score matching was performed (matching each outpatient case to three inpatient cases). The propensity score was calculated using demographic information, Deyo-Charlson comorbidity index, and the additional comorbidity variables. Standardized differences were recalculated for the matched cohort to ensure proper propensity score matching (Table 2). Using this matched cohort, mixed-effects regression models were applied to compare inpatient and outpatient TSA/RSA in terms of the study outcomes (Table 3). In a further refinement of this analysis we estimated inpatient/outpatient differences in terms of all-cause 90-day readmission, SNF discharge and costs, separately for Deyo-Charlson comorbidity categories 0 to >2 (Table 4). This analysis was performed to evaluate the hypothesized beneficial impact of outpatient surgery across patient comorbidity categories.
Table 2.
Univariate analysis of propensity-score matched patient demographics and comorbidities.
| Variable | Inpatient (N) | Inpatient (%) | Outpatient (N) | Outpatient (%) | P-Value | SDD (%) |
|---|---|---|---|---|---|---|
| Age | 0.911 | 5.7 | ||||
| 65-70 | 3752 | 31.7 | 1269 | 32.2 | ||
| 70-75 | 3750 | 31.7 | 1258 | 31.9 | ||
| 75-80 | 2798 | 23.6 | 919 | 23.3 | ||
| 80-85 | 1070 | 9.0 | 355 | 9.0 | ||
| 85+ | 465 | 3.9 | 144 | 3.7 | ||
| Sex | 0.876 | 0.2 | ||||
| Male | 6055 | 51.2 | 2024 | 51.3 | ||
| Female | 5780 | 48.8 | 1921 | 48.7 | ||
| Race | 0.047 | 6.4 | ||||
| White | 11,198 | 94.6 | 3692 | 93.6 | ||
| Black | 242 | 2.1 | 100 | 2.5 | ||
| Other | 395 | 3.3 | 153 | 3.9 | ||
| Charlson Deyo Index | 0.516 | 5.1 | ||||
| 0 | 7217 | 61.0 | 2400 | 60.8 | ||
| 1 | 3183 | 26.9 | 1042 | 26.4 | ||
| 2 | 921 | 7.8 | 310 | 7.9 | ||
| >2 | 514 | 4.3 | 193 | 4.9 | ||
| Year of surgery | 0.795 | 2.4 | ||||
| 2016 | 3428 | 29.0 | 1132 | 28.7 | ||
| 2017 | 4343 | 36.7 | 1435 | 36.4 | ||
| 2018 | 4064 | 34.3 | 1378 | 34.9 | ||
| Comorbidities | ||||||
| Smoking | 541 | 4.6 | 189 | 4.8 | 0.569 | 1.0 |
| Obesity | 1481 | 12.5 | 500 | 12.7 | 0.792 | 0.4 |
| Hypertension | 7036 | 59.5 | 2336 | 59.2 | 0.793 | 0.5 |
| Myocardial Infarction | 471 | 4.0 | 169 | 4.3 | 0.402 | 1.5 |
| Congestive Heart Failure | 333 | 2.8 | 117 | 3.0 | 0.620 | 0.9 |
| Peripheral Vascular Disease | 268 | 2.3 | 100 | 2.5 | 0.330 | 1.7 |
| Cerebrovascular Disease | 82 | 0.7 | 39 | 1.0 | 0.065 | 3.2 |
| Dementia | 51 | 0.4 | 20 | 0.5 | 0.537 | 1.1 |
| COPD | 1650 | 13.9 | 545 | 13.8 | 0.842 | 0.4 |
| Rheumatoid Disease | 433 | 3.7 | 155 | 3.9 | 0.437 | 1.4 |
| Mild Liver Disease | 55 | 0.5 | 20 | 0.5 | 0.738 | 0.6 |
| Severe Liver Disease | 0 | 0.0 | 0 | 0.0 | 1.000 | 0 |
| Renal Disease | 531 | 4.5 | 196 | 5.0 | 0.211 | 2.3 |
| End Stage Renal Disease | 10 | 0.1 | 3 | 0.1 | 0.873 | 0.3 |
| Diabetes without Complications | 1784 | 15.1 | 591 | 15.0 | 0.888 | 0.3 |
| Diabetes with Complications | 302 | 2.6 | 106 | 2.7 | 0.643 | 0.9 |
| Diabetes Insulin Dependent | 210 | 1.8 | 73 | 1.9 | 0.755 | 0.6 |
| Diabetes Non-Insulin Dependent | 1845 | 15.6 | 611 | 15.5 | 0.879 | 0.3 |
SDD, Standardized Difference; COPD, Chronic obstructive pulmonary disease.
