Abstract
Objective
With the advances in biological technologies over the past 20 years, a number of new therapies to promote bone healing have been introduced. Particularly in the spinal surgery field, more unprecedented biological therapeutics become available to enhance spinal fusion success rate along with advanced instrumentation approaches. Yet surgeons may not have been well informed about their safety and efficacy profiles in order to improve clinical practices. Therefore there is a need to summarize the evidence and bring the latest progress to surgeons for better clinical services for patients.
Methods
We comprehensively reviewed the literatures in regard to the biological therapeutics for enhancement of spinal fusion published in the last two decades.
Results
Autograft bone is still the gold standard for bone grafting in spinal fusion surgery due to its good osteoconductive, osteoinductive, and osteogenic abilities. Accumulating evidence suggests that adding rhBMPs in combination with autograft effectively promotes the fusion rate and improves surgical outcomes. However, the stimulating effect on spinal fusion of other growth factors, including PDGF, VEGF, TGF-beta, and FGF, is not convincing, while Nell-1 and activin A exhibited preliminary efficacy. In terms of systemic therapeutic approaches, the osteoporosis drug Teriparatide has played a positive role in promoting bone healing after spinal surgery, while new medications such as denosumab and sclerostin antibodies still need further validation. Currently, other treatment, such as controlled-release formulations and carriers, are being studied for better releasing profile and the administration convenience of the active ingredients.
Conclusion
As the world's population continues to grow older, the number of spinal fusion cases grows substantially due to increased surgical needs for spinal degenerative disease (SDD). Critical advancements in biological therapeutics that promote spinal fusion have brought better clinical outcomes to patients lately. With the accumulation of higher-level evidence, the safety and efficacy of present and emerging products are becoming more evident. These emerging therapeutics will shift the landscape of perioperative therapy for the enhancement of spinal fusion.
Keywords: Spinal degenerative disease, Spinal fusion, Osteobiologics
1. Introduction
Spinal fusion surgical procedure is required most of the time to treat various vertebral bone-related diseases, including spinal degenerative diseases, spinal trauma, spinal deformities, and spinal infections [1]. The primary purpose of the surgery is to restore the spine's mechanical strength by fusing two or more adjacent vertebral bodies when the anatomical structure of the spine has been changed during disease stages. Since Dr. Fritz Lange, a German surgeon, first performed spinal fusion in patients with scoliosis in 1909, the fixation techniques necessary for surgery have improved significantly [2]. The surgical protocols have also been standardized in orthopedics.
Recent studies reported that 1.62 million spinal instrumentations were performed in 2018 in the US, of which 327,000 cases were thoracolumbar fixation and 350,000 cases of cervical fixation [3]. If the estimation is made according to the non-fusion rate of 10–28% previously defined [4,5], hundreds of thousands of people would have had experienced non-fusion or pseudoarthritis after spinal surgery every year in the US alone. Notably, the early loosening rate of pedicle screws was significantly higher in non-fusion patients, which caused 62.5% of subsequent reoperations [6,7]. Therefore, spinal non-fusion is a common and severe postoperative complication that will seriously affect patients' satisfaction, postoperative function, and mental state [7]. Furthermore, as the aging population surges, more people will become aging-related patients who undergo spinal fusion surgery for degenerative spinal diseases every year. Additional comorbidities such as osteoporosis can also impede bone fusion [8]. Thus, the enhancement of spinal fusion is a crucial issue that needs more attention.
A variety of intraoperative techniques have been recommended to help stabilize the spine and facilitate its fusion. Larger-diameter at multiple fixation points, expanded screws, undertapping of the pedicle screw tracks, transpedicular transdiscal screws, and cement augmentation are practical considerations to promote postoperative stabilization in osteoporotic patients [9,10]. For intervertebral fusion, the interbody fusion body made with advanced 3D-printed surfacing or expandable techniques is also available in the market. They could not only contribute to instant postoperative stabilization but also potentially help with osseointegration [11]. However, these fixations hardly fulfill the ultimate aim of achieving in situ rapid solid bony fusion.
Till now, autologous bone, characterized by its good osteoconductivity, osteoinductivity, and osteogenic abilities [1], is still the gold standard of bone grafting for spinal fusion. For decades, autologous iliac bone grafting has been the graft of choice for lumbar spine fusion [12]. However, the paucity of autograft bone and the donor site's complications are hurdles to prevent its widespread use [13], for the following problems remain to be adequately solved: 1) How to improve the fusion rate by stimulating the autograft bone; 2) How to achieve spinal fusion when the autograft bone is not sufficient enough to fill the gap; 3) How to prevent the potential adverse reactions caused by the therapy.
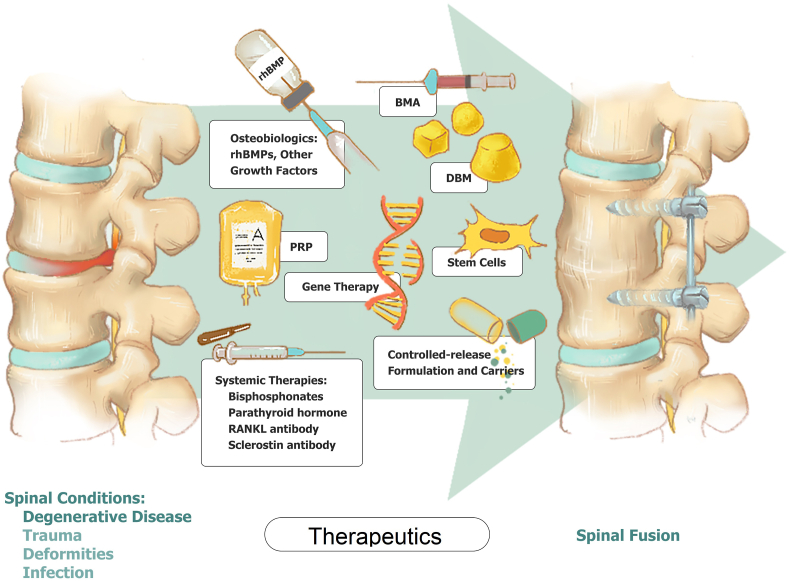
In the past 20 years, developments in novel biological therapeutics have brought various solutions to enhance spinal fusion. However, it is still essential to prove these therapies' clinical efficacy and safety through higher-level evidence for a much wider acceptance. Therefore, this article will give a comprehensive summary of the present and emerging therapies (Fig. 1, Table 1.) to facilize a better understanding.
