Abstract
Reported advantages of early excision for larger burn injuries include reduced morbidity, mortality, and hospital length of stay for adult burn patients. However, a paucity of evidence supports the best option for paediatric burns and the advantages of non-excisional (mechanical) debridement. Procedural sedation and analgesia in the emergency department is a popular alternative to debridement in operating theatres under general anaesthesia. This study aims to evaluate the association between early (< 24 h post-injury) non-excisional debridement under general anaesthesia with burn wound re-epithelialisation time and skin graft requirements. Cohort study of children younger than 17 years who presented with burns of five percent total body surface area or greater. Data from January 2013 to December 2019 were extracted from a prospectively collected state-wide paediatric burns’ registry. Time to re-epithelialisation was tested using survival analysis, and binary logistic regression for odds of skin graft requirementto analyse effects of early non-excisional debridement in the operating theatre. Overall, 292 children met eligibility (males 55.5%). Early non-excisional debridement under general anaesthesia in the operating theatre, significantly reduced the time to re-epithelialisation (14 days versus 21 days, p = 0.029)) and the odds of requiring a skin graft in comparison to paediatric patients debrided in the emergency department under Ketamine sedation (OR: 6.97 (2.14–22.67), p < 0.001. This study is the first to demonstrate that early non-excisional debridement under general anaesthesia in the operating theatre significantly reduces wound re-epithelialisation time and subsequent need for a skin graft in paediatric burn patients. Analysis suggests that ketamine procedural sedation and analgesia in the emergency department used for burn wound debridement is not an effective substitute for debridement in the operating theatre.
Subject terms: Paediatric research, Trauma, Health care
Introduction
Significant advances in the survival of paediatric patients with medium to large burn wounds (> 5% total body surface area (TBSA)), were made in the 20th century1–3. Sepsis, skin grafting requirements, wound re-epithelialization time have become key clinical outcomes to improve burn wound care beyond survival in developed countries. Following a thermal injury, the compromised barrier function of the skin combined with the anatomical characteristics of paediatric skin, render children more susceptible to inflammation and infection 4. The aim of debridement is to remove all non-viable tissue and debris from the injured cutaneous surface. Traditional surgical debridement, using sharp excision, aimed to improve survival by avoiding sepsis, however the sacrifice with this method is the unintentional removal of healthy tissue along with the intentional removal of dead tissue. Non-excisional debridement methods include mechanical (e.g. hydro surgery or abrasion technique) and more recently, enzymatic debridement5,6. Mechanical debridement requires an aggressive scrub using gauze, non-cytotoxic cleanser, and water. Once completed, a more accurate assessment of the size and depth of the burn wound is possible, critical considerations of burn management7.
Many studies have demonstrated that, delayed burn wound re-epithelialisation is associated with an increased risk for hypertrophic scar formation in children8–10. Multiple factors have been found to influence this critical time to re-epithelialisation time in children. Hence, clinicians consider the timing, setting and analgesia at initial debridement of medium to large burn wounds to optimise outcomes such as re-epithelialisation time and requirement for skin graft. Time to wound debridement is dependent on the consideration of a multitude of factors including patient stability, injury severity, body location and TBSA11. Early debridement is thought to reduce the toxic and bacterial burden from a burn wound12–14 and within 24 h of injury has been associated with significantly reduced re-epithelialisation time in adults 15. In children, early excision with immediate wound closure was associated with improved survival, shorter hospitalisation3,13,16 and found to be safe and effective within 72 h of injury1. At the study site, a quaternary paediatric hospital and burns centre, greater than 20,000 paediatric burns patients have been treated over the last 20 years with only two children succumbing to their injuries.
Furthermore, there are several factors in the consideration for early debridement including burn severity, staff expertise, pain, conforming dressing, available resources, location, time of day/week/weekend, and type of analgesia that will be administered. Sub-optimal pain management has been shown to delay paediatric burn wound re-epithelialisation 17. In addition, a recent study reported that parental acute psychological distress influences child procedural-related pain distress 18. Often, minor (TBSA < 5%), and a proportion of medium to large TBSA burns, are initially managed in the emergency department (ED) with procedural sedation and analgesia (PSA). The level of PSA ranges from minimal (anxiolysis with impaired cognitive function) to moderate, where the child would have reduced level of consciousness, respond to verbal commands and maintain adequate spontaneous ventilation 19,20. The resurgence of paediatric PSA with ketamine, either as a single drug or in combination with other PSA agents 21 is due to its potent anaesthetic and analgesic properties and low incidence cardiorespiratory adverse effects22–27 when compared to opioids. For these reasons, ketamine PSA is a popular choice by clinicians treating paediatric burns wounds in EDs 21,28–30. Despite this, ‘emergence reaction’ after ketamine is a well-documented adverse reaction. The rate of hallucination when emerging from a dissociative state occurs at rates between 5 and 14% 31, and is reported to be transient and mild in children32. However, little is known on the efficacy of longer term protective factors of medical trauma.