Table 3.
Propensity-score matched analysis of outcomes and cost variables between inpatient and outpatients ATSA/RTSA cases.
| Outcomes | Inpatient (N) | Inpatient (%) | Outpatient (N) | Outpatient (%) | P-Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| All Cause Readmission within 90 days | 573 | 4.8 | 93 | 2.4 | <0.001 | 0.48 | 0.38 | 0.59 |
| Mortality within 90 days | 22 | 0.2 | 8 | 0.2 | 0.833 | 1.09 | 0.49 | 2.45 |
| Blood Transfusion | 20 | 0.2 | 9 | 0.2 | 0.453 | 1.35 | 0.62 | 2.97 |
| Non-Home Discharge | 3226 | 27.3 | 552 | 14.0 | <0.001 | 0.43 | 0.39 | 0.48 |
| Discharge to SNF | 898 | 7.7 | 25 | 0.6 | <0.001 | 0.08 | 0.05 | 0.12 |
| Readmission within 90 days for: | ||||||||
| Surgical Site Infection | 25 | 0.2 | 5 | 0.1 | 0.297 | 0.60 | 0.23 | 1.57 |
| Pulmonary Embolism | 36 | 0.3 | 7 | 0.2 | 0.191 | 0.58 | 0.26 | 1.31 |
| Deep Vein Thrombosis | 31 | 0.3 | 6 | 0.2 | 0.222 | 0.58 | 0.24 | 1.39 |
| Urinary Tract Infection | 70 | 0.6 | 9 | 0.2 | 0.007 | 0.38 | 0.19 | 0.77 |
| Pneumonia | 50 | 0.4 | 11 | 0.3 | 0.211 | 0.66 | 0.34 | 1.27 |
| Myocardial Infarction | 35 | 0.3 | 9 | 0.2 | 0.487 | 0.77 | 0.37 | 1.61 |
| Acute Kidney Injury | 88 | 0.7 | 23 | 0.6 | 0.297 | 0.78 | 0.49 | 1.24 |
| Cerebrovascular Accident |
20 |
0.2 |
1 |
0.0 |
0.064 |
0.15 |
0.02 |
1.12 |
| Cost variables |
Inpatient (Mean ± SD)($) |
Outpatient (Mean ± SD)($) |
P-value | Cost difference (%) | 95% Confidence Interval | |||
| Hospitalization Cost ($) | 14,896 ± 3854 | 12,296 ± 4591 | <0.001 | −17.5 | −18.3 | −16.6 | ||
| 90-Day Post-Discharge Cost ($) | 1234 ± 5513 | 598 ± 3641 | <0.001 | −15.7 | −23.7 | −7.0 | ||
| 90-Day Total Cost ($) | 16130 ± 6835 | 12894 ± 6178 | <0.001 | −20.1 | −21.1 | −19.1 | ||
SNF, Specialized nursing facility.
Table 4.