Figure 1.
Summary of present and emerging therapeutics for enhancement of spinal fusion. rhBMPs: Recombinant human bone morphogenetic proteins; BMA: Bone marrow aspirate; DBM: Demineralized bone matrix; PRP: Platelet-rich plasma.
Table 1.
Summary of mechanism, efficacy, and unsolved challenges of therapeutics for the enhancement of spinal fusion. BMPs: Bone morphogenetic proteins; MSC: Mesenchymal stem cell; DBM: Demineralized bone matrix; BMA: Bone marrow aspirate; PRP: Platelet-rich plasma.
| Therapeutics | Mechanism | Efficacy | Unsolved challenges |
|---|---|---|---|
| Osteobiologics | |||
| BMPs | Stimulate osteogenic differentiation of MSCs (osteoinduction) and new bone formation [16] | Superior effect in combination with autograft bone | Dosage and safety concerns, lack of slow-release carrier |
| Other Growth Factors | NELL-1: Induce MSCs expansion and new bone formation [35] Activin A: Stimulate osteogenic differentiation of MSCs and new bone formation [36] |
Some have shown efficacy in animal models | Lack of clinical data in human |
| Biological Factor-containing Bone Expanders | |||
| DBM | Offer osteoconductive and osteoinductive capacities [39] | Non-inferior to autograft bone | Lack of mechanical strength and osteoinductive capacity |
| BMA | Offer osteoinductive capacity with cytokines and growth factors secreted by cells [40] | Non-inferior to autograft bone | Intraoperative leakage and lack of mechanical strength |
| PRP | Offer osteoinductive capacities with growth factors [43] | More than five times of concentration as it is in the peripheral blood to take effect | Inconsistent concentration among samples |
| Systemic Therapies | |||
| Bisphosphonates | Inhibit osteolysis and increase bone density [47] | Do not impede the spinal fusion | Low grade of evidence |
| Teriparatide | Stimulate bone formation [[49], [50]] | Correlated with a higher spinal fusion rate | Lack of evidence for routine use |
| Denosumab | Inhibit osteolysis and increase bone density [54] | Correlated with a higher spinal fusion rate | Lack of evidence for routine use |
| Romosozumab | Dual effect | No reports | Unknow efficacy |
| Other Therapies | |||
| Cell and Gene Therapies | |||
| Stem Cells | Facilitate spinal fusion with their osteogenic properties [56] | Not convincing in spinal fusion | GMP facility needed |
| Gene Therapy | Provide a vector expressing growth factors [62] | Provide expression of osteoinductive proteins | Adverse reactions are common |
| Controlled-release Formulation and Carriers | |||
| New Formulations | Controlled release of osteoinductive molecules [63] | Presently not well developed in spinal fusion | Adjustment of releasing profile and sterile |
| Potential Carriers | Controlled release of osteoinductive molecules provide instant mechanical strength [66] | Presently not well developed in spinal fusions, especially for macro-molecules | Optimization of releasing profile and stable combination with the molecule of interest |
2. Osteobiologics
2.1. Bone morphogenetic proteins (BMPs)
2.1.1. BMP-2 and BMP-7
BMPs are a group of kinases belonging to the transforming growth factor-β (TGF-β) Superfamily. Recombinant human BMPs (rhBMPs) can accelerate bone formation and promote osteogenic differentiation [14]. The FDA initially approved rhBMP-7 as a surgical device for nonunion of long bones in 2001 under a humanitarian device exemption. The FDA also approved rhBMP-2 in 2004 as a surgical device for spinal fusion and tibial fractures. These products all underwent registration as drug-device combinations when coupled with collagen sponges or intervertebral fusion cages in clinical use.
The majority of meta-analyses generally confirm the effect of BMPs in spinal fusion practices. For example, Parajón found that, in patients who received minimally invasive transforaminal lumbar interbody fusions (MIS TLIFs), the spinal fusion could reach as high as 99.1% by adding rhBMP-2 in combination with autograft bone. This rate was significantly higher than that of the autograft bone group alone (91.8%) [15]. Other studies have also shown that rhBMP-2 itself promotes a higher spinal fusion rate than that from allograft bone, ceramics, or demineralized bone matrix (DBM). At the same time, the operation time is shorter, and the reoperation rate is also lower. Besides, when compared with using iliac crest bone graft (ICBG) alone, the use of rhBMP-2 can also be presented with lower back pain, upper limb pain, Oswestry Disability Index (ODI) scores, and donor site pain [16,17]. In terms of spinal deformities, application of only 8.75 mg of rhBMPs within each segment rather than the recommended dose of 12 mg can achieve such effects of reducing the incidence of non-fusion significantly (OR = 0.23, 95% CI, p = 0.002, i2 = 0) [18].
Despite all these clinical evidence, however, in some specific fusion procedures, such as transforaminal lumbar interbody fusion (TLIF), the application of rhBMP-2 didn't show its superior efficacy in promoting fusion compared with autograft bone alone (92.7% vs. 92.3%). Based on this evidence, rhBMP-2 administration is unlikely to benefit patients undergoing this type of surgery probably due to the high curative rate [19].
A post-marketing multicenter retrospective study further defines that rhBMP-2 is mainly used for degenerative disc disease (DDD; 32.3% of patients), followed by lumbar spondylolisthesis (29.8%), spinal deformities (14.8%), and spinal nonunion (7.3%). Among these, rhBMP-2 treatment is most widely used in the posterior lumbar fusion (PLF) technique (34.9% of levels) [20].
