Abstract
Sleep health is an important factor across several physical and mental health disorders, and a growing scientific consensus has identified sleep as a critical component of opioid use disorder (OUD), both in the active disease state and during OUD recovery. The goal of this narrative review is to collate the literature on sleep, opioid use, and OUD as a means of identifying therapeutic targets to improve OUD treatment outcomes. Sleep disturbance is common and often severe in persons with OUD, especially during opioid withdrawal, but also in persons on opioid maintenance therapies. There is ample evidence that sleep disturbances including reduced total sleep time, disrupted sleep continuity, and poor sleep quality often accompany negative OUD treatment outcomes. Sleep disturbances are bidirectionally associated with several other factors related to negative treatment outcomes, including chronic stress, stress reactivity, low positive affect, high negative affect, chronic pain, and drug craving. This constellation of outcome variables represents a more comprehensive appraisal of the quality of life and quality of recovery than is typically assessed in OUD clinical trials. To date, there are very few clinical trials or experimental studies aimed at improving sleep health in OUD patients, either as a means of improving stress, affect, and craving outcomes, or as a potential mechanistic target to reduce opioid withdrawal and drug use behaviors. As such, the direct impact of sleep improvement in OUD patients is largely unknown, yet mechanistic and clinical research suggests that therapeutic interventions that target sleep are a promising avenue to improve OUD treatment.
Keywords: opioid use disorder, sleep, sleep disturbance, treatment, circadian
The opioid crisis continues to devastate the lives of numerous individuals and communities across the United States. More than 72,000 Americans died from a drug overdose in 2017, the majority of which were opioid-related (National Center for Health Statistics, 2018). Chronic relapse is one of the defining features of OUD (Goldstein & Volkow, 2002; Volkow & Li, 2004), and is driven in part by stress and negative reinforcement associated with opioid withdrawal (Dunn et al., 2020; Koob, 2013; Koob & Volkow, 2010). To address the high incidence of negative treatment outcomes for opioid use disorder (OUD), public health officials are advocating for the utilization of medications for OUD (MOUD) as a frontline approach to address the ongoing opioid crisis (Collins et al., 2018; Volkow et al., 2014), including the full mu-opioid receptor agonist, methadone (Sees et al., 2000; Strain et al., 1993), the partial mu-opioid agonist/antagonist, buprenorphine (Kraus et al., 2011), and the long-acting mu-opioid receptor antagonist, extended-release naltrexone (XR-NTX; Krupitsky et al., 2011). These MOUDs are gold-standard treatments that confer significant benefits to OUD patients, including decreased illicit drug use, decreased treatment attrition, reduced disease transmission, and improved social functioning (Volkow et al., 2014). Despite the clinical utility of MOUDs, many persons with OUD do not receive MOUDs as part of their treatment (Dunn et al., 2019; Huhn, Hobelmann, et al., 2020). Persons in OUD recovery can broadly be characterized by their MOUD status (e.g., maintenance on an MOUD, or no MOUD), and while persons utilizing MOUDs are more likely to evidence reduced relapse and improved adherence outcomes relative to those on no MOUD, all forms of OUD treatment could and should be improved (Connery, 2015). Novel medication and behavioral strategies are needed to optimize OUD treatment and improve treatment outcomes. One strategy to consider in this effort is to identify and target the core neurobiological systems and behavioral manifestations of OUD that promote relapse and diminish the quality of life. In the present review, we focus on sleep as a potential mechanistic target to improve OUD treatment outcomes.
