Abstract
Enological tannins are assessed as promising alternative to SO2 in order to control oxidative process during winemaking, due to allergic reactions incurred by sulfite sensitive individuals. In the present study, the commercial enological Tara tannin “Vitanil B″ was added, as alternative to the addition of sulfites, at different concentrations (100–500 mg/L) in white wine from grapes of Vitis vinifera L. var. Malagousia in order to enhance antioxidant stability and sensory character of the wine. Considering photometric analyses and chromatic parameters results, tannin addition (300 mg/L) in Malagousia enhanced total phenolic content, antioxidant and antiradical activity and prevented color deterioration, for a storage period of 100 d, compared to control and sulfited wines. Moreover, aroma quality, body, after taste and overall acceptance of wine treated with 300 mg/L tannin, were highly appreciated and received the highest scores. The overall evaluation of tannin addition was performed by Principal Component Analysis, leading to discrimination of wines, according to photometric, color and sensory analysis parameters. Conclusively, tannin addition resulted in a considerable increase of total phenolic content, antioxidant and antiradical activity, compared to the control and sulfited wines, maintaining the sensory parameters and overall acceptance of Malagousia wine.
Keywords: Malagousia white wine, Enological tannin, Phenolics, Antioxidant activity, Sensory evaluation, Color parameters
Graphical abstract
Highlights
-
•
Enological tannins as promising SO2 alternative to control wine oxidation.
-
•
Tara tannin enhanced the antioxidant profile and stability of Malagousia wine.
-
•
Sensory character of tannin-treated Malagousia wine was highly improved.
-
•
Tara tannin prevents color deterioration of Malagousia, for a period of 100 days.
1. Introduction
Greek white wines account for approximately 67% of the annual wine production (Hellenic Ministry of Rural Development and Food, 2021) and, as reported by Tourtoglou et al. (2014), stand high (32.5% over 54.1% of red wine) among the preferences of the Greek consumers. Vitis vinifera L. var. Malagousia (ECP/GR, 2021) is a Greek white grape variety, thought to be extinct and known to very few in the 1970s. However, today, Malagousia is widely considered a world class grape, producing outstanding dry whites, medium pale to lemon green in color with an exceptional aromatic profile, showing hints of peaches, green bell pepper, basil and flowers (EDOAO, 2021). Moreover, according to Nanou et al. (2020), Malagousia wines were described by assessors as having lemon, grapefruit, and citrus blossom character, they also shared some descriptors with Assyrtiko wines, such as mushroom and earthy characters, and had some shared characters, like floral and citrus notes, with Moschofilero samples.
In oenology, the use of SO2 is well known in the wine making areas of central Europe since Middle Ages, because of its antioxidant, antimicrobial and preservative action. Sulfur dioxide (SO2) is widely used as additive during the vinification process (from must pressing to wine bottling).
In addition, this compound prevents the wine browning by inactivation of enzymes such as polyphenoloxidade (PPO), peroxidase (POD), and proteases, and also by inhibition of the Maillard reaction (Garde-Cerdán et al., 2008; Ribereau-Gayon et al., 2006). Moreover, the addition of sulfites protects wine aroma and reduces color loss usually observed during wine aging, by reducing the rate of phenolic polymerization (Oliveira et al., 2011).
Except for all the positive effects of SO2, sulfites are included in the list of allergens of Regulation (EU) 2019/33 (European Commission 2019a); therefore, the negative effects on human health have been the subject of research for many years (Guerrero and Cantos-Villar, 2015; Vally and Thompson, 2003; Qin and Meng, 2009; OIV 2021a; OIV 2021b). Consequently, the maximum concentration of SO2 allowed by legislation in wines has been gradually reduced to 150 and 200 mg/L for dry red and white wines, respectively (Regulation (EU) 2019/934, European Commission 2019b).
Actually, there has been a growing interest in finding other preservatives and innovative technologies, replacing or at least complementing the action of SO2 during the winemaking process. Several methods have been applied as alternatives to SO2 use in wine production, including the following: a) the addition of chemical preservatives such as dimethyl decarbonate (Costa et al., 2008; Sonni et al., 2011), lysozyme (Azzolini et al., 2010; Sonni et al., 2009) and ascorbic acid (Barril et al., 2012); b) processes including high hydrostatic pressure (HHP) Briones-Labarca et al. (2017), pulsed electric field (PEF) (Delsart et al., 2015), ultraviolet irradiation (UV) (Fredericks et al., 2011), high power ultrasound (HPU) (Gracin et al., 2016) and low electric current (LEC) (Silva and van Wyk, 2021), and c) the use of phenolic compounds and plant extracts (Raposo et al., 2016; Sonni et al., 2009), killer toxins and bacteriocins or combined methods (Yildirim and Darici, 2020; Lisanti et al., 2019).
A number of plant materials containing phenolic substances have also been studied as wine additions for their antioxidant properties (Tzachristas et al., 2020; Proestos et al., 2015). Nevertheless, the proposed alternatives should ensure the protection against wine oxidation, the microbiological safety of wine and the maintenance as much as possible of wine sensory characteristics.
Concerning the substitution of SO2 with natural plant extracts, tannins prevent the oxidative phenomena of musts and wines possibly as a consequence of a dual mechanism involving inhibition of enzymes and radical-scavenging activity. They have been used to facilitate the clarification of musts and wines, to contribute to wine structure and to improve the sensory impact of white wines (Sonni et al., 2009, 2011; Panero et al., 2015; Pascual et al., 2017). Hydrolysable tannins, such as gallotannins extracted from oak galls and ellagitannins from oak or chestnut, which are not naturally present in grapes, make up the most sold commercial tannins (Versari et al., 2013).
In the concept of SO2 replacement with natural preservatives, the goal of the study was the application of wine tannins in white wines for their antioxidant stability and sensory improvement. To a further step, the present study aimed to assess the effect of the addition of “Vitanil B″ tannin on Malagousia white wine overall quality, including antioxidant activity, color and sensory characteristics, through statistical evaluation.
2. Materials and Methods
2.1. Reagents and standards
The chemicals 2,4,6-tris(2-pyridyl)-S-triazine (TPTZ), sodium persulfate, ferrous sulfate heptahydrate, iron(III) chloride hexahydrate, ABTS [2,2′-Azinobis (3 ethylbenzothiazoline-6-sulfonic acid)], Trolox (6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid), gallic acid (3,4,5-trihydroxybenzoic acid), and Folin–Ciocalteu phenol reagent, as well as solvents of analytical grade were purchased from Mallinckrodt Chemical Works (St. Louis, MO, USA), Alfa Aesar GmbH (Karlsruhe, Germany), Tokyo Chemical Industry (Japan), and Sigma-Aldrich Chemie GmbH (Germany).
2.2. Process of vinification
A special vinification process for the production of white wine without added sulfites was performed with grapes of Malagousia variety at Roxanis Matsa estate in Kantza, Attica, as described below. Grape berries of Malagousia variety were harvested in plastic containers at optimum maturity stage, after the evaluation of detailed maturation data and organoleptic testing. Upon receipt, the grapes were carefully hand sorted and were kept at 10 °C until further use. Subsequently, the grapes were pressed under inert atmosphere and the resulting grape pulp was gently pressed and left for 24 h. After the removal of wine mud, inoculation with selected yeast strains and addition of nutrients was performed in the must. The alcoholic fermentation was performed in a stainless-steel tank at controlled temperature, ranging from 14 to 18 °C. When the fermentation was completed, the wine lees were removed and the wine was transferred in a new stainless-steel tank. White wine was packed immediately after fermentation in 10 L plastic bags. The quality characteristics of Malagousia wine were measured according to the methods of analysis of the International Organization Of Vine And Wine (OIV, 2021c). Specifically, the corresponding values were: pH=3.33±0.05, alcohol=12.1±0.1%, density=0.9901±0.0004, total acidity expressed as tartaric acid=5.82±0.11 g/L, volatile acidity expressed as acetic acid=0.36±0.04 g/L, fructose=0.9±0.1 g/L, glucose=0.7±0.1 g/L and glycerol=4.8±0.2 g/L.