This study aims to evaluate the effect of non-excisional, mechanical debridement within 24 h of paediatric burns injuries greater than or equal to 5% TBSA, under general anaesthesia in the operation theatre on wound re-epithelialisation and skin graft requirements.
Objectives
The objectives of this study were as follows.
Primary The effect of timing, setting and analgesia for non-excisional debridement of acute, medium to large (≥ 5% TBSA), paediatric burn injuries on time to re-epithelialisation.
Secondary The effect of timing, setting and analgesia for non-excisional debridement of acute, medium to large (≥ 5% TBSA), paediatric burn injuries on skin graft requirements.
Methods
A single-centre, retrospective, cohort study using prospectively collected data from the (De-identified) Paediatric Burns Registry was conducted at a paediatric burn’s referral centre in (De-identified), Australia. All children who presented to the study site, between January 2013 and December 2019, younger than 17 years, with TBSA ≥ 5%, of any burn mechanism were eligible for inclusion. All eligible families who presented to the burns outpatient department or inpatient ward were approached for consent and data collection. Parents who declined for their child’s data to be entered into the Paediatric Burns Registry were excluded from this study. Registry data collection consists of demographic and injury characteristics as well as clinical interventions, hospital interactions and clinical outcomes. Children with full thickness burns were excluded due to their certainty of requiring a skin graft and conversely superficial burns were excluded due to their unlikely requirements of skin grafting 33
All children taken to theatre within 24-h post-burn for an initial, non-excisional debridement in theatre received general anaesthesia. The non-excisional debridement intervention uses an aggressive washing technique with sterile water, soap-free surfactant cleanser (QV Cleanser, Melbourne, Australia) 34, and sterile gauze sponge (Ray-Tec, Johnson & Johnson, NJ, USA) for the removal of all non-viable tissue from the burn wound. This was followed by immediate wound closure with an appropriate cover such as a silver impregnated, or biological dressing as determined by the treating surgeon. Children were then subsequently managed as either in-patient or outpatients, dependent on the severity of burn injury or other concern. The treating burns surgeon determined when burn wound achieved spontaneous re-epithelialisation ≥ 95% or requirement for a skin graft.
Reporting of this cohort study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement 35. Once the study was approved by the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/16/QRCH/61. SSA/16/QRCH/61), data were extracted and deidentified prior to analysis. The study was conducted in accordance to the principles of the Declaration of Helsinki. Written informed consent was obtained from the legal guardians at the time of data registry inclusion.
Data management
Data for each patient was extracted from the (De-identified) Paediatric Burns Registry and included socio-demographic data, TBSA%, burn depth, age of burn in hours at debridement, initial dressing applied, analgesia at debridement, setting of initial debridement, time to re-epithelialisation in days and incidents of skin graft requirements. Data collection was from the time of first presentation to a health service, up until wound re-epithelialisation was achieved or skin grafting undertaken. Data was captured with and stored in FileMaker (Claris International Inc., NSW, Australia).
Statistical methods
The timing, location and analgesia at initial wound debridement were selected as variables of interest for this investigation, with key outcomes being differences in time to re-epithelialisation and need for skin grafting. The dataset was divided into three groups: ‘OT < 24 h’, ‘ED Ketamine PSA’, and ‘Other Settings’ (comparator). The ‘OT < 24 h’ group included paediatric burn patients taken to OT for non-excisional wound debridement under general anaesthetic within the first 24-h following burn injury. The ‘ED Ketamine PSA’ group included children whose debridement were completed in the ED with Ketamine PSA. The ‘Other settings’ group included all other debridement that were not completed in the operation theatre under general anaesthesia within 24 h of injury or in the ED under Ketamine PSA.
Descriptive analysis was carried out for all key variables. Chi-square tests were performed to examine the relationships between purely categoric variables and the context of debridement (OT24hrs, ED Ketamine PSA or Other). Due to non-normal distributions, Kruskal–Wallis tests were used for continuous variables.
Binary logistic regression was used to determine the associations between the three treatment groups and requirement for skin graft, after controlling for burn severity as indicated by TBSA and burn depth.
Cox proportional hazards model was used to examine the effect of treatment group on wound re-epithelialisation time, adjusted for burn severity among those who did not receive a skin graft. A sensitivity analysis was performed to examine the impact of inclusion of patients with a skin graft on the findings of time to re-epithelialisation to include, as skin grafts are an augmented wound closure. Following consultation with the study centre burn surgeons, a dummy value of 28 days was selected to estimate an average time to re-epithelialisation in order to account for all large burns in the cohort. Sensitivity analysis (Supplementary File 1) indicated no substantial changes in the conclusions and the initial model was retained. A value of p < 0.05 was considered statistically significant. Data was analysed with SPSS 27 (IBM Corporation, Armonk, NY, USA) software.