Propensity-score matched analysis between inpatient and outpatient cases categorized in Charlson-Deyo comorbidity index categories.
| Deyo 0 |
Deyo 1 |
Deyo 2 |
Deyo >2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Odds Ratio | 95% CI | P-Value | Odds Ratio | 95% CI | P-Value | Odds Ratio | 95% CI | P-Value | Odds Ratio | 95% CI | P-Value | |||||
| All Cause Readmission within 90 days | 0.49 | 0.36 | 0.67 | <0.001 | 0.42 | 0.27 | 0.65 | <0.001 | 0.87 | 0.45 | 1.58 | 0.656 | 0.40 | 0.19 | 0.85 | 0.017 | |
| Mortality within 90 days | 1.00 | 0.27 | 3.70 | 1.000 | 1.80 | 0.43 | 7.56 | 1.000 | 6.02 | 0.54 | 66.77 | 0.144 | <0.01 | <0.001 | 1.000 | 0.997 | |
| Discharge to SNF |
0.07 |
0.03 |
0.13 |
<0.001 |
0.01 |
0.05 |
0.19 |
<0.001 |
0.13 |
0.05 |
0.35 |
<0.001 |
0.08 |
0.03 |
0.27 |
<0.001 |
|
| Cost Variables | Cost Diff (%) | 95% CI | P-Value | Cost Diff (%) | 95% CI | P-Value | Cost Diff (%) | 95% CI | P-Value | Cost Diff (%) | 95% CI | P-Value | |||||
| Hospitalization Cost ($) | −17.9 | −19.0 | −16.7 | <0.001 | −17.4 | −19.0 | −15.7 | <0.001 | −17.8 | −20.8 | −14.7 | <0.001 | −17.6 | −21.5 | −13.5 | <0.001 | |
| 90-Day Post-Discharge Cost ($) | −10.3 | −20.9 | 1.6 | 0.088 | −19.0 | −32.8 | −2.3 | 0.027 | 26.1 | −10.2 | 77.0 | 0.180 | −39.7 | −61.1 | −6.6 | 0.024 | |
| Total 90-day Cost ($) | −19.9 | −21.1 | −18.6 | <0.001 | −20.2 | −22.0 | −18.3 | <0.001 | −18.3 | −22.0 | −14.5 | <0.001 | −23.2 | −27.7 | −18.4 | <0.001 | |
SNF, Specialized nursing facility.
We aimed to identify the strongest drivers of 90-day total cost in the inpatient and outpatient setting separately (Table 5). Here, we included patient age, sex and the most common individual comorbidities (smoking, obesity, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes mellitus, renal disease, rheumatoid disease and history of a myocardial infarction). Lastly, we analyzed the relationship between medical comorbidities and hospital readmission in the outpatient group separately (Table 6). Throughout, we report odds ratios (OR) with 95% confidence intervals (CI) for the binary outcomes while percent change and 95% CI is reported for the continuous outcomes. Here, we applied the gamma distribution with a log link function (within PROC GLIMMIX in SAS statistical software) as these variables are highly skewed. All analyses were performed using SAS v9.4 statistical software (SAS Institute, Cary, NC).
Table 5.
Significant drivers of total 90-day cost in the inpatient and outpatient setting.