In addition to the products expressed from eukaryotic cells, surgeons also use rhBMP-2 derived from Escherichia coli in posterolateral lumbar fusion surgery. Unfortunately, it has not achieved the same effect as the cell-derived rhBMP-2, and neither the fusion rate nor the clinical scores, including Visual Analogue Scale (VAS), ODI, and SF-36, was not possible superior to the autologous bone application [21].
Another BMP family member, rhBMP-7, is also being used commonly in spinal fusion surgery. Early studies employed it as an adjunct to iliac crest autograft for non-instrumented posterolateral fusions in patients with degenerative spondylolisthesis. In a prospective fashion, patients were managed with one-level fusion performed using either iliac crest autograft or iliac crest with rhBMP-7. At the 12-month follow-up, the autograft plus rhBMP-7 group reached radiographical and clinical success in 80% (8 of 10) patients [22]. Subsequently, multiple studies successfully demonstrated its efficacy in promoting spinal fusion as an alternative to the autogenous bone. In addition, its long-term safety and efficacy results for non-instrumented posterolateral fusion were also appraised [23]. However, the report of its use in other more widely accepted surgical approaches, such as TLIF and oblique lumbar interbody fusion (OLIF), is minimal.
Although the efficacy of BMP-2 and BMP-7 are encouraging, concerns relating to their safety profile are accumulating. FDA announced an alert of complications in 2008 and indicated their use in the cervical spine might lead to swelling of the neck and throat tissue, which is life-threatening [24]. Other evidence of adverse reactions, including heterotopic ossification, osteolysis, infection, arachnoiditis, and increased neurological deficits, continues to mount [25,26]. Scientists have made many efforts to minimize these side effects. One notable finding is that human mesenchymal cells (MSCs) can secrete BMP antagonists, including noggin, gremlin1 and 2, chordin, follistatin, BMP3, and twisted gastrulation. These molecules can mitigate BMPs’ function endogenously through antagonistic effects. Therefore, screening for BMP molecules with lower affinity with their antagonists may significantly improve its efficacy to allow a lower dosage. Thus, for the reduction of BMP dosage could potentially eliminate its adverse reactions [27]. Meanwhile, the bovine-derived collagen sponge, to which the BMP solution is applied on [28], has also probably engaged in the adverse inflammatory reaction and its related complications, suggesting the development of a BMP carrier with less immunogenicity is obligatory.
2.1.2. BMP-6
BMP-6, like other BMPs, has specific effects in its ability to convert stem cells to cartilage and bone-forming cells. In addition, it is resistant to inactivation by BMP antagonists that neutralize BMP-2 and displays a 20-fold higher affinity to BMPR-IA than BMP-7. As a result, much less amount of rhBMP-6 is needed for successful bone formation and healing. Recently, two studies have demonstrated this presumption in treating critical segmental defects and posterolateral lumbar spine fusion in rabbits [29,30]. Furthermore, in combination with synthetic ceramics, rhBMP-6 has shown new bone formation and ceramic particles integrated with the transverse processes resulting in superior biomechanical properties [31]. More importantly, preliminary trials in humans have also demonstrated the potency of rhBMP-6 in the acceleration of bone healing in patients with distal radial fractures and patients undergoing high tibial osteotomy, displaying a good safety profile [32,33].
In summary, BMPs have played a viable role in promoting spinal fusion, and BMP products with better efficacy and better safety profile are unmet medical needs for spinal fusion.
2.1.3. Other growth factors
Besides BMPs, other growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGR), transforming growth factor-beta (TGF-β), and fibroblast growth factor (FGF), are candidates for promoting bone healing. Although they can stimulate cell division and the secretion of matrix proteins, their osteoinductive function in spinal fusion has not been validated [1]. Some new growth factors have attracted more interest, while most are still in the pre-clinical stage [34]. For example, Nell-1 is a secreted osteogenic protein. James et al. in a proof-of-concept study, first tested its effect in the nonhuman primate lumbar spinal fusion model. An apatite-coated β-tricalcium phosphate carrier that slowly releases recombinant human NELL-1 (rhNELL-1) was developed to stimulate the osteogenic differentiation of mesenchymal progenitor cells (MPCs). All samples achieved complete osseous fusion (100% spinal fusion rate) [35]. In another study, Zheng et al. proved that in the beagle model, an activin A/BMP-2 chimera (AB204) could promote spinal fusion compared to rhBMP-2 alone (90% vs. 15%), which indicates that activin A may become an alternative to rhBMP-2 [36].
3. Biological factor-containing bone expanders
3.1. DBM
Demineralized bone matrix (DBM) is a decalcified product obtained by removing the mineral components from cadaver bones [37]. Most of the residual components are type I collagen deemed to have the osteoinductive ability. Besides, DBM also contains BMPs, insulin-like growth factor (IGF), TGF-β, and FGF, which can also offer osteoinductive capacity. Thus, surgeons often use DBM as a bone extender [38].
A meta-analysis showed no significant difference in the posterolateral or intervertebral fusion rate between the DBM- and autograft bone-group for posterolateral lumbar spine fusion and lumbar interbody fusion, suggesting that DBM may be an alternative to the autograft bone [39].
3.2. BMA
Bone marrow aspirate (BMA) is a cell-rich extract obtained from the iliac crest or pedicle. Since BMA does not have structural integrity and is easy to spread when used, it generally needs to be mixed with carriers, such as autologous bone, allogeneic bone, ceramic, or DBM. The osteoinductive ability of BMA is mainly related to cytokines and growth factors secreted by cells [40]. Although the extraction and preparation of BMA are time-consuming, implanting a mixture of BMA, autogenous bone, and rhBMP-2 at the conjunction of the spine can achieve a lumbar fusion rate of 94%, which is favorable during a surgeon's learning process [41].
One prospective study shows that when adopting BMA and beta-tricalcium phosphate complex as bone graft substitutes in anterior lumbar interbody fusion (ALIF), the fusion rate of the treatment segment under X-ray was 85.48%. Furthermore, CT analysis showed the new bone bridge in 77.78% of treated segments. Both are equivalent to that of autograft bone, indicating that the mixture of BMA and beta-tricalcium phosphate is a good substitute for autograft for ALIF [40]。
Another meta-analysis showed that although BMA combined with allograft bone had a higher fusion rate and better safety profile than allograft bone alone, this combination may not have equivalent efficacy as autograft bone or rhBMP-2 [42].