Ethics approval and consent to participate
The study was approved by the Children’s Health Queensland Hospital and Health Service Human Research Ethics Committee (HREC/16/QRCH/61).
Consent for publication
Consent for inclusion in the database was obtained at data collection. Patient information was deidentified while undergoing statistical analysis, maintaining patient privacy and confidentiality.
Results
Participants
Two hundred and ninety-two paediatric burn patients met the inclusion criteria for the study (i.e., aged ≤ 17 years with a burn TBSA ≥ 5%) and were extracted from the (De-identified) Paediatric Burns Registry. Demographic details of the sample population are presented in Table 1. Children under the age of four accounted for over 68% of participants included in this investigation. Males were slightly overrepresented in the sample population—accounting for more than 54% of children overall. No significant differences were found between the ED Ketamine PSA, Other Setting, or the OT < 24hrs for first aid, gender, age, or time to debridement.
Table 1.
Characteristics of patients: debrided in the ED under ketamine PSA, debrided in other settings, and debrided in the OT within 24 h.
| Debridement in the ED with ketamine n = 28 N (%) |
Debridement other settings n = 220 N % |
Debridement in OT within 24 h n = 44 N % |
p value | |
|---|---|---|---|---|
| Burn depth | ||||
| DDPT | 16 (57.1) | 107 (48.6) | 33 (75) | |
| SPT | 12 (42.9) | 113 (51.4) | 11 (25) | 0.005 |
| TBSA % Median (IQR) | 9.5 (8–12.8) | 6 (5–9) | 11.5 (7.3–16) | < 0.001 |
| Gender | 0.665 | |||
| Male | 16 (57.1) | 125 (56.8) | 21 (47.7) | |
| Female | 12 (42.9) | 92 (41.8) | 21 (47.7) | |
| Missing | – | 3 (1.4) | 2 (4.5) | |
| First aid | 0.944 | |||
| Yes | 21 (75) | 168 (76.4) | 32 (72.7) | |
| No | 6 (21.4) | 52 (23.6) | 11 (25) | |
| Missing | 1 (3.6) | – | 1 (2.3) | |
| Age (years) | 0.081 | |||
| 0–4 | 23 (82.1) | 143 (65) | 34 (77.3) | |
| 5–10 | 4 (14.3) | 46 (20.9) | 3 (6.8) | |
| > 10 | 1 (3.6) | 31 (14.1) | 7 (15.9) | |
| Time to re-epithelialisation (Days) Median (IQR) | 21 (12–34) | 17 (12–23) | 14 (10–19) | 0.020 |
| Time to debridement (h:min) | 5:07 (3:26–11:27) | 5:29 (3:44–9:27) | 9:44 (2:57–22:35) | 0.730 |
| Definitive dressing applied | ||||
| Acticoat | 13 (46.6) | 125 (56.8) | 29 (65.9) | |
| Mepilex Ag | 10 (35.7) | 72 (32.7) | 3 (6.8) | |
| Combined silver | 5 (17.9) | 18 (8.2) | 1 (2.3) | |
| Biobrane | – | – | 8 (18.2) | |
| RECELL | – | 1 (0.5) | 2 (4.5) | |
| Flamazine | – | 2 (0.9) | – | |
| Not recorded | – | 2 (0.9) | 1 (2.3) | |
| Grafted | 13 (46.4) | 37 (16.8) | 9 (20.5) | 0.001 |
DDPT deep dermal partial thickness, SPT superficial partial thickness, TBSA total body surface area, ED emergency department, OT operating theatre, IQR interquartile range.
aCombined Silver = Acticoat + Mepilex Ag + Hypafix applied to burns.
Effect of non-excisional debridement on time to re-epithelialisation
A significant difference in time to re-epithelialisation was identified between paediatric patients taken to theatre within 24 h in comparison to those debrided in the ED under Ketamine PSA (p = 0.029). Median time to re-epithelialisation for children taken to theatre for debridement under general anaesthetic was 14 days (IQR 10–19) versus 21 days (IQR: 12–34), p = 0.029 for patients debrided in the ED under Ketamine PSA. Median time to re-epithelialisation for children in the Other Settings group was equal to 17 days (IQR: 12–23).