2.3. Wine and model wine samples preparation
“Vitanil B” (MARTIN VIALATTE, France) was the tannin chosen for the addition in Malagousia white wine. According to product specification, “Vitanil B″ is a commercial enological Tara pod (bean originating from South America) clear tannin extracted from gallic alcohol, perfectly adapted to the fining of white wine. Its main application fields are the clarification of white wines, the protection of must from oxidation and the limitation of the development of taste reduction during fermentation. “Vitanil B” (MARTIN VIALATTE, France), was added at four different concentration levels (ranging from 100 to 500 mg/L) in multiple 100 mL aseptic well-sealed brown glass bottles, containing Malagousia white wine. Model Wine solutions were prepared by adding 6 g/L (+)-tartaric acid in hydroalcoholic mixture 12% vol, and pH was set at about 3.3 by adding 4M sodium hydroxide. Model wines were subjected to the same tannin addition level accordingly to the wine samples. White wine samples containing potassium metabisulphite (200 mg/L), corresponding to 108±4 mg/L of total sulfur dioxide and 43±3 mg/L of free sulfur dioxide, were also used for comparison purposes. The treatment and levels of tannin and sulfur dioxide addition in wine and model wine samples are given in Table 1. All the wine and model wine samples were stored at 4 °C until further analysis. All chemical analyses were performed in triplicate at regular intervals of 25 days for a total period of four months.
Table 1.
Coding of wine and model wine samples, according to treatment.
| Wine Samples |
|||||||
|---|---|---|---|---|---|---|---|
| W | WSO2 | W1 | W2 | W3 | W4 | W5 | |
| Treatment | Control |
Addition of potassium metabisulphite (200 mg/L) |
Addition of Tannin (100 mg/L) |
Addition of Tannin (200 mg/L) |
Addition of Tannin (300 mg/L) |
Addition of Tannin (400 mg/L) |
Addition of Tannin (500 mg/L) |
| Model Wines | |||||||
|
MW0 |
MW1 |
MW2 |
MW3 |
MW4 |
MW5 |
||
| Treatment | 6 g/L tartaric acid in hydroalcoholic mixture 12% vol | Addition of Tannin (100 mg/L) | Addition of Tannin (200 mg/L) | Addition of Tannin (300 mg/L) | Addition of Tannin (400 mg/L) | Addition of Tannin (500 mg/L) | |
2.4. Determination of total phenolic content (TPC)
The total phenolic content (TPC) of wine samples was determined according to a modified micromethod of Folin–Ciocalteu's colorimetric assay (Andreou et al., 2018). The absorbance was measured at 750 nm with a visible spectrophotometer (Spectro 23, Digital Spectrophotometer, Labomed Inc., USA). The results were expressed as mg Gallic acid equivalents (GAE) per L of wine, using a standard curve with a range of 25–2600 mg/L Gallic acid (y = 0.0005x + 0.0783, R2 = 0.9989).
2.5. Scavenging activity on 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS+)
The antiradical activity of the wine samples was determined according to the method described by Lantzouraki et al. (2015). Absorbance was measured at 734 nm with a visible spectrophotometer (Spectro 23, Digital Spectrophotometer, Labomed Inc., USA). Trolox, a water soluble form of vitamin E, was used as a standard compound, and the antiradical activity of each sample was expressed as mg Trolox Equivalents (TE) per L of wine. A standard curve was prepared with a concentration range of 0.20–1.50 mM Trolox (y = 0.2876x – 0.002, R2 = 0.9995).
2.6. Ferric reducing/antioxidant power assay (FRAP)
The Ferric Reducing/Antioxidant Power for each wine sample was evaluated based on the reduction of Fe(III) in the form of ferric-2, 4,6-tripyridyl-s-triazine complex to Fe(II), as described by Lantzouraki et al. (2016). The absorbance was measured at 595 nm with a visible spectrophotometer (Spectro 23, Digital Spectrophotometer, Labomed Inc., USA). A standard curve (y=0.0003x+0.0081, R2=0.9969) was prepared using various concentrations (50–1800 μM) of FeSO4.7H2O stock solutions. The results were expressed as mg Fe(II) per L of wine.
2.7. Color measurement
The chromatic characteristics of wine samples were defined by the colorimetric coordinates L* (lightness), a* (redness/greenness), b* (yellowness/blueness), C (chroma), and h (hue angle in degrees). The above values were measured with a tristimulus chromatometer (model CR-400, Minolta, Tokyo, Japan) calibrated with a white standard plate (L*: 97.83, a*: −0.45, b*: +1.88). Color parameters were evaluated at days 0, 25, 50, 75, 100, and 125. Three random readings per sample were taken and averaged.
2.8. Sensory analysis
The sensory analysis of the wine samples was evaluated using Quantitative Descriptive Analysis (QDA), developed by Tragon Corporation in 1974. A 12-member panel was trained in a number of preliminary sessions, using different wines that represent the range of characteristics that may be tested, in order to develop a common vocabulary for the description of the sensory attributes of wine samples and to familiarise them with scales and procedures. Ten descriptors were included in the analysis: color (intensity), odor (intensity), aroma (quality), oxidation, taste (intensity), taste (balance), body, bitterness, aftertaste and overall acceptance. The wine samples were randomly evaluated by assigning a score between 1.00 (absence of sensation) and 9.00 (extremely intense) in individual booths under incandescent white light, in a special room which met the requirements of ISO 8589:2007.
2.9. Statistical analysis
Spectrophotometric assays were repeated three times. The values were averaged and reported along with their standard deviation (S.D). The data regarding TPC, antiradical-antioxidant activity and color parameters were analyzed with One-Way ANOVA Post Hoc Tests, using Tukey's test for pairwise multiple comparisons with statistical significance (P<0.05). Regarding sensory scores, for all attributes assessed, F-value and P-values of each effect of the ANOVA and of the post-hoc Duncan discrimination test were calculated. The correlation among the results was performed by Spearman rank-order test. All statistical calculations including Principal Components and Classification Analysis procedure were performed with the STATISTICA package (STATISTICA software Statsoft Inc, 2004).