3.3. PRP
Platelet-rich plasma (PRP) facilitates bone fusion in animal experiments, but its enhancement of spinal fusion in humans is still controversial [43]. One of the hurdles is that the preparation protocols cannot be standardized, making it challenging to keep the count and the concentration of platelets consistent [14].
According to 5 prospective studies, the bone healing effect of the high platelet concentration group was more significant than that of the control group (P < 0.05). The speed of new bone formation was also faster (P < 0.05), with the bone healing time shorter within six months after surgery (P < 0.05). They also emphasized that it is better to increase the growth factor concentration to more than five times as it is in the peripheral blood to ensure PRP's effect. Otherwise, the effect will not be satisfactory [43]. Another study with a 10-year follow-up found that the mixture of PRP and autograft bone had a wider area of bone formation (P < 0.05) and less bone resorption (P < 0.05) at 3 and 6 months after surgery than autograft bone alone. Therefore, PRP is still considered a low-cost option for spinal fusion [44].
Other meta-analyses believe that the role of PRP in spinal fusion is minimal. The use of PRP cannot improve the patient's VAS pain score nor spinal fusion rate. They also agree that platelet count or growth factor concentration is the crucial limiting factor essential to the effect [45,46].
4. Systemic therapies
4.1. Bisphosphonates and PTH
Bisphosphonates and parathyroid hormone (PTH) analogs are the primary drugs for treating osteoporosis, and their use in spinal fusion has become more popular. However, people used to concern about the inhibitory effect of bisphosphonates on spinal fusion [47]. In terms of PTH, Teriparatide may facilitate fusion through its anabolic effect [48].
A recent meta-analysis concludes that bisphosphonates do not impede the successful fusion of the spine, whereas they are related to the prevention of cage subsidence and spinal fractures. Compared with bisphosphonates, Teriparatide has a higher correlation with spinal fusion rates [49]. Another meta-study that studied the literatures from 1980 to 2015 came to a similar conclusion. Patients in the PTH group have a higher spinal fusion rate than the bisphosphonate group. Thus, intermittent use of PTH will help promote fusion, but it still needs more study to support its routine use [50].
There is also evidence that the use of Teriparatide in osteoporotic patients undergoing spinal fusion surgery can increase their BMD, reduce the risk of fractures, increase the fusion rate, and reduce the rate of hardware failure. However, further study is needed to understand the proper duration of teriparatide use and its feasibility to male patients [51].
The level of evidence that supports the use of bisphosphonates for spinal fusion is low. One of the retrospective studies found that bisphosphonates could accelerate spinal fusion, shorten fusion time, and reduce the risk of adjacent vertebral compression fractures and screw loosening [48].
4.2. Denosumab
Denosumab is a fully human monoclonal antibody to the receptor activator of nuclear factor κB ligand (RANKL) and has been used for the treatment of postmenopausal osteoporosis. It inhibits the osteoclast-mediated bone resorption by interfering with the interaction between RANKL and RANK receptors on the osteoclast [52].
A recent prospective study revealed that the combination of denosumab and Teriparatide could accelerate the spinal fusion of patients undergoing posterior lumbar interbody fusion surgery. By the end of the one-year follow-up, the combination group achieved a significantly higher bone union rate compared to the Teriparatide group (82% vs. 36%, P < 0.05) [53]. Another prospective, open-label study evaluated the efficacy of denosumab treatment on the pedicle screw fixation following spinal fusion surgeries. The result showed that both pullout strength of pedicle screws and compression force of the vertebra increased significantly at 12 and 24 months following denosumab treatment (both P < 0.05), indicating that the spinal fusion may be potentially enhanced due to solid instrumentation [54].
Nevertheless, there has been no sufficient evidence for its routine postoperative use.
4.3. Romosozumab
Romosozumab is a humanized monoclonal antibody that binds and inhibits sclerostin, with a dual effect of increasing bone formation and decreasing bone resorption [55]. Unfortunately, there have been no reports of romosozumab in the clinical trial for enhancing bone fusion in spinal surgery. However, it is expected that romosozumab may robustly promote the healing of the vertebrae considering its proven efficacy in osteoporosis treatment.
5. Cell and gene therapies
5.1. Stem cells
Bone marrow-derived MSC(BMSC) is another option to facilitate spinal fusion with its osteogenic properties. Clinical studies have shown that autologous and allogeneic bone mesenchymal stem cell sources result in high percentages of spinal fusion and are much less morbid than the current gold standard of autograft [56].
An analysis that included 15 stem cell studies showed that the spinal fusion rate was within the range of 60% and 100%, with an average of 87.1%. However, the difference between commercially available BMSC and BMA is not significant. BMSC products are supposed to be more applicable in patients with poor bone marrow quality [57].
The clinical application of autologous stem cells requires extraction of bone marrow and expansion in a good manufacturing practice (GMP) facility. Cells are loaded in tricalcium phosphate carrier for single-segment spinal fusion in patients with degenerative disc disease. They found that cells successfully expanded, and the postoperative VAS and ODI scores both improved. Furthermore, the radiographic results showed that 80% of patients achieved spinal fusion at the last follow-up with no complications related to the operation. Nevertheless, the study lacks a control group to compare it with other approaches [58].
Allogeneic stem cells are more common in the enhancement of bone healings than autologous stem cells. A meta-analysis showed that 90% of patients who used allogenic stem cell products achieved successful fusion 12 months after surgery with improved function and pain. However, the inconsistency among different interventions is significant, and the evidence supporting the efficacy and safety of allogeneic MSC is still insufficient. It is doubtful that the clinical efficacy is a true reflection of the cell [59].