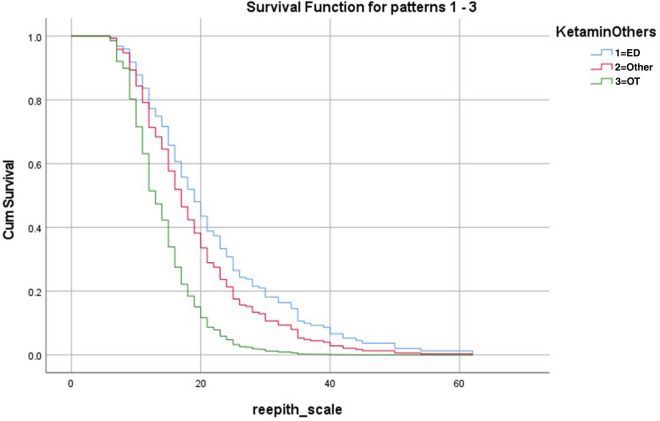
Cox regression analysis determined there was a significant effect of the setting of the initial non-excisional debridement of burn injuries on burn wound time to re-epithelialisation after adjusting for burn severity (Table 2). In comparison to children who received non-excisional wound debridement in the OT within 24-h post-burn, paediatric patients who underwent non-excisional debridement under Ketamine PSA in the ED had a 61% reduced chance of reaching 95% re-epithelialisation (Hazards Ratio = 0.39, 95% CI 0.21–0.72, p < 0.001). Furthermore, children debrided in ‘Other Settings’ had a 49% reduced chance of reaching 95% re-epithelialisation in comparison to children who received non-excisional debridement under general anaesthetic in theatre within 24 h (Hazards Ratio = 0.51, 95% CI 0.33–0.78, p = 0.001). Deep dermal partial thickness (DDPT) injuries demonstrated a significant effect on time to re-epithelialisation (p < 0.001), confirming their significant incorporation into the model. A Cox Regression Survival Plot (Fig. 1) demonstrates the significant shortened time for the OT<24hrs Group compared to Other Settings and Ketamine PSA in ED Groups.
Table 2.
Time to re-epithelialisation Cox regression (hazard ratios) n = 233.
| Variable | Sub-group | Hazard ratio (95% CI) | p value |
|---|---|---|---|
| TBSA | 0.97 (0.93–1.01) | 0.150 | |
| Burn depth | DDPT | 0.52 (0.39–0.67) | < 0.001 |
| SPT | 1 | ||
| Debridement sub-group | Ketamine in the ED | 0.39 (0.21–0.72) | < 0.001 |
| Other | 0.51 (0.33–0.78) | 0.001 | |
| OT within 24 h | 1 |
NB: All grafted patients N = 59, were excluded from this analysis.
DDPT deep dermal partial thickness, SPT superficial partial thickness, TBSA total body surface area, ED emergency department, OT operating theatre, CI confidence interval.
*1 = reference group for regression.
Figure 1.
Effect of non-excisional debridement on re-epithelialisation time (Cox survival Plot).
The additional model, using a proxy value of 28 days for grafted patients reflected similar patterns of Hazard Ratios described in Table 2 (Supplementary File 1).
Effect of non-excisional debridement on requirement for skin graft
In the binary regression, children in the Ketamine PSA group had almost seven times the odds of requiring a skin graft compared to those receiving non-excisional debridement under general anaesthesia within 24 h of injury (Odds Ratio = 6.97, 95% CI 2.14–22.67, p < 0.001), even after controlling for variables known to influence rates of grafting such deep partial thickness burn depth and TBSA% (Table 3). No significant difference in odds of split thickness skin grafting was identified between paediatric patients in the Other Settings group compared to those in the OT<24hrs group (Odds Ratio = 0.51, 95% CI 0.33–0.78, p = 0.126).
Table 3.
Odds of grafting following non-excisional debridement n = 292.
| Variable | Sub-group (N) | Odds ratio (95% CI) | p value |
|---|---|---|---|
| TBSA | 1.14 (1.05–1.24) | 0.001 | |
| Burn depth | DDPT | 6.6 (3.03–14.18) | < 0.001 |
| SPT | *1 | ||
| Debridement sub-group | Ketamine in the ED | 6.97 (2.14–22.67) | < 0.001 |
| Other | 2.1 (0.81–5.62) | 0.126 | |
| OT within 24 h | *1 |
DDPT deep dermal partial thickness, SPT superficial partial thickness, TBSA total body surface area, ED emergency department, OT operating theatre, CI confidence interval.
*1 = reference group for regression.
Discussion
This study is the first to demonstrate that early initial, non-excisional debridement of acute paediatric burns under general anaesthesia in an operating theatre, significantly reduces the wound re-epithelialisation time and subsequent requirements for skin graft. Non-excisional burn wound debridement completed in the operating theatre within 24 h of burn injury resulted in a wound re-epithelialisation time of 7 days faster when compared to Ketamine PSA in the ED. The odds for requiring a skin graft were significantly increased when non-excisional debridement was not completed in theatre under general anaesthetic within 24 h of injury. These findings add to the evidence supporting early debridement of acute burn injuries in children 3,12,37,38.