Over the past decade, a growing scientific consensus has identified sleep as a critical component of OUD development, maintenance, and treatment. A recent FDA public meeting that included patients with OUD identified sleep disturbance as a primary contributor to relapse and treatment attrition (Food and Drug Administration, 2018). Subsequently, the NIH committed nearly $25 million to fund a research program titled “Sleep Dysfunction as a Core Feature of Opioid Use Disorder and Recovery” as part of the Helping End Addiction Long-Term (HEAL) Initiative. Sleep disturbance is common and often severe in persons with OUD (Eacret et al., 2020), and is a core symptom of several mental health conditions that have high co-occurrence with OUD such as major depressive disorder (Tsuno et al., 2005). Sleep has a bidirectional relationship with emotional processes known to affect OUD treatment outcomes including low positive and high negative affect (Minkel et al., 2012; Ong et al., 2017). Furthermore, there is a well-established link between sleep and stress, and both high chronic stress and stress reactivity are associated with negative OUD treatment outcomes (Koob, 2008; Sinha, 2007, 2008; Van Bockstaele et al., 2010). Despite these links, sleep disturbance is not a primary consideration in most OUD treatment programs, and is typically a secondary or tertiary outcome in OUD research, if it is assessed at all. The omission of sleep assessments in OUD treatment research is just one aspect of a larger issue, namely an inconsistent metric of OUD “recovery,” and a vague understanding of what “recovery” means to individuals with OUD. In this review, we use the term recovery to denote improvements in mental health, physical health, and quality of life, with an understanding that there are individual differences in patient-driven endpoints regarding lifestyle, social functioning, and the desire to reduce or eliminate drug use (Hay et al., 2019; Witkiewitz et al., 2020). Extant literature on sleep and OUD is complicated by a limited understanding of how the mu-opioid receptor system, in the presence of either MOUDs (mu-opioid receptor full agonist, partial agonist, and antagonist), or no MOUD, may influence sleep in recovery. Further complicating sleep research in persons with OUD are mechanisms contributing to sleep disturbance (a) during active opioid use, (b) during opioid withdrawal, (c) at different time points in recovery and/or in persons with different definitions of their own recovery endpoints, and (d) in the context of dysregulated circadian rhythms of stress.
This narrative review will highlight mechanisms by which opioids disrupt sleep, the relationship between sleep and emotional processes in persons with OUD, and the potential role of sleep improvement in promoting long-term, healthy recovery in persons in OUD treatment. The goal of this narrative review is to collate the evidence addressing sleep disturbance in persons with OUD and provide an actionable research agenda to evaluate the hypothesis that behavioral and pharmacological interventions targeting sleep disturbance might improve OUD treatment outcomes.
Circadian Rhythms That Influence Both Sleep and Opioid Use Disorder
Circadian rhythms are biological processes that follow a ~24-hr pattern, and circadian rhythms of stress hormones and several neurotransmitter systems regulate the sleep–wake cycle. The suprachiasmatic nucleus of the hypothalamus is the master clock of the sleep–wake cycle and nearly all neurons in the suprachiasmatic nucleus produce the neurotransmitter, γ-aminobutyric acid (GABA; Moore & Speh, 1993). GABA projections to the locus coeruleus inhibit noradrenergic neurotransmission and are partially responsible for the onset of sleep and the progression of sleep architecture (Gervasoni et al., 1998). GABA receptor agonists are generally sedating and are often used to treat acute sleep disturbance and anxiety (Gottesmann, 2002), and preclinical models of OUD have demonstrated that GABA neurotransmission in the central amygdala complex and basal ganglia portion of the cortico-basal gangliathalamocortical loop (i.e., the neural reward system) is associated with opioid reinforcement, tolerance, and withdrawal (Kallupi et al., 2020; Kang-Park et al., 2015; Matsui et al., 2014; Xi & Stein, 2002). Both opioid and GABA agonists shift diurnal patterns of activity in preclinical models (Vansteensel et al., 2003). Persons in OUD recovery often exhibit altered sleep–wake patterns and report sleep disturbances, and epidemiological studies suggest that many individuals with OUD co-use benzodiazepines, possibly to self-treat anxiety and insomnia symptoms that are common in this population (Hernandez et al., 2018; Hirschtritt et al., 2017; Jones et al., 2012). The role of GABA in the sleep–wake cycle is primarily related to sedation, yet circadian arousal and stress systems also play a key role in both sleep and OUD.