3. Results and discussion
3.1. Photometric analyses
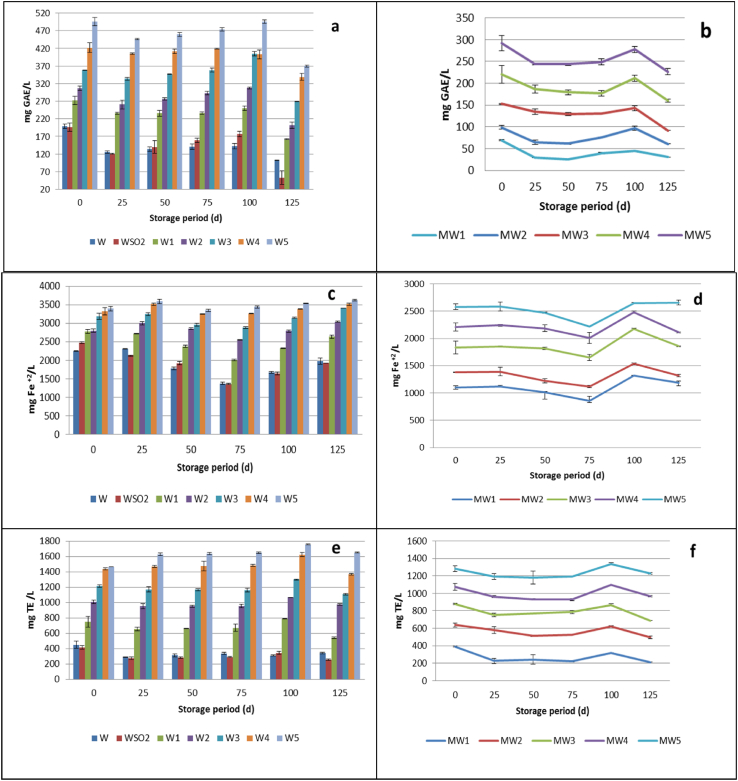
The evolution of total phenolic content (TPC) of Malagousia wine samples, treated with different tannin addition levels, over storage time, is presented in Fig. 1a. TPC value of the studied wine (199.20±6.36 mg/L) was similar with those reported by other researchers for white wines which ranging between 81 and 423 mg/L (Tourtoglou et al., 2014; Olejar et al., 2015; Jagatić Korenika et al., 2020; Tekos et al., 2021).
Fig. 1.
Evolution of total phenolic content, Ferric Reducing/Antioxidant Power, and radical scavenging activity over storage time, of (a, c, e) Wine samples and (b, d, f) Model Wine samples, respectively.
The addition of tannin resulted in a considerable and proportional increase of phenolic content, compared to the phenolic content of control and sulfited wines. This increase was remarkable until 100 d of storage period; however, at the end of experiment (125 d) a significant decrease in total phenolic content was observed at all tannin addition levels.
In addition to analysing the total phenolic content of wine samples, the ferric reducing/antioxidant power and ABTS•+ radical scavenging activity of the wines (expressed as mg Fe+2/L and mg Trolox equivalents/L, respectively) were also measured at the same time intervals previously mentioned (Fig. 1c and e).
The addition of tannin resulted in a considerable increase of ferric reducing/antioxidant power and radical scavenging activity, compared to the respective ones of control and sulfited wines (Fig. 1c and e). However, after 25 d of storage time, the antioxidant power decreased gradually until 75 d of storage, whereas a considerable increase was observed at 100 d, and finally, at the end of experiment, the antioxidant power of all wine samples was reduced (Fig. 1c). The ABTS•+ radical scavenging activity of wine samples treated with tannin remained constant until 75 d of storage time, then it increased at 100 d of storage and at the final time period, the radical scavenging activity was reduced (Fig. 1e).
In accordance to the above findings, Jagatić Korenika et al. (2020), reported that the addition of ascorbic acid and enological tannins in Sauvignon blanc wine, resulted to significant increase of total phenolic content and antiradical activity. Moreover, enological tannins were suggested by other researchers to enhance the wine antioxidant activity (Ribereau-Gayon et al., 2006; Canuti et al., 2012; Rinaldi and Moio, 2018; Yildirim and Darici, 2020).
Model wine samples, prepared by adding 6 g/L (+)-tartaric acid in hydroalcoholic mixture 12% vol and treated with different concentrations of tannin, were also evaluated in comparison with the wine samples, in order to examine the antioxidant and antiradical activity of tannins without the interference of wine matrix. Control model wine (MW0) was prepared and analyzed for TPC, antioxidant and antiradical activity. However, the results showed negligible values (data not shown). It was observed that the addition of tannin in model wine solutions caused a similar effect in total phenolic content (Fig. 1b), ferric reducing/antioxidant power (Fig. 1d) and radical scavenging activity (Fig. 1f), compared with the respective of the wines. A remarkable finding was that the variations in total phenolic content, antiradical activity and antioxidant power followed the same trend in the model wine solutions as in the respective one of wine samples.
“Vitanil B″ tannin chosen for the experiment is a hydrolysable gallotannin consisting of polymers formed by esterification between D-glucose and gallic acid. Therefore, the increase of total phenolic content and antioxidant power observed over time could be explained by the hydrolysis of glycosylated forms of gallic acid, resulting to aglycone structures, whereas the subsequent decrease may be due to their susceptibility to oxidation (Ribéreau-Gayon et al., 2000).
The most important phenolic substances in white wines, both in terms of quantity and ability to participate in redox reactions, are hydroxycinnamic acids as well as hydroxybenzoic acids and flavanols (Pati et al., 2014). Among the phenolic acids, caffeic, caftaric and gallic acids predominate. Catechin is the most abundant flavonoid in white wines, and it commonly constitutes up to 20% of the total phenolic content (Abramovic et al., 2015). During the vinification process, phenolic compounds are susceptible to enzymatic and non-enzymatic oxidation. Enzymatic oxidation occurs entirely in grape must, whereas non-enzymatic oxidation occurs in wine in the presence of transition metal ions and involves the oxidation of polyphenols into quinones, which are unstable and participate in polymerization processes to produce colored dimers (Oliveira et al., 2011). These compounds are possibly rearranged to form new dihydroxybenzene groups, more sensitive to oxidation due to their lower redox potential compared to their initial phenols (Singleton, 1987).
The main mechanisms of the action of sulfur dioxide as antioxidant agent are the direct oxygen scavenging, the reaction with hydrogen peroxide produced by the oxidation of polyphenols in wine and the reduction of the quinones formed during the oxidation process back to their phenol form (Yildirim and Darici, 2020). Gallotannins possess antioxidant capacities mainly originating by many phenolic hydroxyl groups and electron-donating groups at benzene ring; therefore, they protect the wine against chemical oxidation by direct consumption of dissolved oxygen and by scavenging peroxyl radicals (Pascual et al., 2017). Moreover, the degree of polymerization, highly related to the molecular weight of tannins, is another characteristic that influences antioxidant properties. Tannins of high molecular weight and with a great amount of hydroxyl groups in their structure possess high antioxidant properties, while the formation of tannin–protein complexes reduces this antioxidant ability (Fraga-Corral et al., 2020).
Spearman Rank Order correlation coefficients showed a very strong positive relationship among total phenolic content (TPC) and antioxidant power (FRAP assay) (r=0.869) as well as between TPC and ABTS radical scavenging activity (r=0.917). Additionally, Spearman rank order correlation between ABTS antiradical activity and antioxidant power (FRAP assay) was also very strong (r=0.904). This finding indicates that the presence of phenolic compounds is strongly related to the antioxidant and antiradical activities of the examined samples of Malagousia wine. Similarly, high correlation coefficients were also observed between the total polyphenols and flavanols and the antioxidant activity values in selected Hellenic varietal white wines, including Malagousia, after 3 and 6 months storage (Kallithraka et al., 2009), as well as between TPC and Ferric reducing antioxidant power (FRAP) (r=0.970) and between TPC and DPPH (2,2-diphenyl-1-picrylhydrazyl) assays (r=0.987) of North Macedonian Wines (Mitrevska et al., 2020).