In addition, emerging BMSC-substitute composite also shows preliminary efficacies. Recently, a novel tissue-engineered bone graft with silicon-substituted calcium phosphate (Si-CaP), autogenous fine particulate bone powder (AFPBP), and BMSCs was tested in the rabbit spinal fusion model. The study aimed to reduce the amount of autogenous bone with no compromise in spinal fusion rate. Interestingly, the Si-CaP/AFPBP/BMSCs group showed similar bone formation to those that only adopted AFPBP. Thus, the potential use of this composite as a potent substitute to autograft bone is demonstrated [60].
5.2. Gene therapy
The purpose of gene therapy is to induce osteogenic differentiation and local bone formation by delivering and expressing the targeted gene [61]. Both non-viral DNA and viral DNA delivery systems are available. Non-viral delivery is presumably safer but can only provide short-term expression of the target gene. Wegman et al. used alginate hydrogel, an anionic copolymer of b-D-mannuronic acid and a-l-lucoronic acid residues, as a delivery vehicle for BMP-2 to induce osteogenic differentiation and bone matrix formation [62]. There are also studies using adenovirus vectors to express BMP-2. In animal experiments, the formation of bone bridges between the vertebrae and a reduction of the spine flexion and extension angle can be observed. Ninety percent of the animals successfully achieved spinal fusion after four weeks [63]. The adverse events of the viral vector are mainly caused by the immune response. In addition to direct virus injection into the host, autograft cells can also be transfected in vitro and implanted during surgery [1].
6. Controlled-release formulation and carriers
Burst release is undesirable when delivering an osteoinductive protein, as this may lead to bone shell formation and other adverse reactions related to inflammations. Therefore, controlled-release formulations and carriers are critical to the safety and success of spinal fusion.
6.1. New formulations
The development of osteoinductive protein carriers has been ongoing. The original carrier for rhBMP-2 is an absorbable collagen sponge whose sustained-release effect was not evident. Hsu et al. lately tested a peptide amphiphile to deliver BMP-2, aiming at improving the controlled release and reducing the dosage. Another study focused on polyelectrolyte complex as a BMP-2 carrier, in which they observed controlled bone growth [64]. Both methods are purely adjustment in formulations, and they have a chance to apply for approval as drugs instead of drug-device combinations.
6.2. Potential carriers
Collagen hydroxyapatite carrier is not new. However, optimization of its controlled-release property, osteoconductivity, and osteoinductive activity is difficult. A type I collagen embedded with Mg-HA nanoparticles (RegenOSS) was tested for adult scoliosis surgery. The 3-year follow-up showed that 95.1% of patients achieved spinal fusions, demonstrating this approach's feasibility and preliminary clinical safety [65].
In addition to collagen, nanomaterials prepared from various substances may also have excellent carrier properties [38]. For example, one complex produced with poly (lactide-co-glycolide) (PLGA) and Mg-HA nanoparticles is developed to deliver BMP-2. Animal experiments showed that this scaffold could inhibit the inflammatory response and promote BMP-2 induced bone formation in a posterolateral spinal fusion model, indicating its clinical application in subsequent studies [66].
As additive manufacturing technology improves, 3D-printed biomaterials have become more widely accepted. 3D-printer can use biodegradable materials to produce implants with good porosity for better osteointegration. In addition to the osteoconductive ability, it is expected that the material will gain osteoinductive and osteogenic ability with printing procedures. In one study, human adipose-derived stem cells (ADSCs) transfected with rhBMP-2 lentiviral vector were loaded on 3D-printed Hyperelastic Bone®, and it was found that the fusion score and rate of this group was significantly higher than other groups. So this therapy successfully integrated gene and stem cell therapy with 3D-printed biomaterials and exhibited superior efficacy [38].
Interestingly, vectors that interact with osteoclasts are also being developed. The absorption profile of this material is different from that of other materials (polylactic acid/polyglycolic acid, PLA/PGA, and tricalcium phosphate, TCP, materials). Instead, osteoclasts adhere to it and initiate its degradation. Hence the modeling and remodeling rates match the normal bone formation, which may benefit the quality of the newly-formed bone. Since BMP-2 embeds with the material during manufacture, a slow release of BMP-2 is likely to happen after its implantation [67].
The thermosensitive hydrogel is a formulation that can also harden in situ and provide a controlled release. Qu et al. developed a composite of thermosensitive hydrogel and PDLLA electrospun nanofiber membrane, which can fill irregular defects with mechanical strength and low immunogenicity and serve as a carrier for BMP- 2 nanoparticles. These characteristics assure the composite promising to spinal fusion [68].
As the world's older population continues to grow, the number of spinal fusion cases increases yearly. Critical advancements in biological therapeutics that promote spinal fusion have brought better clinical outcomes to patients lately. With the accumulation of higher-level evidence, the safety and efficacy of these osteobiologics have become more evident. At the same time, randomized controlled trials are still the most reliable tool for collecting this evidence.
Among the emerging therapeutics, upgrades in BMP are very likely to happen in the foreseeable future. At the same time, stem cell and gene therapy are also very promising due to their significant advancements in recent years. Although the anti-osteoporotic biologics have not been universally accepted for perioperative use by orthopedic surgeons, it may be of great importance to initiate such clinical studies and acquire more higher-level evidence. Controlled-release formulations and advanced carriers may also likely improve the drug's efficacy and safety profile. These emerging therapeutics will shift the landscape of perioperative therapy for the enhancement of spinal fusion.
Funding
This work was supported by the Capital Research and Translational Application for Clinical Diagnosis and Treatment [Z201100005520073].
Declaration of competing interest
The author(s) have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by the Capital Research and Translational Application for Clinical Diagnosis and Treatment (No. Z201100005520073).
Contributor Information
Yidan Zhang, Email: yidan.zhang@angitiabio.com.
Yu Jiang, Email: jiangyu@bjmu.edu.cn.
Da Zou, Email: zd_puth@163.com.
Baozhi Yuan, Email: baozhi.yuan@angitiabio.com.
Hua Zhu Ke, Email: david.ke@angitiabio.com.
Weishi Li, Email: puh3liweishi@163.com.