| Variables | Reference | Reference Mean Cost ($)\ |
Cost Difference ($) | Cost Difference (%) | 95% CI | P-Value | |
|---|---|---|---|---|---|---|---|
| A. Inpatient | |||||||
| Age (years) | |||||||
| 70-75 | 65–70 | 15,922 | 242 | 1.5 | 1.0 | 2.1 | <0.001 |
| 75-80 | 364 | 2.3 | 1.7 | 2.8 | <0.001 | ||
| 80-85 | 620 | 3.9 | 3.2 | 4.6 | <0.001 | ||
| 85+ | 1073 | 6.7 | 5.8 | 7.7 | <0.001 | ||
| Sex | |||||||
| Female | Male | 16,283 | −81 | −0.5 | −0.9 | −0.1 | 0.014 |
| Comorbidities | |||||||
| Smoking | – | 16,260 | −71 | −0.4 | −1.4 | 0.5 | 0.378 |
| Obesity | – | 16,177 | 417 | 2.6 | 2.1 | 3.1 | <0.001 |
| Congestive Heart Failure | – | 16,157 | 1397 | 8.7 | 7.7 | 9.6 | <0.001 |
| COPD | – | 16,159 | 417 | 2.6 | 2.1 | 3.1 | <0.001 |
| Dementia | – | 16,242 | 738 | 4.6 | 2.8 | 6.4 | <0.001 |
| Diabetes Mellitus | – | 16,244 | −118 | −0.7 | −1.2 | −0.2 | 0.006 |
| Renal Disease | – | 16,150 | 806 | 5.0 | 4.3 | 5.7 | <0.001 |
| Rheumatoid Disease | – | 16,243 | 157 | 1.0 | 0.1 | 1.2 | 0.025 |
| Myocardial Infarction |
– |
16,216 |
485 |
3.0 |
2.0 |
3.9 |
<0.001 |
| B. Outpatient | |||||||
| Age (years) | |||||||
| 70-75 | 65–70 | 12,735 | 217 | 1.7 | 1.2 | 2.2 | <0.001 |
| 75-80 | 316 | 2.5 | 1.9 | 3.1 | <0.001 | ||
| 80-85 | 544 | 4.3 | 3.6 | 5.0 | <0.001 | ||
| 85+ | 885 | 7.0 | 6.0 | 7.9 | <0.001 | ||
| Sex | |||||||
| Female | Male | 12,876 | −28 | −0.2 | −0.6 | 0.2 | 0.283 |
| Comorbidities | |||||||
| Smoking | – | 12,884 | −54 | −0.4 | −1.4 | 0.6 | 0.396 |
| Obesity | – | 12,838 | 360 | 2.8 | 2.3 | 3.3 | <0.001 |
| Congestive Heart Failure | – | 12,884 | 1126 | 8.7 | 7.8 | 9.7 | <0.001 |
| COPD | – | 12,877 | 343 | 2.7 | 2.1 | 3.2 | <0.001 |
| Dementia | – | 12,887 | 633 | 4.9 | 3.1 | 6.8 | <0.001 |
| Diabetes Mellitus | – | 12,878 | −84 | −0.7 | −1.2 | −0.1 | 0.014 |
| Renal Disease | – | 12,858 | 676 | 5.3 | 4.5 | 6.0 | <0.001 |
| Rheumatoid Disease | – | 12,885 | 145 | 1.1 | 0.3 | 2.0 | 0.010 |
| Myocardial Infarction | – | 12,896 | 376 | 2.9 | 2.0 | 3.9 | <0.001 |
Table 6.
Multivariate analysis of risk factors for 90-day all cause readmission in the outpatient setting.
| Variables | Reference | Odds ratio | 95% CI | P-Value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 70-75 | 65–70 | 1.62 | 0.90 | 2.91 | 0.107 |
| 75-80 | 1.73 | 0.93 | 3.23 | 0.084 | |
| 80-85 | 3.46 | 1.75 | 6.85 | <0.001 | |
| 85+ | 2.08 | 0.68 | 6.31 | 0.194 | |
| Sex | |||||
| Female | Male | 1.03 | 0.68 | 1.57 | 0.887 |
| Race | |||||
| Black | White | 0.37 | 0.05 | 2.66 | 0.320 |
| Other | 1.26 | 0.45 | 3.54 | 0.661 | |
| Comorbidities | |||||
| Smoking | – | 2.24 | 1.05 | 4.77 | 0.037 |
| Obesity | – | 1.07 | 0.58 | 1.97 | 0.832 |
| Congestive Heart Failure | – | 1.62 | 0.62 | 4.26 | 0.330 |
| COPD | – | 0.84 | 0.45 | 1.57 | 0.589 |
| Dementia | – | <0.001 | <0.001 | 1 | 0.927 |
| Diabetes Mellitus | – | 2.27 | 1.42 | 3.61 | <0.001 |
| Renal Disease | – | 1.41 | 0.66 | 3.04 | 0.378 |
| Rheumatoid Disease | – | 1.93 | 0.82 | 4.56 | 0.132 |
| Myocardial Infarction | – | 1.06 | 0.41 | 2.74 | 0.903 |
3. Results
We identified 108,889 patients who received an elective TSA or RSA between 2016 and 2018; 3947 cases (3.6%) were defined as outpatient. Study results are presented separately for each study question.