3.2. Chromatic parameters
In the CIELab color system, color is described by the five parameters L*, a*, b* and h*. L* represents the color lightness, ranging from 0 (black) to 100 (white), a* describes the green/red part of the color (a* < 0 green, a* > 0 red), b* the yellow/blue part (b* > 0 yellow, b* < 0 blue) and h* the tone (hue) of color. Table 2 displays the evolution of color parameters (L, a*, b* and h*) of tannin enriched and sulfited wine samples, over the storage time. Some significant variations in lightness (L*) were observed in control, tannin enriched and sulfited wine samples, over storage time, resulting in a slight decrease of lightness at the end of experiment in most cases (125 d). However, in tannin enriched wine samples, lightness was not seriously affected until the fifth sampling date (100 d).
Table 2.
Effect of tannin addition and storage time on the chromatic parameters (L, a*, b* and hue) of wine samples.
| Wine samples | L* (Lightness) |
|||||
|---|---|---|---|---|---|---|
| Storage period (d) | ||||||
| 0 | 25 | 50 | 75 | 100 | 125 | |
| W | 54,01±0,12 aA | 53,71±0,05 aA | 58,07±0,05bA | 57,59±0,29 cA | 57,24±0,11 cA | 52,95±0,06 dA |
| WSO2 | 57,49±0,06 aB | 58,87±0,14bB | 55,34±0,21 cB | 57,23±0,07adAC | 55,35±0,40 cB | 56,59±0,38dBC |
| W1 | 56,31±0,06 aC | 56,99±0,03bCE | 58,20±0,05 dA | 57,38±0,08 cA | 57,00±0,14bA | 57,12±0,28bcC |
| W2 | 56,81±0,03aD | 57,56±0,14bD | 58,25±0,05 cA | 54,76±0,03 dB | 56,82±0,07 aA | 56,49±0,34aBC |
| W3 | 57,24±0,03abE | 57,36±0,36abD | 57,60±0,10 aC | 57,70±0,31 aA | 57,16±0,10abA | 56,97±0,02bC |
| W4 | 56,66±0,04acD | 56,88±0,09abE | 57,43±0,06bC | 56,78±0,37 aC | 56,08±0,30 cC | 53,88±0,14dD |
|
W5 |
57,05±0,2 aF |
56,63±0,06 aE |
57,67±0,15bC |
56,15±0,06cD |
56,69±0,22aAC |
56,05±0,31 cB |
|
a* (redness/greenness) |
||||||
| W | −0,13±0,02aAB | −0,60±0,00bA | −0,95±0,01 dA | −0,75±0,01cAB | −0,77±0,02 cA | −0,63±0,03bAC |
| WSO2 | 0,24±0,01 aC | −1,11±0,04bB | −0,65±0,02cdB | −0,71±0,01 dB | −0,61±0,02 cB | −0,70±0,02dABC |
| W1 | 0,03±0,00aD | −0,72±0,00cdC | −0,82±0,00eC | −0,81±0,02deAC | −0,57±0,04bB | −0,71±0,04cAB |
| W2 | −0,08±0,02 aA | −0,91±0,03bDE | −0,86±0,02bD | −0,77±0,01cAB | −0,55±0,04 dB | −0,74±0,02 cB |
| W3 | −0,23±0,01 aE | −0,95±0,02bE | −0,74±0,00 cE | −0,88±0,02 dC | −0,60±0,03eB | −0,70±0,0cABC |
| W4 | −0,07±0,00 aA | −0,82±0,02bcF | −0,88±0,01bD | −0,73±0,01cAB | −0,46±0,04 dC | −0,62±0,03eC |
|
W5 |
−0,20±0,02aBE |
−0,85±0,01bDF |
−0,85±0,01bCD |
−0,56±0,02cD |
−0,46±0,02 dC |
−0,64±0,02eAC |
|
b* (yellowness/blueness) |
||||||
| W | 2,39±0,03 aA | 3,56±0,00bA | 5,97±0,03eA | 5,03±0,04dAD | 5,91±0,04eA | 4,80±0,04 cA |
| WSO2 | 1,18±0,01 aB | 5,14±0,03bB | 3,73±0,07 cB | 3,39±0,07 dB | 3,76±0,07 cB | 3,90±0,12 cB |
| W1 | 2,29±0,06 aC | 4,20±0,02bC | 5,32±0,03 cC | 5,50±0,03 dC | 5,37±0,03cdCD | 5,30±0,07 cC |
| W2 | 2,27±0,03 aC | 5,34±0,02bdD | 5,69±0,02cD | 5,24±0,02dD | 5,54±0,02bcD | 5,56±0,21bcCD |
| W3 | 3,07±0,04aD | 5,94±0,02bcE | 5,00±0,06 dE | 6,01±0,10 cE | 7,14±0,10eE | 5,82±0,06bD |
| W4 | 2,45±0,02 aA | 5,42±0,06bcD | 5,66±0,03cdD | 4,84±0,05eA | 5,22±0,05bC | 5,88±0,12dD |
|
W5 |
3,05±0,03aD |
5,20±0,04bB |
5,93±0,05 cA |
3,86±0,03 dF |
4,64±0,03eF |
5,26±0,13bC |
|
h* (hue angle) |
||||||
| W | 93,16±0,31aAD | 99,54±0,09bAC | 99,04±0,09bcA | 98,45±0,11 cA | 97,42±0,31 dA | 97,29±0,33 dA |
| WSO2 | 78,45±0,50 aB | 102,23±0,31 cB | 100,24±0,45bB | 101,85±0,31 cB | 99,17±0,41bB | 100,14±0,35bB |
| W1 | 89,19±1,38 aC | 99,71±0,26 cC | 98,70±0,15cdACD | 98,34±0,15cdA | 96,05±0,35bC | 97,61±0,31bdA |
| W2 | 91,89±0,47 aA | 99,65±0,30bAC | 98,62±0,10cACD | 98,32±0,14 cA | 95,74±0,48dCE | 97,54±0,18eA |
| W3 | 94,25±0,15aD | 99,13±0,11bAD | 98,41±0,13cCD | 98,38±0,05 cA | 94,79±0,23aD | 96,93±0,38dAC |
| W4 | 91,65±0,14 aA | 98,56±0,14bD | 98,90±0,06bAD | 98,60±0,42bA | 95,03±0,28cDE | 96,03±0,35 dC |
| W5 | 93,73±0,41aD | 99,32±0,12bAC | 98,16±0,02 cC | 98,30±0,01 cA | 95,70±0,23dCDE | 96,98±0,46eAC |
The results represent mean ± standard deviation (N=3). Different small letters after each value, in the same row, and different capital letters, in the same column, indicate statistically significant differences (p<0.05).
As regards the a* values, the higher the value of a* is, the more it tends to red. The addition of tannin did not seriously affect the a* value of wine samples throughout the storage period, compared with the control. Nevertheless, after 25 d, a* values decreased and were very slightly moved toward green area. Therefore, Tara tannin “Vitanil B″ could confer protection from pinking phenomena, which is an undesirable result of white wines oxidative modifications (Cosme et al., 2019).
Unlike to changes of a*, tannin addition led to increase of b* (yellow/blue color component) values, more noticeable at the third level of tannin addition (300 mg/L-Wine sample W3) at 100 d of storage time. It was observed that tannin addition enhanced yellowness of wine samples in contrast to sulfur dioxide addition.
Finally, tannin addition caused a slight decrease in hue angle values (h*) and movement to the yellow zone (90°), compared to control and sulfited wines. Interestingly and in accordance with the b* values, this relocation to the yellow zone was more intense at the third level of tannin addition (300 mg/L-Wine sample W3) at 100 d of storage time.