References
- 1.Katsuura Y., Shafi K., Jacques C., Virk S., Iyer S., Cunningham M. New strategies in enhancing spinal fusion. HSS J. 2020;16(2):177–182. doi: 10.1007/s11420-020-09749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarpada S.P., Morris M.T., Burton D.A. Spinal fusion surgery: a historical perspective. J Orthop. 2017;14(1):134–136. doi: 10.1016/j.jor.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petscavage-Thomas J., Ouyang T., Bible J. Spine fixation hardware: an update. AJR Am J Roentgenol. 2020;215(3):534–544. doi: 10.2214/AJR.20.22810. [DOI] [PubMed] [Google Scholar]
- 4.Formica M., Vallerga D., Zanirato A., Cavagnaro L., Basso M., Divano S., et al. Fusion rate and influence of surgery-related factors in lumbar interbody arthrodesis for degenerative spine diseases: a meta-analysis and systematic review. Musculoskel Surg. 2020;104(1):1–15. doi: 10.1007/s12306-019-00634-x. [DOI] [PubMed] [Google Scholar]
- 5.Irmola T.M., Hakkinen A., Jarvenpaa S., Marttinen I., Vihtonen K., Neva M. Reoperation rates following instrumented lumbar spine fusion. Spine. 2018;43(4):295–301. doi: 10.1097/BRS.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 6.Martin B.I., Mirza S.K., Comstock B.A., Gray D.T., Kreuter W., Deyo R.A. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32(3):382–387. doi: 10.1097/01.brs.0000254104.55716.46. [DOI] [PubMed] [Google Scholar]
- 7.Ushirozako H., Hasegawa T., Ebata S., Ohba T., Oba H., Mukaiyama K., et al. Impact of early intervertebral osseous union after posterior lumbar interbody fusion on health-related quality of life. Global Spine J. 2020 doi: 10.1177/2192568220953813. 2192568220953813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe K., Katsumi K., Ohashi M., Shibuya Y., Hirano T., Endo N., et al. Surgical outcomes of spinal fusion for osteoporotic vertebral fracture in the thoracolumbar spine: comprehensive evaluations of 5 typical surgical fusion techniques. J Orthop Sci. 2019;24(6):1020–1026. doi: 10.1016/j.jos.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Karikari I.O., Metz L.N. Preventing pseudoarthrosis and proximal junctional kyphosis: How to deal with the osteoporotic spine. Neurosurg Clin. 2018;29(3):365–374. doi: 10.1016/j.nec.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Lv Q.B., Gao X., Pan X.X., Jin H.M., Lou X.T., Li S.M., et al. Biomechanical properties of novel transpedicular transdiscal screw fixation with interbody arthrodesis technique in lumbar spine: a finite element study. J Orthopaedic Transl. 2018;15:50–58. doi: 10.1016/j.jot.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnard J.L., Parr W.C.H., Choy W.J., Walsh W.R., Mobbs R.J. 3D-printed spine surgery implants: a systematic review of the efficacy and clinical safety profile of patient-specific and off-the-shelf devices. Eur Spine J. 2020;29(6):1248–1260. doi: 10.1007/s00586-019-06236-2. [DOI] [PubMed] [Google Scholar]
- 12.Boden S.D. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27(16 Suppl 1):S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 13.Silber J.S., Anderson D.G., Daffner S.D., Brislin B.T., Leland J.M., Hilibrand A.S., et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28(2):134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Yoo J.S., Ahn J., Patel D.S., Hrynewycz N.M., Brundage T.S., Singh K. An evaluation of biomaterials and osteobiologics for arthrodesis achievement in spine surgery. Ann Transl Med. 2019;7(Suppl 5) doi: 10.21037/atm.2019.06.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parajon A., Alimi M., Navarro-Ramirez R., Christos P., Torres-Campa J.M., Moriguchi Y., et al. Minimally invasive transforaminal lumbar interbody fusion: meta-analysis of the fusion rates. What is the Optimal Graft Material? Neurosurgery. 2017;81(6):958–971. doi: 10.1093/neuros/nyx141. [DOI] [PubMed] [Google Scholar]
- 16.Li G., Li P., Chen Q., Thu H.E., Hussain Z. Current updates on bone grafting biomaterials and recombinant human growth factors implanted biotherapy for spinal fusion: a review of human clinical studies. Curr Drug Deliv. 2019;16(2):94–110. doi: 10.2174/1567201815666181024142354. [DOI] [PubMed] [Google Scholar]
- 17.Feng J.T., Yang X.G., Wang F., He X., Hu Y.C. Efficacy and safety of bone substitutes in lumbar spinal fusion: a systematic review and network meta-analysis of randomized controlled trials. Eur Spine J. 2020;29(6):1261–1276. doi: 10.1007/s00586-019-06257-x. [DOI] [PubMed] [Google Scholar]
- 18.Poorman G.W., Jalai C.M., Boniello A., Worley N., McClelland S., 3rd, Passias P.G. Bone morphogenetic protein in adult spinal deformity surgery: a meta-analysis. Eur Spine J. 2017;26(8):2094–2102. doi: 10.1007/s00586-016-4841-5. [DOI] [PubMed] [Google Scholar]
- 19.Khan T.R., Pearce K.R., McAnany S.J., Peters C.M., Gupta M.C., Zebala L.P. Comparison of transforaminal lumbar interbody fusion outcomes in patients receiving rhBMP-2 versus autograft. Spine J. 2018;18(3):439–446. doi: 10.1016/j.spinee.2017.08.230. [DOI] [PubMed] [Google Scholar]
- 20.Vincentelli A.F., Szadkowski M., Vardon D., Litrico S., Fuentes S., Steib J.P., et al. rhBMP-2 (Recombinant Human Bone Morphogenetic Protein-2) in real world spine surgery. A phase IV, National, multicentre, retrospective study collecting data from patient medical files in French spinal centres. OTSR. 2019;105(6):1157–1163. doi: 10.1016/j.otsr.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Cho J.H., Lee J.H., Yeom J.S., Chang B.S., Yang J.J., Koo K.H., et al. Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J. 2017;17(12):1866–1874. doi: 10.1016/j.spinee.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Vaccaro A.R., Chiba K., Heller J.G., Patel T., Thalgott J.S., Truumees E., et al. Bone grafting alternatives in spinal surgery. Spine J. 2002;2(3):206–215. doi: 10.1016/s1529-9430(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 23.White A.P., Vaccaro A.R., Hall J.A., Whang P.G., Friel B.C., McKee M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31(6):735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz D.G. In: Health CfDaR, editor. Food and Drug Administration; 2008. FDA public health notification: lifethreatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion; pp. 1–3. [Google Scholar]