The evolution and improvements of paediatric burn care have been reflected by an exceptional increase in survival rates over the last 50 years. Thus, expanding the focus of burn care to decreasing the risk of scar formation. Chipp et al. demonstrated the linear relationship between time to re-epithelialisation and risk of scaring with every additional day taken to re-epithelialise, multiplying the risk of hypertrophic scaring by 1.13810. In addition to this Chipp et al. challenged traditional dogma of healing within 3 weeks to be oversimplified in the paediatric cohort, emphasizing that every effort should be made to reach re-epithelialisation as quickly as possible10.
The initial phase of burn wound healing is typified by inflammation and haemostasis that confine the extent of injury and cleanse the wound 39. Burn wound conversion causes deepening of the burn wound due to ongoing ischaemia and inflammation 40. Early tangential excision is thought to address this inflammatory phase by removal of non-viable tissue from the wound, first described by Janzekovic 41. Lu et al. demonstrated the influence of tangential excision within 24 h post-burn injury of deep partial wounds was a significant reduction of inflammatory markers (IL8, MPO and MDA) when compared to non-debrided areas of the wound 14. We hypothesise that non-excisional debridement, in comparison to excisional debridement, is likely to preserve more healthy tissue and contribute towards the removal of the considerable burden of these inflammatory markers.
In the ED, wound debridement may occur with the parent present during the procedure. Some parents experience distress observing this process. There is evidence to support that parental distress and anxiety directly correlates to the child’s burn wound healing time42,43. It is postulated that general anaesthesia in the operation theatre provides an environment for complete burn wound debridement, adequate wound closure, and optimal peri-procedural analgesia. Traditionally, clinicians have been reluctant to subject young children to frequent general anaesthesia due to concerns for neurotoxicity after exposure to anaesthetic drugs44,45. Recently, three large paediatric studies (GAS46, PANDA47, and MASK 48) have identified no correlation between single anaesthesia exposure and reduced cognition45. Whilst this is a promising finding for the safety of children, limited research has been conducted examining the influence of debridement setting and analgesia within 24 h of injury on clinical outcomes such as re-epithelialisation time and skin graft requirements for medium to large burns.
Debridement under general anaesthesia provides a controlled environment where peri-procedural analgesia can be optimised. Brown et al. showed that wound re-epithelialisation was delayed by 2.2% for every increase of one point on the Faces Pain Scale Revised 17. It is postulated that whilst under a general anaesthesia, the injured child is not able to formulate a memory of the painful procedure that may contribute towards increased anticipatory distress 49 during subsequent dressing changes. Further studies would be required to explore this hypothesis. Another proposed benefit of general anaesthesia for initial debridement is that burn surgeons can select the most appropriate wound management approach and achieve complete coverage of the burn wound. This is not always possible in a busy emergency department, for a child who has been given peri-procedural analgesia with or without adjunct distraction techniques.
Efforts to address the complex physiological activity of an acute burn injury, specifically to disrupt wound progression, are increasingly visible in scientific burns literature. The early application of negative pressure wound therapy in paediatric burn wounds has shown decreased time to re-epithelialisation, with suggested cost savings due to decreased proportions of skin grating requirements 36. Additionally, effective adherence to 20 min of cool running water within the first three hours of burn injury has resulted in significantly reduced odds of skin grafting amongst other patient outcomes 50,51. More recently Holbert et al. highlighted the characteristics of burn wounds associated with higher pain levels 52. Acknowledging the impact risk factors and interventions have on the time to re-epithelialisation and subsequent risk of scarring are important considerations in tailoring acute burn treatment pathways. Bundling these individual interventions together may lead to additional improvements in patient outcomes. More studies would be necessary to explore this hypothesis.
The setting for this study is the sole paediatric tertiary burns centre, housing five burns surgeons, treating > 1200 new burns per annum, covering a land mass of 1.85 million km2/715,447.3 miles2. This study was performed to enable decision makers with evidence to facilitate access to the best treatment options for optimal clinical outcomes and define treatment pathways into the future. In patients who went to operating theatre in less than 24 h, there were two patients included who received RECELL. Although a recent literature review could not reach a definitive role of autologous skin cell suspension in re-epithelialisation, there is widespread anecdotal acknowledgment of the positive experience of the intervention. However, with only two participants receiving RECELL, it is highly unlikely to have impacted the reliability of results presented.
The cost and access to an operating theatre is not always an easy accomplishment in a busy tertiary hospital. Whilst this intervention can be perceived as an early burden on hospital resources, other studies have shown that early intervention investments improve the longer term patient outcome benefits and ultimately overall cost effectiveness 53,54. To better define this benefit, future work should incorporate a formalised cost effectiveness analysis, to strengthen discussions with hospital executives to consider prioritising operating theatres for this intervention.