Wine color deterioration, namely wine browning, is a major problem occurring in white wines during storage, affecting the shelf life of the product. This particular defect arises from the oxidation of phenolic compounds and the subsequent polymerization of the resulting products in order to generate colored compounds in the yellow-brown spectral region. Undesirable color changes in white wines may result after polymerization reactions between phenols and other compounds in the wine, such as acetaldehyde, or between phenols and oxidation products of tartaric acid (López-Toledano et al., 2006). More recently, Bührle et al. (2017) studied the occurrence and importance of xanthylium derivatives, yellow to orange pigments formed by dimerization of flavanols, in white wine and they concluded that these compounds might play a role in color formation as intermediate products in polymerization and browning. Given the above concerns and in accordance with the results of the present study, Vignault et al. (2019) reported that enological tannins inhibit laccase activity and protect the color of white wines from browning.
Therefore, taking into consideration the results of photometric analyses as well as those of chromatic parameters, it can be assumed that the addition of tannin (300 mg/L) in Malagousia white wine enhances total phenolic content, ferric reducing/antioxidant power, radical scavenging activity and prevents color deterioration, for a storage period of 100 d.
3.3. Sensory analysis
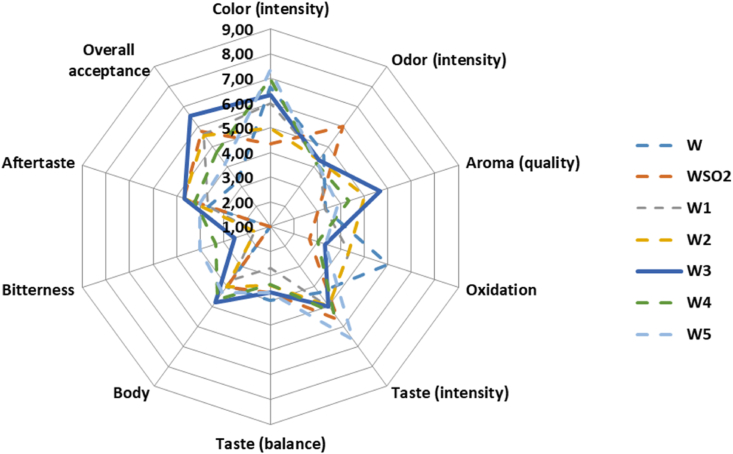
The tannin enriched and sulfited Malagousia wine samples were evaluated for their sensory properties, at days 0, 25, 50, 75, 100, and 125, as explained in the Materials and Methods section. Taking into account the conclusions drawn by the aforementioned photometric and chromatic results, a characteristic radar plot listing 10 attributes and the panel scores obtained, after 100 d of storage, is presented in Fig. 2.
Fig. 2.
Sensory scoring of tannin enriched and sulfited Malagousia wine samples, after 100 d of storage.
Color, taste intensity, aroma quality and body of wine samples were significantly affected after tannin addition, mostly at the upper levels of tannin addition. However, in the oxidation test results, the tannin enriched wine samples received lower scores than the control wine, nevertheless higher than the respective one of the sulfited wine, indicating the efficacy of tannin as antioxidant agent. Odor intensity was scored with 6 in sulfited wine, followed by the control wine and the tannin enriched wines. Therefore, the increased score of odor intensity in sulfited wine is possibly related to the presence of sulfur dioxide. Concerning the bitterness, a clear difference was observed in tannin enriched wines, compared to control and sulfited wine, whereas taste balance of tannin enriched wines was evaluated as comparable with the respectives of control and sulfited wines.
Finally, the aroma quality, the body, the after taste and the overall acceptance of wine sample treated with 300 mg/L tannin, were highly appreciated and received the highest scores by the assessors (Fig. 2).
Moreover, a correlation analysis among photometric assays, color parameters and sensory attributes in Malagousia wine samples, after 100 d storage time, was listed in Table 3. Total phenolic content (TPC), antiradical activity (ArA), and antioxidant power (AP) were very strongly positive correlated with chromatic parameter a* and with bitterness; nevertheless, they were fairly strong negative correlated with hue. Lightness (L*) was strongly positive correlated with chromatic parameter b* and oxidation; while chromatic parameter a* was strongly positive correlated with taste intensity and bitterness and b* strongly related with aroma quality. Strong correlations were also observed between color intensity, either positive with bitterness, or negative with odor intensity. Aroma quality was strongly positive correlated with body and moderately with aftertaste and overall acceptance. Finally, overall acceptance was strongly positive correlated with aftertaste.
Table 3.
Correlation analysis between photometric analyses, color parameters and sensory attributes in Malagousia wine samples, after 100 days storage time (Correlation is significant at the 0.05 level).
| Antiradical activity | Antioxidant power | L* | a* | b* | h* | Color (intensity) | Odor (intensity) | Aroma (quality) | Oxidation | Taste (intensity) | Taste (balance) | Body | Bitterness | Aftertaste | Overall acceptance | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 0,981 | 0,972 | 0,105 | 0,809 | 0,173 | −0,773 | 0,570 | −0,634 | 0,584 | −0,517 | 0,631 | −0,074 | 0,681 | 0,956 | 0,184 | 0,124 |
| Antiradical activity | 0,984 | 0,092 | 0,844 | 0,160 | −0,815 | 0,607 | −0,701 | 0,565 | −0,471 | 0,566 | −0,144 | 0,663 | 0,962 | 0,124 | 0,054 | |
| Antioxidant Power | 0,202 | 0,792 | 0,305 | −0,888 | 0,570 | −0,754 | 0,685 | −0,423 | 0,457 | −0,179 | 0,677 | 0,911 | 0,175 | 0,157 | ||
| L* | −0,318 | 0,795 | −0,532 | 0,468 | −0,693 | 0,379 | 0,702 | −0,444 | −0,031 | −0,019 | 0,036 | −0,430 | −0,177 | |||
| a* | −0,286 | −0,526 | 0,236 | −0,388 | 0,270 | −0,706 | 0,722 | −0,430 | 0,357 | 0,801 | 0,218 | 0,228 | ||||
| b* | −0,638 | 0,344 | −0,584 | 0,716 | 0,382 | −0,587 | 0,066 | 0,420 | 0,043 | 0,004 | 0,182 | |||||
| h* | −0,604 | 0,922 | −0,760 | 0,078 | −0,028 | 0,305 | −0,568 | −0,693 | 0,003 | −0,158 | ||||||
| Color (intensity) | −0,724 | 0,138 | 0,164 | 0,167 | 0,115 | 0,465 | 0,714 | −0,576 | −0,552 | |||||||
| Odor (intensity) | −0,541 | −0,237 | 0,045 | 0,298 | −0,322 | −0,616 | 0,328 | 0,163 | ||||||||
| Aroma (quality) | −0,178 | −0,111 | 0,051 | 0,677 | 0,374 | 0,562 | 0,467 | |||||||||
| Oxidation | −0,679 | 0,131 | −0,476 | −0,474 | −0,602 | −0,554 | ||||||||||
| Taste (intensity) | 0,024 | 0,196 | 0,673 | 0,146 | −0,004 | |||||||||||
| Taste (balance) | 0,367 | −0,041 | 0,202 | −0,413 | ||||||||||||
| Body | 0,648 | 0,412 | 0,153 | |||||||||||||
| Bitterness | −0,022 | −0,103 | ||||||||||||||
| Aftertaste | 0,683 | |||||||||||||||
| Overall acceptance |
The oxidative degradation of white wines rapidly leads to a loss of their sensorial qualities, particularly the loss of characteristic floral and fruity aromas of young wines or the formation of new non typical aromas associated with the deterioration of wine and subsequently to undesirable chromatic changes (development of brown color) (Ferreira et al., 2002). Although it is well established that the addition of tannins contributes to the structure of wine and improves many sensory attributes, such as the mouth feel and the color stability of wine, they are not always perfectly integrated and wines may lose their equilibrium, resulting in a hardening of the wine and an increase in bitter sensations (Crespy, 2002).