- 25.Epstein N.E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int. 2013;4(Suppl 5):S343–S352. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Y.D., Jiang W.M., Yang H.L., Shi J.H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J Orthopaedic Transl. 2020;24:166–174. doi: 10.1016/j.jot.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May R.D., Frauchiger D.A., Albers C.E., Tekari A., Benneker L.M., Klenke F.M., et al. Application of cytokines of the bone morphogenetic protein (BMP) family in spinal fusion - effects on the bone, intervertebral disc and mesenchymal stromal cells. Curr Stem Cell Res Ther. 2019;14(8):618–643. doi: 10.2174/1574888X14666190628103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein N.E. Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int. 2011;2:10. doi: 10.4103/2152-7806.76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grgurevic L., Oppermann H., Pecin M., Erjavec I., Capak H., Pauk M., et al. Recombinant human bone morphogenetic protein 6 delivered within autologous blood coagulum restores critical size segmental defects of ulna in rabbits. JBMR Plus. 2019;3(5) doi: 10.1002/jbm4.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vukicevic S., Grgurevic L., Erjavec I., Pecin M., Bordukalo-Niksic T., Stokovic N., et al. Autologous blood coagulum is a physiological carrier for BMP6 to induce new bone formation and promote posterolateral lumbar spine fusion in rabbits. J Regen Med Tissue Eng. 2020;14(1):147–159. doi: 10.1002/term.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokovic N., Ivanjko N., Pecin M., Erjavec I., Karlovic S., Smajlovic A., et al. Evaluation of synthetic ceramics as compression resistant matrix to promote osteogenesis of autologous blood coagulum containing recombinant human bone morphogenetic protein 6 in rabbit posterolateral lumbar fusion model. Bone. 2020;140 doi: 10.1016/j.bone.2020.115544. [DOI] [PubMed] [Google Scholar]
- 32.Durdevic D., Vlahovic T., Pehar S., Miklic D., Oppermann H., Bordukalo-Niksic T., et al. A novel autologous bone graft substitute comprised of rhBMP6 blood coagulum as carrier tested in a randomized and controlled Phase I trial in patients with distal radial fractures. Bone. 2020;140 doi: 10.1016/j.bone.2020.115551. [DOI] [PubMed] [Google Scholar]
- 33.Chiari C., Grgurevic L., Bordukalo-Niksic T., Oppermann H., Valentinitsch A., Nemecek E., et al. Recombinant human BMP6 applied within autologous blood coagulum accelerates bone healing: randomized controlled trial in high tibial osteotomy patients. J Bone Miner Res. 2020;35(10):1893–1903. doi: 10.1002/jbmr.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cottrill E., Ahmed A.K., Lessing N., Pennington Z., Ishida W., Perdomo-Pantoja A., et al. Investigational growth factors utilized in animal models of spinal fusion: systematic review. World J Orthoped. 2019;10(4):176–191. doi: 10.5312/wjo.v10.i4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James A.W., Shen J., Tsuei R., Nguyen A., Khadarian K., Meyers C.A., et al. NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. JCI Insight. 2017;2(12) doi: 10.1172/jci.insight.92573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng G.B., Yoon B.H., Lee J.H. Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle's posterolateral lumbar spine model. Spine J. 2017;17(10):1529–1536. doi: 10.1016/j.spinee.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H., Yang L., Yang X.G., Wang F., Feng J.T., Hua K.C., et al. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg. 2019;11(5):725–737. doi: 10.1111/os.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plantz M.A., Hsu W.K. Recent research advances in biologic bone graft materials for spine surgery. Curr Rev Musculoskel Med. 2020;13(3):318–325. doi: 10.1007/s12178-020-09620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han S., Park B., Lim J.W., Youm J.Y., Choi S.W., Kim D.H., et al. Comparison of fusion rate between demineralized bone matrix versus autograft in lumbar fusion : meta-analysis. J Korean Neurosurg Soc. 2020;63(6):673–680. doi: 10.3340/jkns.2019.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lechner R., Putzer D., Liebensteiner M., Bach C., Thaler M. Fusion rate and clinical outcome in anterior lumbar interbody fusion with beta-tricalcium phosphate and bone marrow aspirate as a bone graft substitute. A prospective clinical study in fifty patients. Int Orthop. 2017;41(2):333–339. doi: 10.1007/s00264-016-3297-x. [DOI] [PubMed] [Google Scholar]
- 41.Nandyala S.V., Fineberg S.J., Pelton M., Singh K. Minimally invasive transforaminal lumbar interbody fusion: one surgeon's learning curve. Spine J. 2014;14(8):1460–1465. doi: 10.1016/j.spinee.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 42.Buser Z., Hsieh P., Meisel H.J., Skelly A.C., Brodt E.D., Brodke D.S., et al. Use of autologous stem cells in lumbar spinal fusion: a systematic review of current clinical evidence. Global Spine J. 2020 doi: 10.1177/2192568220973190. 2192568220973190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manini D.R., Shega F.D., Guo C., Wang Y. Role of platelet-rich plasma in spinal fusion surgery: systematic review and meta-analysis. Adv Orthoped. 2020 doi: 10.1155/2020/8361798. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imagama S., Ando K., Kobayashi K., Ishikawa Y., Nakamura H., Hida T., et al. Efficacy of early fusion with local bone graft and platelet-rich plasma in lumbar spinal fusion surgery followed over 10 years. Global Spine J. 2017;7(8):749–755. doi: 10.1177/2192568217696690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M.S., Moon S.H., Kim T.H., Oh J.K., Yoon W.Y., Chang H.G. Platelet-rich plasma for the spinal fusion. J Orthop Surg. 2018;26(1) doi: 10.1177/2309499018755772. 2309499018755772. [DOI] [PubMed] [Google Scholar]