3.1. What patient characteristics appear to be used to select patients for outpatient TSA/RSA?
Overall, patients undergoing outpatient (compared to inpatient) TSA/RSA were younger (71.6 versus 73.0 years old, standardized difference 0.20). They were also more often male (51.3% versus 42.4%, standardized difference 0.18), and generally had a lower comorbidity burden (60.8% versus 52.2% with a Deyo-Charslon comorbidity burden of 0), standardized difference 0.23). Inpatient/outpatient differences in terms of individual comorbidities were most pronounced for obesity (12.7% versus 17.4%), congestive heart failure (CHF; 3.0% versus 5.3%), chronic obstructive pulmonary disease (COPD; 13.8% versus 17.8%), renal disease (5.0% versus 9.0%) and diabetes (DM) with complications (2.7% versus 4.8%); all p < 0.0001 and with standardized differences >0.1 (Table 1).
3.2. To what extent is outpatient (compared to inpatient) TSA/RSA as safe and more economic?
Propensity score matching resulted in a more balanced distribution of variables between groups (Table 2) as reflected by non-significant p-values and standardized differences <0.1.
Outpatient (compared to inpatient) TSA/RSA was associated with decreased odds of all-cause 90-day readmission (OR 0.48: 95% CI: 0.38–0.59, p < 0.0001), and readmission with a diagnosis code for UTI (OR 0.38: 95% CI: 0.19–0.77, p = 0.007). No differences were observed for readmission due to DVT, PE, MI, AKI, transfusion, pneumonia, SSI or 90-day mortality. Lastly, outpatient (compared to inpatient) TSA/RSA was associated with decreased odds of discharge to a SNF (OR 0.08: 95% CI: 0.06–0.12, p < 0.0001); Table 3.
Outpatient (compared to inpatient) TSA/RSA was associated with a 17.5% reduction in hospitalization cost (CI: 16.6%–18.3%; p < 0.0001), a 15.7% reduction in total 90-day post-discharge cost (CI: 7%–23.6%; p < 0.0001), and a 20.1% reduction in total 90-day cost (19.1%–21.1%; p < 0.001), This translated to a mean per-case cost saving of $2600, $637, and $3236 respectively; Table 3.
3.3. Subgroup analyses stratified by Deyo-Charlson comorbidity index
Among patients with a Deyo-Charlson comorbidity index of 0 and 1, outpatient TSA/RSA surgery was associated with lower all-cause 90-day readmission; this effect was absent among patients with a Deyo-Charlson comorbidity index score of ≥2. Outpatient TSA/RSA surgery was associated with lower rates of SNF discharge across all Deyo-Charlson comorbidity groups. A similar pattern was observed in terms of total 90-day cost. Table 4.
Which comorbidities are the strongest drivers of increased cost in inpatient and outpatient TSA/RSA and which patient subgroups should be avoided in outpatient TSA/RSA?
For inpatient TSA/RSA, all studied comorbidities except for smoking were associated with significantly increased total 90-day cost per patient. The largest 90-day increase was seen for patients with a history of CHF which added on average $1397 per patient, followed by age >85 (+$1072), and history of renal disease (+$806). Table 5. For outpatient TSA/RSA all comorbidities except for smoking and diabetes were found to be significant drivers of increased 90-day total cost with CHF (+$1125) representing the largest increase in cost per patient of all the comorbidities, followed by age >85 (+$885) and renal disease (+$676). (Table 5).
In the outpatient TSA/RSA group, smokers (OR 2.24 CI: 1.05–4.77, p-value = 0.037), diabetic patients (OR 2.27 CI:1.42–3.61, p-value <0.001) and patients aged 80–85 (OR 3.46 CI: 1.75–6.85, p-value<0.001) had higher 90-day all cause readmission rates (Table 6).