Orexin (a.k.a. hypocretin) producing neurons are primarily localized in the lateral hypothalamus and perifornical area of the brain; however, orexinergic neurons project to several subcortical/brainstem regions that are responsible for regulating wakefulness/arousal, diurnal neuroendocrine stress signaling, food, drink (and consequently drug) consumption, and mood (Date et al., 1999; Sakurai et al., 1998). Orexin signaling follows a circadian rhythm whereby increased orexin signaling activates the wake phase relative to the rest/sleep phase (Baumann & Bassetti, 2005). Within the sleep phase, decreased orexin signaling is essential for the onset of rapid-eye-movement (REM) sleep (Kantor et al., 2009). In addition, antagonizing the orexin system improves sleep continuity by modulating circadian rhythms of the sleep/wake cycle as opposed to causing sedation (Scammell & Saper, 2007). There is robust evidence from preclinical models of OUD demonstrating that increased orexin signaling contributes to arousal and stress reactivity in substance use disorders including OUD, and orexin receptor antagonists attenuate opioid withdrawal symptoms (Harris & Aston-Jones, 2006; Harris et al., 2005; Martin-Fardon et al., 2010). Stress reactivity is one of the neurobiological hallmarks of OUD (Koob, 2008; Koob et al., 2014; Koob & Volkow, 2010), and stress/sleep interactions are a critical intermediate target that might bidirectionally affect OUD behaviors. Furthermore, hyperarousal is a hallmark of insomnia (Riemann et al., 2010), a sleep disorder that is common in OUD and characterized by difficulty initiating/maintaining sleep with accompanying daytime impairments.
Orexinergic projections from the lateral hypothalamus directly and indirectly activate neuroendocrine stress signaling via the hypothalamic–pituitary–adrenal axis (HPA-axis; Al-Barazanji et al., 2001; Kuru et al., 2000; Russell et al., 2001), which also influences diurnal rhythms of the sleep/wake cycle, including the timing, continuity, and duration of sleep relative to the daily light/dark cycle (Kok et al., 2002; Saper et al., 2005). The HPA-axis becomes dysregulated during the course of OUD, which is evident in recovery; for example, persons inducted to methadone display decreased systemic corticotropin-releasing hormone and cortisol levels relative to healthy controls, and persons in opioid withdrawal display decreased corticotropin-releasing hormone but increased cortisol relative to healthy controls (Zhang et al., 2008). Thus, it is likely that chronic opioid exposure is associated with HPA-axis dysfunction, and that opioid withdrawal increases the adrenal stress response. In addition, stress signaling via the neurotransmitter corticotropin-releasing factor directly activates orexinergic neurons (Winsky-Sommerer et al., 2004), thereby exacerbating stress response and promoting hyperarousal consistent with drug craving (Boutrel et al., 2005). It is not clear how circadian rhythms of stress via neuroendocrine and neurotransmitter signaling might reregulate over time in recovery, but these biological stress signals are interrelated and their dysregulation provides a mechanistic target for sleep disturbances in persons with OUD.
Opioid Use and Objective Measures of Sleep
In general, sleep can be assessed using several metrics, including sleep quantity (e.g., total sleep time), sleep continuity (e.g., sleep onset latency, wake after sleep onset, and sleep efficiency), sleep architecture (e.g., the duration and percent of time spent in each stage of sleep: N1–3, and rapid-eye movement [REM] sleep), sleep quality (e.g., self-reported restfulness), and events that fragment sleep, such as sleep apnea or restless legs (Crivello et al., 2019; Fell et al., 1996; Terrill et al., 2015). Disturbances of any of these parameters can result in bidirectional and feedforward decrements to mental and physical health (Atkinson & Davenne, 2007; Freeman et al., 2017). Acute doses of opioids disrupt sleep continuity in animal (Cronin et al., 1995; Gauthier et al., 2011; Moreton et al., 1976; Watson et al., 2007) and human studies (Dimsdale et al., 2007; Howe et al., 1980; Kay, 1975a; Kay et al., 1969). For example, acute opioid administration reduces neural activity and GABA neurotransmission in the pontine reticular formation, an area of the brainstem involved in sleep and eye movement (Cronin et al., 1995; Watson et al., 2007), and disrupts sleep architecture by reducing REM sleep in rodent models (Gauthier et al., 2011). In humans, acute opioid administration also disrupts sleep architecture by reducing time spent in stage N3, also referred to as slow-wave sleep (Dimsdale et al., 2007), although acute opioid use may increase total sleep time for persons with acute pain (Cutrufello et al., 2020).