There are noteworthy limitations in this study. Firstly, the observational data set is at risk of selection bias associated with restricting the data selection and subsequent analysis to participants with completed data for outcomes. Secondly, although detailed training was provided for data collectors, the possibility of variability in data entry into the proformas cannot be eliminated. Lastly, the cohort is small and results could be bolstered with a greater sample size in subsequent studies in this area.
Conclusion
Early non-excisional debridement of acute burns under general anaesthesia in children reduces wound re-epithelialisation time and requirements for skin grafting. Effective non-excisional debridement can be achieved under general anaesthesia, aggressive mechanical debridement with warm water, sterile surgical gauze, and a soap-free surfactant cleanser.
Supplementary Information
Acknowledgements
The investigators would like to thank the participants and their families as well as the clinical staff of the study site for their continued support. Especially Dr Craig McBride, Dr Bhavesh Patel and Dr Romi Das Gupta for their valuable input into constructing a clinically meaningful approach to data analysis.
Abbreviations
- TBSA
Total body surface area
- CI
Confidence interval
- IQR
Inter-quartile range
- OR
Odds ratio
- FT
Full thickness
- DDPT
Deep dermal partial thickness
- SPT
Superficial partial thickness
- OT
Operating theatre
- IQR
Interquartile range
- ED
Emergency Department
- GA
General anaesthesia
- PSA
Procedural sedation and analgesia
- M
Male
- F
Female
- IL8
Interleukin-8
- MPO
Myeloperoxidase
- MDA
Malondialdehyde
- GAS
General Anaesthesia Spinal Trial (46)
- PANDA
Paediatric Anaesthesia Neurodevelopmental Assessment Trial (47)
- MASK
Mayo Anaesthesia Safety in Kids Trial (48)
Author contributions
B.G., R.K. and A.B. collectively developed the concept and protocol for this study. Z.D. and L.J. are both biostatisticians that led the statistical methodology and analysis. M.H. assisted with data analysis and drafting of manuscript. All authors participated in the drafting of the manuscript.
Funding
This project is unfunded. Dr Griffin is a Senior Research Fellow, funded by the National Health Medical Research Council (Australia), Centre of Research Excellence in Wiser Wound Care (APP1196436).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03141-x.
References
- 1.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J. Trauma. 1986;26(2):149–152. doi: 10.1097/00005373-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J. Trauma. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 3.Tompkins RG, Remensnyder JP, Burke JF, Tompkins DM, Hilton JF, Schoenfeld DA, et al. Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann. Surg. 1988;208(5):577–585. doi: 10.1097/00000658-198811000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong F, Galzote C, Duan Y. Change in skin properties over the first 10 years of life: A cross-sectional study. Arch. Dermatol. Res. 2017;309(8):653–658. doi: 10.1007/s00403-017-1764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmondson SJ, Ali Jumabhoy I, Murray A. Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials. Burns. 2018;44(7):1721–1737. doi: 10.1016/j.burns.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Wormald JC, Wade RG, Dunne JA, Collins DP, Jain A. Hydrosurgical debridement versus conventional surgical debridement for acute partial-thickness burns. Cochrane Database Syst. Rev. 2020;9:CD012826. doi: 10.1002/14651858.CD012826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams FN, Lee JO. Pediatric burn infection. Surg. Infect. (Larchmt). 2021;22(1):54–57. doi: 10.1089/sur.2020.218. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J. Trauma. 1983;23(10):895–898. [PubMed] [Google Scholar]
- 9.Lonie S, Baker P, Teixeira RP. Healing time and incidence of hypertrophic scarring in paediatric scalds. Burns. 2017;43(3):509–513. doi: 10.1016/j.burns.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, Wilson Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: Every day counts. Burns Trauma. 2017;5(1):3. doi: 10.1186/s41038-016-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugherty THFMDMS, Ross AMD, Neumeister MWMDF. Surgical excision of burn wounds. Clin. Plastic Surg. 2017;44(3):619–625. doi: 10.1016/j.cps.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Wilder D, Rennekampff HO. Debridement of burn wounds—Rationale and options. Handchir Mikrochir Plast Chir. 2007;39(5):302–307. doi: 10.1055/s-2007-989227. [DOI] [PubMed] [Google Scholar]
- 13.Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145–150. doi: 10.1016/j.burns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Lu SL, Liao ZJ, Xiang J, Wang ZY, Yang LY, Jin SW, et al. Influence of tangential excision within 24 postburn hours on the local wound inflammatory response in patients with deep partial thickness burn. Zhonghua Shao Shang Za Zhi. 2005;21(1):24–26. [PubMed] [Google Scholar]