4. Discussion
Using data on >100,000 patients undergoing TSA/RSA surgery we were able to demonstrate benefits of outpatient (compared to inpatient) surgery across nearly all comorbidity groups. However, readmission risk benefits were specifically pronounced in patients with the lowest comorbidity burden. We also identified the most important cost drivers among inpatient and outpatient RSA/TSA.
Outpatient TSA/RSA holds potential at improving efficiency, increasing patient satisfaction while reducing overall healthcare costs.2,6,11, 12, 13 However, reliable, and generalizable data is necessary to demonstrate safety. We have found, surgeons are selecting younger, healthier, and male patients, a conclusion in line with previous literature.13 More specifically, surgeons tended to select against patients with COPD, CHF and patients with complications stemming from DM. Despite this, nearly 14% of the outpatient group had a history of COPD, and almost 3% had diabetes with end-organ damage, comorbidities considered either high risk or absolute contraindications.9,14 Interestingly, surgeons did not appear to select out smokers, and smokers were found to be at higher readmission risk, consistent with a previous study.15
Our analysis confirmed that even after controlling for patient comorbidity, age and demographics, outpatient TSA/RSA was associated with a lower all-cause readmission rates and significantly lower costs than inpatient TSA/RSA in terms of hospitalization cost, post-surgical costs and 90-day total cost (a 20.1% reduction translating to a mean per-patient savings of $3236). With arthroplasty growing at a rate of 10–15% per annum, this translates to significant potential savings for the Medicare system.16,17 In subgroup analyses, we found, the improvement in readmission rate (associated with outpatient surgery) was lost in our sicker cohorts (i.e. those with a Deyo-Charlson score of ≥2). Consistent with multiple papers demonstrating these medical comorbidities are associated with worse outcomes in TSA.14,15,18, 19, 20
Increasingly it is necessary to understand the costs and risks associated with individual patient comorbidities to maintain economic viability for surgeons, especially if bundle based episodic care payments are initiated. Recent proposals to revise the Medicare hospital outpatient prospective payment system (OPPS) and the Medicare ambulatory surgical center (ASC) payment have been put forward.21 If proposed changes are implemented, such as the removal of the “inpatient only” procedure list, significant changes with regards to delivery of care as well as reimbursement for shoulder arthroplasty can be anticipated. Having a better understanding of drivers of risk and cost will be essential to ensuring a sustainable practice.
To maintain equitable access to care, surgeons and payers must account for the elevated medical risk and cost some patients pose the system. Our analysis demonstrates smokers, diabetic patients, and patients aged 80–85 all had increased 90-day readmission rates relative to our standardized controls. Of note, while our 80–85 age group experienced a higher readmission rate, our 85+ age group did not. We believe statistical significance was not obtained due to the small number of patients in this group, and that if the numbers had been larger, the same trend in higher readmission rate would be seen in this group as well.
In the inpatient setting, nearly every comorbidity studied was a significant driver of increased cost. History of CHF, age >85, and a history of renal produced the largest increase in cost. Likewise, in our outpatient group as the same 3 comorbidity groups represented the largest risk factors for increased cost. The exclusion of these patient comorbidities due to elevated cost profiles from outpatient consideration is consistent with a recent study which eliminates these groups from outpatient TSA consideration based on elevated medical risk.9 In our opinion, because these comorbidities are associated with such a significant increase in cost, in addition to a higher rate of adverse outcomes, patients with these comorbidities would likely require risk adjustments in a potential bundle or should be excluded from future bundled payment models altogether to maintain equitable access to care.
After comorbidity matching patients and removing the cost of the surgery and initial hospitalization, patients performed in the outpatient setting demonstrated a 15.7% reduction in 90-day post-discharge cost. We postulate that this additional cost savings may be due to lower utilization rates of inpatient rehabilitation services. Our data suggests routine post-operative hospital admissions likely increase SNF utilization even if it may not be medically necessary, representing a significant increase in cost to the system. We also hypothesize that higher SNF utilization may be at least partially responsible for the higher 90-day readmission rate seen among our inpatient group which was present even after propensity matching patient comorbidities. Additional research is needed to definitively state why comorbidity matched patients have higher SNF discharge. It is possible that this may be a result of lack of social support at home which is not identified appropriately preoperatively. In order to expand outpatient TSA, these patients must be appropriately identified, and home accommodations made preoperatively.