Chronic opioid exposure causes persistent sleep disturbance in morphine and/or methadone-maintained animals (Pačesová et al., 2016; Robert et al., 1999; Young et al., 1975), and methadone and/or buprenorphine-maintained humans (Dunn et al., 2018; Kay, 1975b). Interactions between the orexin and opioid systems suggest that orexinergic signaling could be partially responsible for sleep disturbance in persons with OUD. Preclinical studies have demonstrated that orexinergic neurons express mu-opioid receptors (Georgescu et al., 2003), and orexin mRNA levels increase during opioid withdrawal (Laorden et al., 2012; Zhou et al., 2006). Further, a study examining postmortem human brains found that persons who engage in long-term heroin use had 54% more orexinergic neurons in the lateral hypothalamus than age/sex-matched controls, suggesting that chronic opioid exposure is associated with increased orexin signaling (Thannickal et al., 2018). There is also evidence from human research that dysregulation of the HPA-axis, especially increased cortisol signaling during opioid withdrawal and postwithdrawal periods (Gerra et al., 2003; Shi et al., 2009), might be partially responsible for reductions in total sleep time in early recovery from OUD (Bunce et al., 2015). Moreover, a pronounced and protracted rebound in REM sleep patterns has also been observed following opioid withdrawal in animals (Colasanti et al., 1975; Khazan & Colasanti, 1972) and humans (Mehtry et al., 2014). Taken together, these studies suggest that sleep disturbance is prevalent both during active OUD and during opioid withdrawal and postwithdrawal periods.
Further evidence of the relationship between chronic opioid use and sleep disturbance comes from studies of persons utilizing opioid maintenance therapies (OMT). Methadone-maintained patients display reduced total sleep time and disrupted sleep architecture when compared with healthy controls and/or national norms (Sharkey et al., 2009, 2011; Xiao et al., 2010). Persons utilizing methadone or buprenorphine maintenance therapies might also overreport their sleep quantity and continuity, as evidenced by a study using portable electroencephalography (EEG) in the natural environment; in this study, objective measures of sleep revealed shorter total sleep time and more sleep disruptions (e.g., wake after sleep onset) than self-report assessments (Finan et al., 2020). Interestingly, this study did not find significant differences in objective measures of sleep between buprenorphine and methadone-maintained patients. Another study using portable polysomnography in methadone-maintained patients found generally high concordance between objective and subjective measures for a single-night sleep study (Sharkey et al., 2011). In general, there is a paucity of studies that compare objective measures of sleep such as EEG or full polysomnography (primary objective measures) or actigraphy (secondary objective measure) in persons on different MOUDs, including a notable gap in the literature of studies objectively comparing OMT to XR-NTX. Regardless, objective measures of sleep within persons undergoing opioid withdrawal, postwithdrawal, and opioid maintenance conditions suggest that sleep disturbance is a prevalent and persistent issue in OUD recovery.
Opioid Use Disorder and Sleep Quality
Self-reported sleep quality is an important outcome in sleep research that can supplement objective measures or act as a stand-alone assessment of sleep during OUD recovery. Persons with OUD undergoing supervised withdrawal report significant sleep disturbances, including increased sleep onset latency, reduced total sleep time, and poor sleep quality (Oyefeso et al., 1997), and reduced postwithdrawal sleep quality is associated with increased drug craving (Lydon-Staley et al., 2017). Many OUD patients report substantial sleep disturbances upon entering OMT (Hartwell et al., 2014; Nordmann et al., 2016; Peles et al., 2006, 2011; Stein et al., 2004), and continue to report sleep disturbances and poor sleep quality during treatment (Dunn et al., 2018). Likewise, drug use is associated with later bedtimes and reduced self-reported and objectively measured total sleep time in persons in OMT (Bertz et al., 2019).
There have been few direct comparisons of sleep quality across MOUDs. One study found that individuals maintained on oral naltrexone (for ~10 months) had better sleep relative to individuals maintained on methadone (Staedt et al., 1996), and a recent secondary analysis of a randomized clinical trial found that persons on XR-NTX endorsed persistent insomnia at a lower rate than persons on oral buprenorphine (Latif et al., 2019); however, this study relied on once monthly, retrospective self-reports of sleep disturbance and there is a pressing need to further our understanding of the trajectory and consequence of sleep disturbance in OUD patients who are using different forms of MOUDs.