Additionally, Li et al. (2020) have investigated the sensory qualities of tannin addition in red wines and concluded that the wine added hydrolysable tannin obtained high score in aroma and bouquet with balanced and well after taste; however, the wine had astringent flavor and bitterness which was consistent with the sensory properties of ellagitannins.
3.4. Principal Component Analysis (PCA)
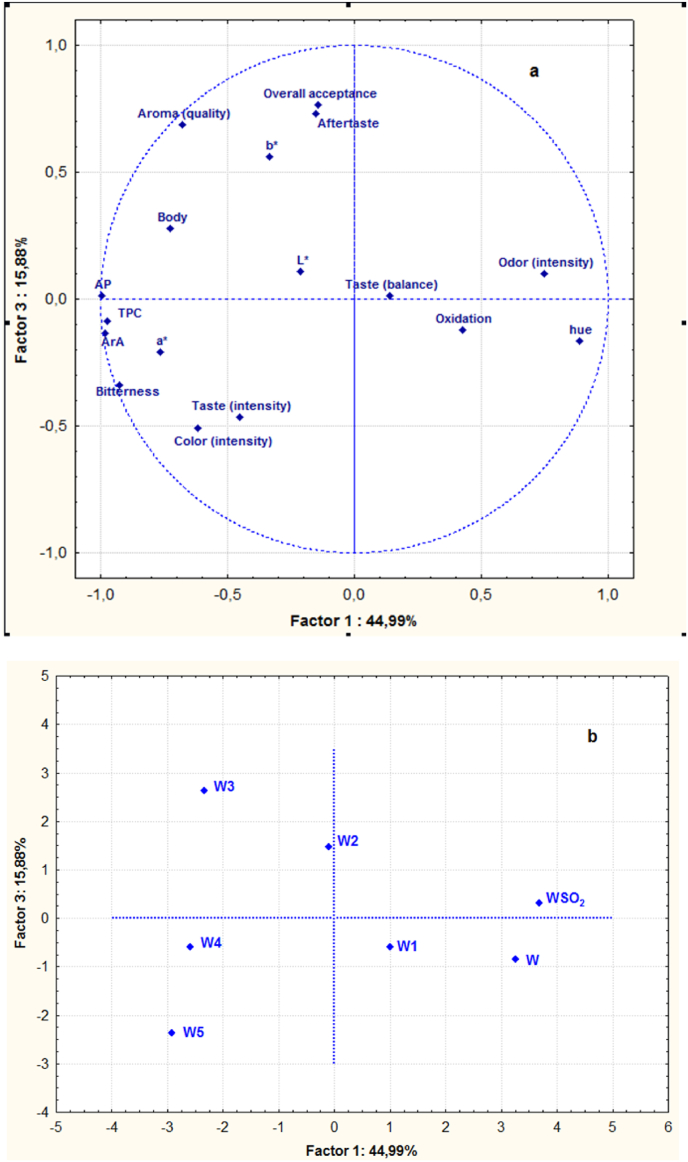
Principal component analysis (PCA) was carried out in order to summarize the relative differences amongst wine samples, in relation to their photometric analyses, color and sensory analysis parameters. In PCA analysis, the first six PC explain 100% of the total variation in all parameters (Fig. 3). The first and third PCs (PC1 and PC3) accounted for 44.99 and 15.88% of the variation, respectively. The PC2 explained 24.33% of the variability, the PC4 10.16%, the PC5 2.71% and the PC6 1.93%. In the loading plot shown (Fig. 3a), odor intensity, oxidation, taste balance and hue were located on the right side, positively linked with PC1, whereas other color parameters (L*, a*, b*), the rest sensory attributes and AP, TPC and ArA were projected at the negative values of PC1. Concerning the variables located far from the origin of PC1and PC3 (Fig. 3a), odor intensity and hue were opposed to AP, TPC, ArA and a*, on the first factor axis and aroma quality was opposed to taste and color intensity on the third factor axis. The PCA of wine characteristics (Fig. 3a) showed a high correlation between overall acceptance and aftertaste, and among AP, TPC and ArA. In Fig. 3b, Malagousia wine samples were projected in the plane defined by the first and third principal components and were clearly differentiated from each other. Control wine, sulfited wine and the wine treated with 100 mg/L tannin were located on the right side of the figure and were characterized by odor intensity, oxidation and hue. Wines treated with the highest levels of tannin (W4 and W5) were located on the left side of the figure, where taste, color intensity, a* parameter and bitterness lay. Finally, the wine treated with 300 mg/L tannin (W3) was characterized with higher contributions of aroma quality, overall acceptance, aftertaste, body, b* parameter and L*.
Fig. 3.
(a) Projection of photometric analyses, color and sensory analysis parameters in the plane defined by the first and third principal components. (b) Projection of the variables of Malagousia wine samples studied in the plane defined by the first and third principal components (TPC: Total phenol content; AP: Antioxidant Power; ArA: Antiradical Activity). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusions
The issue of reducing or supplementing the amount of SO2 has always been considered as a challenge for wine industry. The intention of the present study was to apply an enological Tara tannin in Malagousia white wine, as a protective compound against oxidative damage, as well as to evaluate the wine quality, including antioxidant/antiradical activity and sensory characteristics. The results of the study demonstrated that the addition of tannin in Malagousia white wine influenced considerably total phenolic content, antioxidant power, radical scavenging activity and hindered color degradation, for a storage period of 100 d. The 300 mg/L tannin dosage was suggested as a better choice, because it combines high efficacy against oxidative degradation in the sulphite-free white wine coupled with better sensorial acceptance and color deterioration prevention, compared with the higher tannin dosages. Conclusively and based on the above findings, Principal Component Analysis pointed out the differences and enabled the discrimination among Malagousia wine samples, thus leading us to the hypothesis that the prospect of substituting sulfites with tannins is a promising alternative that might positively contribute to the production of wine with enhanced antioxidant and sensory profile.
CRediT authorship contribution statement
Irini F. Strati: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Project administration. Panagiotis Tataridis: Resources, Data curation, Validation. Adnan Shehadeh: Investigation, Resources. Arhontoula Chatzilazarou: Investigation, Data curation, Validation. Vasileios Bartzis: Software, Formal analysis. Anthimia Batrinou: Investigation, Writing – review & editing. Vassilia J. Sinanoglou: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Irini F. Strati, Email: estrati@uniwa.gr.
Vassilia J. Sinanoglou, Email: vsina@uniwa.gr.