As we transition from a fee-for-service to value-based care models, identifying patient populations which can be safely performed in an outpatient setting will be crucial to improve value-based metrics. We believe studies such as ours which utilize large databases represent an objective and data-driven approach to identify potential patient selection algorithms for outpatient shoulder arthroplasty.
5. Limitations
The limitations of our study include the inherent limitations of most database studies, including unknown disease severity, anesthesia protocols, and post-operative rehabilitation methodology. In addition, our results are based on the quality of provider coding and identification of specific complications via the ICD-10 coding system. Also, there are additional confounding variables inherent to patient selection for outpatient surgery which are not represented in the Medicare database and therefore not included in this study. These factors include things such availability of ambulatory surgery centers, anesthesiology indications, as well as regional practice differences. In addition, given the current reimbursement structure of Medicare making it difficult to get these surgeries approved as outpatient events, with inconsistent reimbursement for implants, these surgeries though deemed “outpatient” because patients have an LOS of 0, most are not performed in an ambulatory surgery center but rather in a traditional hospital and thus patients are not truly getting an “outpatient” surgery in the same way outpatient knee arthroplasty is performed. However, as we were seeking to measure all-cause readmission, mortality, and cost variables in a specific population for which this database is entirely inclusive we feel it was the best possible study design to answer our preliminary study questions.
6. Conclusion
We summarize that while there is no substitute for proper clinical judgement in determining patient selection, our data driven analysis is clearly in support of wider indications for outpatient elective shoulder arthroplasty with a recommendation for a comorbidity-based risk adjustment model to any future shoulder arthroplasty bundle to ensure equitable access to care.
Funding
No outside funding was obtained for the creation of this study.
CRediT authorship contribution statement
Andrew Carbone: Methodology, Investigation, Writing – original draft. Alexander J. Vervaecke: Methodology, Investigation, Writing – original draft. Ivan B. Ye: Software, Formal analysis, Data curation. Akshar V. Patel: Data curation, Resources. Bradford O. Parsons: Conceptualization, Resources, Writing – review & editing. Leesa M. Galatz: Conceptualization, Resources, Writing – review & editing. Jashvant Poeran: Software, Investigation, Resources, Writing – review & editing. Paul Cagle: Conceptualization, Resources, Writing – review & editing, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2021.11.016.
Contributor Information
Ivan B. Ye, Email: ivan.ye@icahn.mssm.edu.
Paul Cagle, Email: Paul.cagle@mountsinai.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Day J.S., Lau E., Ong K.L., Williams G.R., Ramsey M.L., Kurtz S.M. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Brolin T.J., Mulligan R.P., Azar F.M., Throckmorton T.W. Neer Award 2016: outpatient total shoulder arthroplasty in an ambulatory surgery center is a safe alternative to inpatient total shoulder arthroplasty in a hospital: a matched cohort study. J Shoulder Elbow Surg. 2017;26(2):204–208. doi: 10.1016/j.jse.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Leroux T.S., Basques B.A., Frank R.M., et al. Outpatient total shoulder arthroplasty: a population-based study comparing adverse event and readmission rates to inpatient total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(11):1780–1786. doi: 10.1016/j.jse.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Leroux T.S., Zuke W.A., Saltzman B.M., et al. Safety and patient satisfaction of outpatient shoulder arthroplasty. JSES Open Access. 2018;2(1):13–17. doi: 10.1016/j.jses.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer J.D., Chan P.H., Prentice H.A., Hatch J., Dillon M.T., Navarro R.A. Same-day discharge is not inferior to longer length of in-hospital stay for 90-day readmissions following shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29(5):898–905. doi: 10.1016/j.jse.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Gregory J.M., Wetzig A.M., Wayne C.D., Bailey L., Warth R.J. Quantification of patient-level costs in outpatient total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(6):1066–1073. doi: 10.1016/j.jse.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Erickson B.J., Shishani Y., Jones S., et al. Outpatient vs. inpatient reverse total shoulder arthroplasty: outcomes and complications. J Shoulder Elbow Surg. 2020;(608):1–6. doi: 10.1016/j.jse.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services O of I.G. 2000. Prospective Payment System for Hospital Outpatient Service. [Google Scholar]
- 9.Fournier M.N., Brolin T.J., Azar F.M., Stephens R., Throckmorton T.W. Identifying appropriate candidates for ambulatory outpatient shoulder arthroplasty: validation of a patient selection algorithm. J Shoulder Elbow Surg. 2019;28(1):65–70. doi: 10.1016/j.jse.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid 2020. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/DUA_-_NewLDS Limited Data Set. Centers for Medicare & Medicaid Services.