- 46.Muthu S., Jeyaraman M., Ganie P.A., Khanna M. Is platelet-rich plasma effective in enhancing spinal fusion? Systematic overview of overlapping meta-analyses. Global Spine J. 2021 doi: 10.1177/2192568220988278. 2192568220988278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang T., Park S.Y., Hong S.H., Lee J.H., Lee S.H., Park J.H. Bone union after spinal fusion surgery using local bone in long-term bisphosphonate users: a prospective comparative study. Archiv Osteoporos. 2019;14(1):74. doi: 10.1007/s11657-019-0628-8. [DOI] [PubMed] [Google Scholar]
- 48.Ding Q., Chen J., Fan J., Li Q., Yin G., Yu L. Effect of zoledronic acid on lumbar spinal fusion in osteoporotic patients. Eur Spine J. 2017;26(11):2969–2977. doi: 10.1007/s00586-017-5286-1. [DOI] [PubMed] [Google Scholar]
- 49.Buerba R.A., Sharma A., Ziino C., Arzeno A., Ajiboye R.M. Bisphosphonate and teriparatide use in thoracolumbar spinal fusion: a systematic review and meta-analysis of comparative studies. Spine. 2018;43(17):E1014–E1023. doi: 10.1097/BRS.0000000000002608. [DOI] [PubMed] [Google Scholar]
- 50.Stone M.A., Jakoi A.M., Iorio J.A., Pham M.H., Patel N.N., Hsieh P.C., et al. Bisphosphonate's and intermittent parathyroid hormone's effect on human spinal fusion: a systematic review of the literature. Asian Spine J. 2017;11(3):484–493. doi: 10.4184/asj.2017.11.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhary N., Lee J.S., Wu J.Y., Tharin S. Evidence for use of teriparatide in spinal fusion surgery in osteoporotic patients. World Neurosurg. 2017;100:551–556. doi: 10.1016/j.wneu.2016.11.135. [DOI] [PubMed] [Google Scholar]
- 52.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 53.Ide M., Yamada K., Kaneko K., Sekiya T., Kanai K., Higashi T., et al. Combined teriparatide and denosumab therapy accelerates spinal fusion following posterior lumbar interbody fusion. OTSR. 2018;104(7):1043–1048. doi: 10.1016/j.otsr.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Tani S., Ishikawa K., Kudo Y., Tsuchiya K., Matsuoka A., Maruyama H., et al. The effect of denosumab on pedicle screw fixation: a prospective 2-year longitudinal study using finite element analysis. J Orthop Surg Res. 2021;16(1) doi: 10.1186/s13018-021-02360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 56.Shah V.P., Hsu W.K. Stem cells and spinal fusion. Neurosurg Clin. 2020;31(1):65–72. doi: 10.1016/j.nec.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Robbins M.A., Haudenschild D.R., Wegner A.M., Klineberg E.O. Stem cells in spinal fusion. Global Spine J. 2017;7(8):801–810. doi: 10.1177/2192568217701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanco J.F., Villaron E.M., Pescador D., da Casa C., Gomez V., Redondo A.M., et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res Ther. 2019;10(1):63. doi: 10.1186/s13287-019-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh P.C., Buser Z., Skelly A.C., Brodt E.D., Brodke D., Meisel H.J., et al. Allogenic stem cells in spinal fusion: a systematic review. Global Spine J. 2019;9(1 Suppl):22S–38S. doi: 10.1177/2192568219833336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui L., Xiang S., Chen D., Fu R., Zhang X., Chen J., et al. A novel tissue-engineered bone graft composed of silicon-substituted calcium phosphate, autogenous fine particulate bone powder and BMSCs promotes posterolateral spinal fusion in rabbits. J Orthopaedic Transl. 2021;26:151–161. doi: 10.1016/j.jot.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalb S., Mahan M.A., Elhadi A.M., Dru A., Eales J., Lemos M., et al. Pharmacophysiology of bone and spinal fusion. Spine J. 2013;13(10):1359–1369. doi: 10.1016/j.spinee.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Wegman F., Bijenhof A., Schuijff L., Oner F.C., Dhert W.J., Alblas J. Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur Cell Mater. 2011;21:230–242. doi: 10.22203/ecm.v021a18. discussion 42. [DOI] [PubMed] [Google Scholar]
- 63.Olabisi R.M., Lazard Z., Heggeness M.H., Moran K.M., Hipp J.A., Dewan A.K., et al. An injectable method for noninvasive spine fusion. Spine J. 2011;11(6):545–556. doi: 10.1016/j.spinee.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Virk S., Qureshi S., Sandhu H. History of spinal fusion: where we came from and where we are going. HSS J. 2020;16(2):137–142. doi: 10.1007/s11420-020-09747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giorgi P., Capitani D., Sprio S., Sandri M., Tampieri A., Canella V., et al. A new bioinspired collagen-hydroxyapatite bone graft substitute in adult scoliosis surgery: results at 3-year follow-up. J Appl Biomater Funct Mater. 2017;15(3):e262–e270. doi: 10.5301/jabfm.5000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bedair T.M., Lee C.K., Kim D.S., Baek S.W., Bedair H.M., Joshi H.P., et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J Tissue Eng. 2020;11 doi: 10.1177/2041731420967591. 2041731420967591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eppell S.J., Tong W., McMasters J., Soenjaya Y., Barbu A.M., Ko A., et al. Minor review: an overview of a synthetic nanophase bone substitute. Materials. 2018;11(9) doi: 10.3390/ma11091556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu Y., Wang B., Chu B., Liu C., Rong X., Chen H., et al. Injectable and thermosensitive hydrogel and PDLLA electrospun nanofiber membrane composites for guided spinal fusion. ACS Appl Mater Interfaces. 2018;10(5):4462–4470. doi: 10.1021/acsami.7b17020. [DOI] [PubMed] [Google Scholar]