References
- Abramovic H., Kosmerl T., Ulrih N.P., Cigic B. Contribution of SO2 to antioxidant potential of white wine. Food Chem. 2015;174:147–153. doi: 10.1016/j.foodchem.2014.11.030. [DOI] [PubMed] [Google Scholar]
- Andreou V., Strati I.F., Fotakis C., Liouni M., Zoumpoulakis P., Sinanoglou V.J. Herbal distillates: a new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018;253:171–178. doi: 10.1016/j.foodchem.2018.01.162. [DOI] [PubMed] [Google Scholar]
- Azzolini M., Tosi E., Veneri G., Zapparoli G. Evaluating the efficacy of lysozyme against lactic acid bacteria under different winemaking scenarios. South Afr. J. Enol. Vitic. 2010;31(2):99–105. doi: 10.21548/31-2-1406. [DOI] [Google Scholar]
- Barril C., Clark A.C., Scollary G.R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal. Chim. Acta. 2012;732:186–193. doi: 10.1016/j.aca.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Briones-Labarca V., Perez-Wom M., Habib G., Giovagnoli-Vicuña C., Cañas-Sarazua R., Tabilo-Munizaga G., Salazar F.N. Oenological and quality characteristic on young white wines (sauvignon blanc): effects of high hydrostatic pressure processing. J. Food Qual. 2017 doi: 10.1155/2017/8524073. 2017. [DOI] [Google Scholar]
- Bührle F., Gohl A., Weber F. Impact of xanthylium derivatives on the color of white wine. Molecules. 2017;22:1376. doi: 10.3390/molecules22081376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuti V., Puccioni S., Giovani G., Salmi M., Rosi I., Bertuccioli M. Effect of oenotannin addition on the composition of Sangiovese wines from grapes with different characteristics. Am. J. Enol. Vitic. 2012;63(2):220–231. doi: 10.5344/ajev.2012.11091. [DOI] [Google Scholar]
- Cosme F., Andrea-Silva J., Filipe-Ribeiro L., Moreira A.S.P., Malheiro A.C., Coimbra M.A., Domingues M.R.M., Nunes F.M. vol. 12. EDP Sciences; 2019. The origin of pinking phenomena in white wines: an update. (BIO Web of Conferences). 02013. [DOI] [Google Scholar]
- Costa A., Barata A., Malfeito-Ferreira M., Loureiro V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008;25(2):422–427. doi: 10.1016/j.fm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Crespy A. Les tanins œnologiques: origines, propriétés - le cas des tanins de raisin. Revue des Œnologues. 2002;104:17–19. [Google Scholar]
- Delsart C., Grimi N., Boussetta N., Sertier C.M., Ghidossi R., Peuchot M.M., Vorobiev E. Comparison of the effect of pulsed electric field or high voltage electrical discharge for the control of sweet white must fermentation process with the conventional addition of sulfur dioxide. Food Res. Int. 2015;77:718–724. doi: 10.1016/j.foodres.2015.04.017. [DOI] [Google Scholar]
- ECP/GR- European Cooperative Programme for Plant Genetic Resources . 2021. (holding Institution GRC010, Accession Number P07#41BG-35 & P07#70B-29,30, and Holding Institution FRA139, Accession Number FRA139-0Mtp663;) the European Vitis Database.http://www.eu-vitis.de/index.php (last accessed 10/06/2021) [Google Scholar]
- EDOAO National inter-professional organization of vine & wine. 2021. https://winesofgreece.org/varieties/malagousia (last accessed 10/06/2021)
- European Commission . 2019. Delegated Regulation (EU) 2019/33 of 17 October 2018 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Applications for Protection of Designations of Origin, Geographical Indications and Traditional Terms in the Wine Sector, the Objection Procedure, Restrictions of Use, Amendments to Product Specifications, Cancellation of Protection, and Labelling and Presentation.http://data.europa.eu/eli/reg_del/2019/33/oj [Google Scholar]
- European Commission . 2019. Delegated Regulation (EU) 2019/934 of 12 March 2019 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Wine-Growing Areas where the Alcoholic Strength May Be Increased, Authorised Oenological Practices and Restrictions Applicable to the Production and Conservation of Grapevine Products, the Minimum Percentage of Alcohol for By-Products and Their Disposal, and Publication of OIV Files.http://data.europa.eu/eli/reg_del/2019/934/oj [Google Scholar]
- Ferreira A.C.S., De Pinho P.G., Rodrigues P., Hogg T. Kinetics of oxidative degradation of white wines and how they are affected by selected technological parameters. J. Agric. Food Chem. 2002;50(21):5919–5924. doi: 10.1021/jf0115847. [DOI] [PubMed] [Google Scholar]
- Fraga-Corral M., García-Oliveira P., Pereira A.G., Lourenço-Lopes C., Jimenez-Lopez C., Prieto M.A., Simal-Gandara J. Review: technological application of tannin-based extracts. Molecules. 2020;25:614. doi: 10.3390/molecules25030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks I.N., Du Toit M., Krügel M. Efficacy of ultraviolet radiation as an alternative technology to inactivate microorganisms in grape juices and wines. Food Microbiol. 2011;28(3):510–517. doi: 10.1016/j.fm.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Garde-Cerdán T., Marsellés-Fontanet A.R., Arias-Gil M., Ancín-Azpilicueta C., Martín-Belloso O. Influence of SO2 on the evolution of volatile compounds through alcoholic fermentation of must stabilized by pulsed electric fields. Eur. Food Res. Technol. 2008;227(2):401–408. doi: 10.1007/s00217-007-0734-5. [DOI] [Google Scholar]
- Gracin L., Jambrak A.R., Juretić H., Dobrović S., Barukčić I., Grozdanović M., Smoljanić G. Influence of high power ultrasound on Brettanomyces and lactic acid bacteria in wine in continuous flow treatment. Appl. Acoust. 2016;103:143–147. doi: 10.1016/j.apacoust.2015.05.005. [DOI] [Google Scholar]
- Guerrero R.F., Cantos-Villar E. Demonstrating the efficiency of sulphur dioxide replacements in wine: a parameter review. Trends Food Sci. Technol. 2015;42:27–43. doi: 10.1016/j.tifs.2014.11.004. [DOI] [Google Scholar]
- Hellenic Ministry of Rural Development and Food Statistcs, 2019-2020 data reported. Hellenic Republic. 2021 [Google Scholar]
- Jagatić Korenika A.M., Biloš J., Kozina B., Tomaz I., Preiner D., Jeromel A. Effect of different reducing agents on aromatic compounds, antioxidant and chromatic properties of sauvignon blanc wine. Foods. 2020;9(8):996. doi: 10.3390/foods9080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallithraka S., Salacha M.I., Tzourou I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: accelerated browning test versus bottle storage. Food Chem. 2009;113:500–505. doi: 10.1016/j.foodchem.2008.07.083. [DOI] [Google Scholar]
- Lantzouraki D.Z., Sinanoglou V.J., Zoumpoulakis P.G., Glamočlija J., Ćirić A., Soković M., Heropoulos G., Proestos C. Antiradical–antimicrobial activity and profile of pomegranate (Punica granatum L.) juices from different cultivars: a comparative study. RSC Adv. 2015;5(4):2602–2614. doi: 10.1039/c4ra11795f. [DOI] [Google Scholar]
- Lantzouraki D.Z., Sinanoglou V.J., Zoumpoulakis P., Proestos C. Comparison of the antioxidant and antiradical activity of pomegranate (Punica granatum L.) by ultrasound-assisted and classical extraction. Anal. Lett. 2016;49(7):969–978. doi: 10.1080/00032719.2015.1038550. [DOI] [Google Scholar]