- 11.Leroux T.S., Basques B.A., Frank R.M., et al. Outpatient total shoulder arthroplasty: a population-based study comparing adverse event and readmission rates to inpatient total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(11):1780–1786. doi: 10.1016/j.jse.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Steinhaus M.E., Shim S.S., Lamba N., Makhni E.C., Kadiyala R.K. Outpatient total shoulder arthroplasty: a cost-identification analysis. J Orthop. 2018;15(2):581–585. doi: 10.1016/j.jor.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basques B.A., Erickson B.J., Leroux T., et al. Comparative outcomes of outpatient and inpatient total shoulder arthroplasty. Bone Jt J. 2017;99B(7):934–938. doi: 10.1302/0301-620X.99B7.BJJ-2016-0976.R1. [DOI] [PubMed] [Google Scholar]
- 14.Lee R., Lee D., Mamidi I.S., Probasco W.V., Heyer J.H., Pandarinath R. Patients with chronic obstructive pulmonary disease are at higher risk for pneumonia, septic shock, and blood transfusions after total shoulder arthroplasty. Clin Orthop Relat Res. 2019;477(2):416–423. doi: 10.1097/CORR.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells D.B., Holt A.M., Smith R.A., Brolin T.J., Azar F.M., Throckmorton T.W. Tobacco use predicts a more difficult episode of care after anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(1):23–28. doi: 10.1016/j.jse.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Day J.S., Lau E., Ong K.L., Williams G.R., Ramsey M.L., Kurtz S.M. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Dillon M.T., Chan P.H., Inacio M.C.S., Singh A., Yian E.H., Navarro R.A. Yearly trends in elective shoulder arthroplasty , 2005 – 2013. Arthritis Care Res. 2017;69(10):1574–1581. doi: 10.1002/acr.23167. [DOI] [PubMed] [Google Scholar]
- 18.Fu M.C., Boddapati V., Dines D.M., Warren R.F., Dines J.S., Gulotta L.V. The impact of insulin dependence on short-term postoperative complications in diabetic patients undergoing total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(12):2091–2096. doi: 10.1016/j.jse.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Belmont P., Kusnezov N., Dunn J., Bader J., Kilcoyne K., Waterman B. Predictors of hosptal readmission after total shoulder arthroplasty. Orthopedics. 2017;40(1):1–10. doi: 10.3928/01477447-20160915-06. [DOI] [PubMed] [Google Scholar]
- 20.Lovy A.J., Keswani A., Beck C., Dowdell J.E., Parsons B.O. Risk factors for and timing of adverse events after total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(6):1003–1010. doi: 10.1016/j.jse.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Center for Medicare and Medicaid, D of H and HS Hospital outpatient prospective payment and ambulatory surgical center payment systems and quality reporting programs; new categories for hospital outpatient department prior authorization process; clinical laboratory fee schedule; laboratory date of serv. Fed Regist. 2020;85(156):48772–49082. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.