- 15.Shao F, Ren WJ, Meng WZ, Wang GZ, Wang TY. Burn wound bacteriological profiles, patient outcomes, and tangential excision timing: A prospective, Observational study. Ostomy Wound Manag. 2018;64(9):28–36. [PubMed] [Google Scholar]
- 16.Burke FJ, Bondoc CC, Quinby CW. Primary burn excision and immediate grafting: A method shortening illness. J. Trauma Injury Infect. Crit. Care. 1974;14(5):389–395. doi: 10.1097/00005373-197405000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Brown NJ, Kimble RM, Gramotnev G, Rodger S, Cuttle L. Predictors of re-epithelialization in pediatric burn. Burns. 2014;40(4):751–758. doi: 10.1016/j.burns.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Brown EA, De Young A, Kimble R, Kenardy J. Impact of parental acute psychological distress on young child pain-related behavior through differences in parenting behavior during pediatric burn wound care. J. Clin. Psychol. Med. Settings. 2019;26(4):516–529. doi: 10.1007/s10880-018-9596-1. [DOI] [PubMed] [Google Scholar]
- 19.Meredith JR, O'Keefe KP, Galwankar S. Pediatric procedural sedation and analgesia. J. Emerg. Trauma Shock. 2008;1(2):88–96. doi: 10.4103/0974-2700.43189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramalho CE, Bretas PMC, Schvartsman C, Reis AG. Sedation and analgesia for procedures in the pediatric emergency room. J. Pediatr. (Rio. J.). 2017;93(Suppl 1):2–18. doi: 10.1016/j.jped.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Brunette KE, Anderson BJ, Thomas J, Wiesner L, Herd DW, Schulein S. Exploring the pharmacokinetics of oral ketamine in children undergoing burns procedures. Paediatr. Anaesth. 2011;21(6):653–662. doi: 10.1111/j.1460-9592.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds S. Ketamine: Not just for pediatric sedation? Clin. Pediatr. Emerg. Med. 2017;18(4):286–291. [Google Scholar]
- 23.Bellolio MF, Puls HA, Anderson JL, Gilani WI, Murad MH, Barrionuevo P, et al. Incidence of adverse events in paediatric procedural sedation in the emergency department: A systematic review and meta-analysis. BMJ Open. 2016;6(6):e011384. doi: 10.1136/bmjopen-2016-011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahajan C, Dash HH. Procedural sedation and analgesia in pediatric patients. J. Pediatr. Neurosci. 2014;9(1):1–6. doi: 10.4103/1817-1745.131469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bredmose PP, Grier G, Davies GE, Lockey DJ. Pre-hospital use of ketamine in paediatric trauma. Acta Anaesthesiol. Scand. 2009;53(4):543–545. doi: 10.1111/j.1399-6576.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 26.Graudins A, Meek R, Egerton-Warburton D, Oakley E, Seith R. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: A randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann. Emerg. Med. 2015;65(3):248–254.e1. doi: 10.1016/j.annemergmed.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira JESL, Lee JY, Bellolio F, Homme JL, Anderson JL. Intranasal ketamine for acute pain management in children: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020;38(9):1860–1866. doi: 10.1016/j.ajem.2020.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norambuena C, Yañez J, Flores V, Puentes P, Carrasco P, Villena R. Oral ketamine and midazolam for pediatric burn patients: A prospective, randomized, double-blind study. J. Pediatr. Surg. 2013;48(3):629–634. doi: 10.1016/j.jpedsurg.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Thompson EM, Andrews DD, Christ-Libertin C. Efficacy and safety of procedural sedation and analgesia for burn wound care. J. Burn Care Res. 2012;33(4):504–509. doi: 10.1097/BCR.0b013e318236fe4f. [DOI] [PubMed] [Google Scholar]
- 30.Canpolat DG, Esmaoglu A, Tosun Z, Akin A, Boyaci A, Coruh A. Ketamine-propofol vs ketamine-dexmedetomidine combinations in pediatric patients undergoing burn dressing changes. J. Burn Care Res. 2012;33(6):718–722. doi: 10.1097/BCR.0b013e3182504316. [DOI] [PubMed] [Google Scholar]
- 31.Karlow N, Schlaepfer CH, Stoll CRT, Doering M, Carpenter CR, Colditz GA, et al. A systematic review and meta-analysis of ketamine as an alternative to opioids for acute pain in the emergency department. Acad. Emerg. Med. 2018;25(10):1086–1097. doi: 10.1111/acem.13502. [DOI] [PubMed] [Google Scholar]
- 32.Dolansky G, Shah A, Mosdossy G, Rieder M. What is the evidence for the safety and efficacy of using ketamine in children? Paediatr. Child Health. 2008;13(4):307–308. doi: 10.1093/pch/13.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Israel JS, Greenhalgh DG, Gibson AL. Variations in burn excision and grafting: A survey of the American Burn Association. J. Burn Care Res. 2017;38(1):e125–e132. doi: 10.1097/BCR.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 34.Kempf M, Kimble, R., Cuttle, L., editor. The cytotoxicity of QV gentle wash compared to Chlorhexidine Gluconate on Primary human epidermal Keratinocyte (Hek) cells. Australia and New Zealand Burns Association, Annual Scientific Meeting 2017; Auckland, New Zealand.