Opioid Use and Sleep Apnea
In humans, chronic opioid use (Schwarzer et al., 2015), either for pain (Mogri et al., 2009) or as an OMT (Charpentier et al., 2010; DeVido et al., 2015; Farney et al., 2013), is associated with the development of hypoxemia and sleep-disordered breathing. There are numerous mechanisms by which opioids disrupt breathing during sleep, the detailed exposition of which is beyond the scope of this article and has been thoroughly addressed in other reviews (Van Ryswyk & Antic, 2016; Webster et al., 2008). In brief, one of the primary mechanisms of opioid-related sleep-disordered breathing involves opioid receptors that are distributed throughout the parabrachial complex. The parabrachial complex controls the breathing rate and helps maintain upper airway muscle tone via sleep-related chemosensory regulation (Damasceno et al., 2014). Exogenous opioids modulate parabrachial complex function during sleep, both by binding directly to mu-opioid receptors and interacting with chemosensory neurons (Cutrufello et al., 2020). Importantly, chemoreceptors, located on the dendrites of chemosensory neurons within the carotid body (Wicher & Marion-Poll, 2018), are integral to the detection of hypoxia and altered breathing during NREM sleep (Skatrud & Dempsey, 1983). Chronic opioid use desensitizes the chemoreceptor function within this system during sleep, leading to both central sleep apneas and obstructive apneas via upper airway occlusion (Teichtahl et al., 2005; Wang et al., 2005).
In a study of patients on methadone maintenance therapy, neither central sleep apnea nor obstructive sleep apnea were associated with differences in subjective sleep quality (Sharkey et al., 2010). However, sleep apnea is globally associated with increased risk of stroke (Redline et al., 2010; Yaggi et al., 2005) and cardiovascular disease (Kendzerska et al., 2014) and, on a day-to-day level, leads to excessive daytime sleepiness by increasing nocturnal arousals and fragmenting sleep, even in the absence of frank awakenings (Guilleminault et al., 1988). Increased daytime sleepiness is associated with an array of daytime cognitive impairments (Lal et al., 2012; Ohayon & Vecchierini, 2002). Both obstructive and central sleep apnea can be effectively treated with continuous positive airway pressure (Aurora et al., 2012; Ballester et al., 1999; Montserrat et al., 2001). Limited studies among patients on long-term opioid therapy for chronic pain (Jaoude et al., 2016; Kendzerska et al., 2020; Wasef et al., 2020) and OMT (Troitino et al., 2014) have demonstrated poor patient adherence and reluctance to initiate continuous positive airway pressure treatment. However, these studies have been small and/or reliant on retrospective chart review, so there is a significant need for additional research in this domain.
Sleep, Stress, and Emotional Processes in Persons With Opioid Use Disorder
There is a clear link between stress and the onset (Briand & Blendy, 2010; Koob et al., 2014; Meier et al., 2014; Mills et al., 2007; Sinha, 2008), and progression (Briand & Blendy, 2010; Goeders, 2003; Koob, 2008; Kreek et al., 2005; Sinha, 2008) of OUD, and both high tonic stress and stress reactivity contribute to relapse risk in persons in early recovery (Koob, 2008; Sinha, 2007). In animal models, acute opioid exposure reduces HPA-axis-mediated stress response (Koob, 2008), partially through inhibition of the locus coeruleus (Aghajanian & Wang, 1987; Korf et al., 1974; Van Bockstaele et al., 2010), and animal models of chronic opioid exposure demonstrate that stress reactivity is partially mediated by the HPA-axis (Covington & Miczek, 2001; Xu et al., 2004). Stress reactivity may lead to bouts of sleep disturbance in humans, and explain, in part, the persistent sleep disturbances observed in persons in OUD treatment. Persons who use opioids report that sleep disturbance and anxiety symptoms motivate their continued opioid use (Barth et al., 2013; Burke et al., 2008). Longitudinal studies are needed to evaluate the critical transition from opioid misuse to progressively more severe OUD in order to evaluate the degree to which the sleep/stress interaction drives relapse risk trajectories over time.