- Li L., Li Z., Wei Z., Yud W., Cui Y. Effect of tannin addition on chromatic characteristics, sensory qualities and antioxidant activities of red wines. RSC Adv. 2020;10:7108. doi: 10.1039/C9RA09846A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M.T., Blaiotta G., Nioi C., Moio L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019;18(2):455–479. doi: 10.1111/1541-4337.12422. [DOI] [PubMed] [Google Scholar]
- López-Toledano A., Mayen M., Merida J., Medina M. Yeasts used to delay browning in white wines. Food Chem. 2006;97(3):498–504. doi: 10.1016/j.foodchem.2005.05.030. [DOI] [Google Scholar]
- Mitrevska K., Grigorakis S., Loupassaki S., Calokerinos A.C. Antioxidant activity and polyphenolic content of North Macedonian wines. Appl. Sci. 2020;10 doi: 10.3390/app10062010. 2010. [DOI] [Google Scholar]
- Nanou E., Mavridou E., Milienos F.S., Papadopoulos G., Tempère S., Kotseridis Y. Odor characterization of white wines produced from indigenous Greek grape varieties using the frequency of attribute citation method with trained assessors. Foods. 2020;9:1396. doi: 10.3390/foods9101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIV . 2021. International Code of Oenological Practices. Part II Oenological Treatments and Practices, II.1.1-18. Paris, France. [Google Scholar]
- OIV . first ed. OIV publications; Paris, France): 2021. OIV Collective Expertise Document. SO2 and Wine: a Review. March 2021. [Google Scholar]
- OIV . International Organisation Of Vine And Wine; Paris: 2021. Compendium of International Methods of Analysis of Wines and Musts. Edition 2021. [Google Scholar]
- Olejar K.J., Fedrizzi B., Kilmartin P.A. Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem. 2015;183:181–189. doi: 10.1016/j.foodchem.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Oliveira C.M., Ferreira A.C.S., De Freitas V., Silva A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011;44:1115–1126. doi: 10.1016/j.foodres.2011.03.050. [DOI] [Google Scholar]
- Panero L., Motta S., Petrozziello M., Guaita M., Bosso A. Effect of SO2, reduced glutathione and ellagitannins on the shelf life of bottled white wines. Eur. Food Res. Technol. 2015;240(2):345–356. doi: 10.1007/s00217-014-2334-5.10.1007/s00217-014-2334-5. [DOI] [Google Scholar]
- Pascual O., Vignault A., Gombau J., Navarro M., Gómez-Alonso S., García-Romero E., Canals J.M., Hermosín-Gutíerrez I., Teissedre P.L., Zamora F. Oxygen consumption rates by different oenological tannins in a model wine solution. Food Chem. 2017;234:26–32. doi: 10.1016/j.foodchem.2017.04.148. [DOI] [PubMed] [Google Scholar]
- Pati S., Crupi P., Benucci I., Antonacci D., Di Luccia A., Esti M. HPLC-DAD–MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Food Res. Int. 2014;66:207–215. doi: 10.1016/j.foodres.2014.09.017. [DOI] [Google Scholar]
- Proestos C., Sflomos K., Zoumpoulakis P., Tatarides P., Sinanoglou V.J. Botanical extracts used as wine preservatives. Int. J. Agricult. Sci. Food Technol. 2015;1(1) doi: 10.17352/2455-815X.000003. 007-011. [DOI] [Google Scholar]
- Qin G.H., Meng Z.Q. Effects of sulphur dioxide derivatives on expression of oncogenes and tumour suppressor genes in human bronchial epithelial cells. Food Chem. Toxicol. 2009;47(4):734–744. doi: 10.1016/j.fct.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Raposo R., Ruiz-Moreno M.J., Garde-Cerdán T., Puertas B., Moreno-Rojas J.M., Zafrilla P., Gonzalo-Diago A., Guerrero R.F., Cantos-Villar E. Replacement of sulfur dioxide by hydroxytyrosol in white wine: influence on both quality parameters and sensory. LWT-Food Sci. Technol. 2016;65:214–221. doi: 10.1016/j.lwt.2015.08.005. [DOI] [Google Scholar]
- Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdier D. vol. II. John Willey & Sons Ltd; New York: 2000. Phenolic compounds; pp. 129–185. (Handbook of Enology). [Google Scholar]
- Ribereau-Gayon P., Dubourdieu D., Doneche B., Lonvaud A. second ed. vol. 1. Wiley; Chichester: 2006. White winemaking; pp. 397–443. (Handbook of Enology: the Microbiology of Wine and Vinifications). [DOI] [Google Scholar]
- Rinaldi A., Moio L. Effect of enological tannin addition on astringency subqualities and phenolic content of red wines. J. Sensory Stud. 2018;33(3) doi: 10.1111/joss.12325. [DOI] [Google Scholar]
- Silva F.V., van Wyk S. Emerging non-thermal technologies as alternative to SO2 for the production of wine. Foods. 2021;10(9):2175. doi: 10.3390/foods10092175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: observations and practical implications. Am. J. Enol. Vitic. 1987;38(1):69–77. [Google Scholar]
- Sonni F., Cejudo Bastante M.J., Chinnici F., Natali N., Riponi C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: influence on volatile composition of white wines. J. Sci. Food Agric. 2009;89(4):688–696. doi: 10.1002/jsfa.3503. [DOI] [Google Scholar]
- Sonni F., Chinnici F., Natali N., Riponi C. Pre-fermentative replacement of sulphur dioxide by lysozyme and oenological tannins: effect on the formation and evolution of volatile compounds during the bottle storage of white. Food Chem. 2011;129:1193–1200. doi: 10.1016/j.foodchem.2011.05.104. [DOI] [PubMed] [Google Scholar]
- STATISTICA software (Statsoft Inc., 2004, Tulsa, OK, USA).
- Tekos F., Makri S., Skaperda Z.V., Patouna A., Terizi K., Kyriazis I.D., Kotseridis Y., Mikropoulou E.V., Papaefstathiou G., Halabalaki M., Demetrios K. Assessment of antioxidant and antimutagenic properties of red and white wine extracts in vitro. Metabolites. 2021;11(7):436. doi: 10.3390/metabo11070436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtoglou C., Nenadis N., Paraskevopoulou A. Phenolic composition and radical scavenging activity of commercial Greek white wines from Vitis vinifera L. cv. Malagousia. J. Food Compos. Anal. 2014;33:166–174. doi: 10.1016/j.jfca.2013.12.009. [DOI] [Google Scholar]
- Tzachristas A., Pasvanka K., Liouni M., Calokerinos A.C., Tataridis P., Proestos C. Effect of Hippophae rhamnoides L. Leaves treatment on the antioxidant capacity, total phenol content and sensory profile of Moschofilero wines vinified with and without added sulphites. Appl. Sci. 2020;10:3444. doi: 10.3390/app10103444. [DOI] [Google Scholar]
- Vally H., Thompson P.J. Allergic and asthmatic reactions to alcoholic drinks. Allergic and asthmatic reactions to alcoholic drinks. Addiction Biol. 2003;8:3–11. doi: 10.1080/1355621031000069828. [DOI] [PubMed] [Google Scholar]
- Versari A., Du Toit W., Parpinello G.P. Oenological tannins: a review. Aust. J. Grape Wine Res. 2013;19:1–10. doi: 10.1080/1355621031000069828. [DOI] [Google Scholar]
- Vignault A., Pascual O., Jourdes M., Moine V., Fermaud M., Roudet J., Canals J.M., Teissedre P.L., Zamora F. Impact of enological tannins on laccase activity. Oeno One. 2019;53(1):27–38. doi: 10.20870/oeno-one.2019.53.1.2361. [DOI] [Google Scholar]
- Yildirim H.K., Darici B. Alternative methods of sulfur dioxide used in wine production. J. Microbiol. Biotechnol. Food Sci. 2020;9(4):675–687. doi: 10.15414/jmbfs.2020.9.4.675-687. [DOI] [Google Scholar]