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 36.Frear CC, Cuttle L, McPhail SM, Chatfield MD, Kimble RM, Griffin BR. Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children. Br. J. Surg. 2020;107(13):1741–1750. doi: 10.1002/bjs.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor SC, Arsonnaud S, Czernielewski J. The Taylor Hyperpigmentation Scale: A new visual assessment tool for the evaluation of skin color and pigmentation. J. Am. Acad. Dermatol. 2005;52(3):P170. [PubMed] [Google Scholar]
- 38.Harish V, Li Z, Maitz PKM. The optimal timing of outpatient Biobrane application for superficial and mid dermal partial thickness burns: Evidence for the '12-hour rule'. Burns. 2019;45(4):936–941. doi: 10.1016/j.burns.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Ter Horst B, Chouhan G, Moiemen NS, Grover LM. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018;123:18–32. doi: 10.1016/j.addr.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salibian AA, Rosario ATD, Severo LAM, Nguyen L, Banyard DA, Toranto JD, et al. Current concepts on burn wound conversion-A review of recent advances in understanding the secondary progressions of burns. Burns. 2016;42(5):1025–1035. doi: 10.1016/j.burns.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.JanŽEkoviČ Z. A new concept in the early excision and immediate grafting of burns. J. Trauma Injury Infect. Crit. Care. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 42.Brown EA, De Young A, Kimble R, Kenardy J. Impact of parental acute psychological distress on young child pain-related behavior through differences in parenting behavior during pediatric burn wound care. J. Clin. Psychol. Med. Settings. 2019;26(4):516–529. doi: 10.1007/s10880-018-9596-1. [DOI] [PubMed] [Google Scholar]
- 43.Brown EA, Egberts M, Wardhani R, De Young A, Kimble R, Griffin B, et al. Parent and clinician communication during paediatric burn wound care: A qualitative study. J. Pediatr. Nursing Nursing Care Children Families. 2020;55:147–154. doi: 10.1016/j.pedn.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Heer IJ, Tiemeier H, Hoeks SE, Weber F. Intelligence quotient scores at the age of 6 years in children anaesthetised before the age of 5 years. Anaesthesia. 2017;72(1):57–62. doi: 10.1111/anae.13687. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblatt A, Kremer M, Swanson B, Shah R. Anesthesia exposure in the young child and long-term cognition: An integrated review. AANA J. 2019;87(3):231–242. [PubMed] [Google Scholar]
- 46.Shukla A, Chowdhary V. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. Acta Paediatr. 2019;108(11):2115–2116. doi: 10.1111/apa.14943. [DOI] [PubMed] [Google Scholar]
- 47.Sun LS, Li G, Miller TLK, Salorio C, Byrne MW, Bellinger DC, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA J. Am. Med. Assoc. 2016;315(21):2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 2018;129(1):89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Racine NM, Riddell RR, Khan M, Calic M, Taddio A, Tablon P. Systematic review: Predisposing, precipitating, perpetuating, and present factors predicting anticipatory distress to painful medical procedures in children. J. Pediatr. Psychol. 2016;41(2):159–181. doi: 10.1093/jpepsy/jsv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin BR, Frear CC, Babl F, Oakley E, Kimble RM. Cool running water first aid decreases skin grafting requirements in pediatric burns: A cohort study of two thousand four hundred ninety-five children. Ann. Emerg. Med. 2020;75(1):75–85. doi: 10.1016/j.annemergmed.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Wood FM, Phillips M, Jovic T, Cassidy JT, Cameron P, Edgar DW. Water first aid is beneficial in humans post-burn: Evidence from a bi-national cohort study. PLoS ONE. 2016;11(1):e0147259. doi: 10.1371/journal.pone.0147259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holbert MD, Kimble RM, Jones LV, Ahmed SH, Griffin BR. Risk factors associated with higher pain levels among pediatric burn patients: A retrospective cohort study. Reg. Anesth. Pain Med. 2021;46(3):222. doi: 10.1136/rapm-2020-101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee Kee E, Stockton K, Kimble RM, Cuttle L, McPhail SM. Cost-effectiveness of silver dressings for paediatric partial thickness burns: An economic evaluation from a randomized controlled trial. Burns. 2017;43(4):724–732. doi: 10.1016/j.burns.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Frear CC, Griffin BR, Cuttle L, Kimble RM, McPhail SM. Cost-effectiveness of adjunctive negative pressure wound therapy in paediatric burn care: evidence from the SONATA in C randomised controlled trial. Sci. Rep. 2021;11(1):16650. doi: 10.1038/s41598-021-95893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.