In healthy adults, mu-opioid receptor polymorphisms that are associated with opioid misuse are also associated with increased activity of the HPA-axis in response to the mu-opioid receptor antagonist, naloxone (Chong et al., 2006). Persons receiving oral naltrexone for OUD display considerable variability in diurnal systemic cortisol during treatment (Kosten et al., 1986), and may be vulnerable to stress and drug-cue induced craving (Hyman et al., 2007); the studies of stress response during oral naltrexone treatment have been performed in small samples and there is no published evidence of these effects in persons on XR-NTX.
Studies using ecological momentary assessments have also demonstrated that momentary levels of stress and related psychophysiological states such as low positive affect, high negative affect, and pain are associated with opioid craving (Huhn et al., 2016; Mun et al., 2020) and that low positive affect, or anhedonia, partially mediates the relationship between sleep quality and opioid craving in postwithdrawal OUD patients (Lydon-Staley et al., 2017). These studies have focused on within-day and/or point prevalence associations of psychological states and drug use behavior, for example, whether stress is associated with craving at the moment. Studies employing intensive biological measures of HPA-axis function demonstrate that momentary observations of stress in patients with OUD likely adhere to a diurnal rhythm. For example, persons on long-term methadone maintenance display circadian rhythms of HPA-axis measures (including cortisol) that are either blunted (Zhang et al., 2008) or similar to healthy controls (Kreek et al., 1983), and the acute agonist effects of methadone may blunt HPA-axis response to experimental and natural stress (Kreek et al., 1984; Mendelson et al., 1975; Renault et al., 1972; Walter et al., 2008). Methadone maintenance treatment is effective in persons with co-occurring OUD and posttraumatic stress disorder (Trafton et al., 2006), suggesting that it may stabilize the HPA-axis response to stressful events. However, sleep disturbance remains a major issue in patients maintained on both methadone and buprenorphine (Brady, 2001; Dunn et al., 2018; Finan et al., 2020) and drug craving is associated with sleep disturbance during the early stages of opioid abstinence (Fathi et al., 2020).
Drug use and craving are also associated with depressive symptoms, negative affect, and anhedonia in methadone-maintained patients (Epstein et al., 2009; Huhn et al., 2019; Huhn, Brooner, et al., 2020; Mun et al., 2020). Given that sleep disturbance is a symptom of depressive disorders (Tsuno et al., 2005), it is likely that there is a link among sleep, psychiatric symptoms, and treatment outcomes in this population. Indeed, sleep problems have been independently associated with increased psychiatric distress and decreased daily functioning in methadone patients (Burke et al., 2008; Gros et al., 2013), further supporting the treatment of sleep as a target to improve treatment outcomes in persons with OUD. Taken together, the evidence suggests that diligent tracking of sleep, stress, affect, and craving in OUD patients during early abstinence and initiation on OMT could assist in clinical decision-making including the intensity of behavioral treatment.
Discussion: Clinical Implications of Sleep Disturbance in Persons With Opioid Use Disorder
Sleep disturbance is commonly reported by individuals who actively use opioids and those in OUD recovery. Evidence demonstrates that sleep is a major issue both during opioid withdrawal and while being maintained on MOUDs (Finan et al., 2020; Mehtry et al., 2014), yet there is very little controlled research aimed at improving sleep in these populations. This is in contrast to similar conditions such as alcohol use disorder, where several clinical trials have examined the effect of medications for alcohol use disorder on sleep and the relationship between sleep and relapse outcomes (Fortuna et al., 2018; Panin & Peana, 2019). Sleep is an important outcome domain in OUD research, not only because it is intertwined with the quality of life, but also because it is mechanistically linked to the proximal drivers of opioid use, including stress, affect, and craving (Figure 1). Thus, sleep improvement is a logical target to improve the model of care for persons with OUD.
Figure 1. Bidirectional Associations That Decrease Quality of Life in Recovery and Lead to Opioid Relapse.
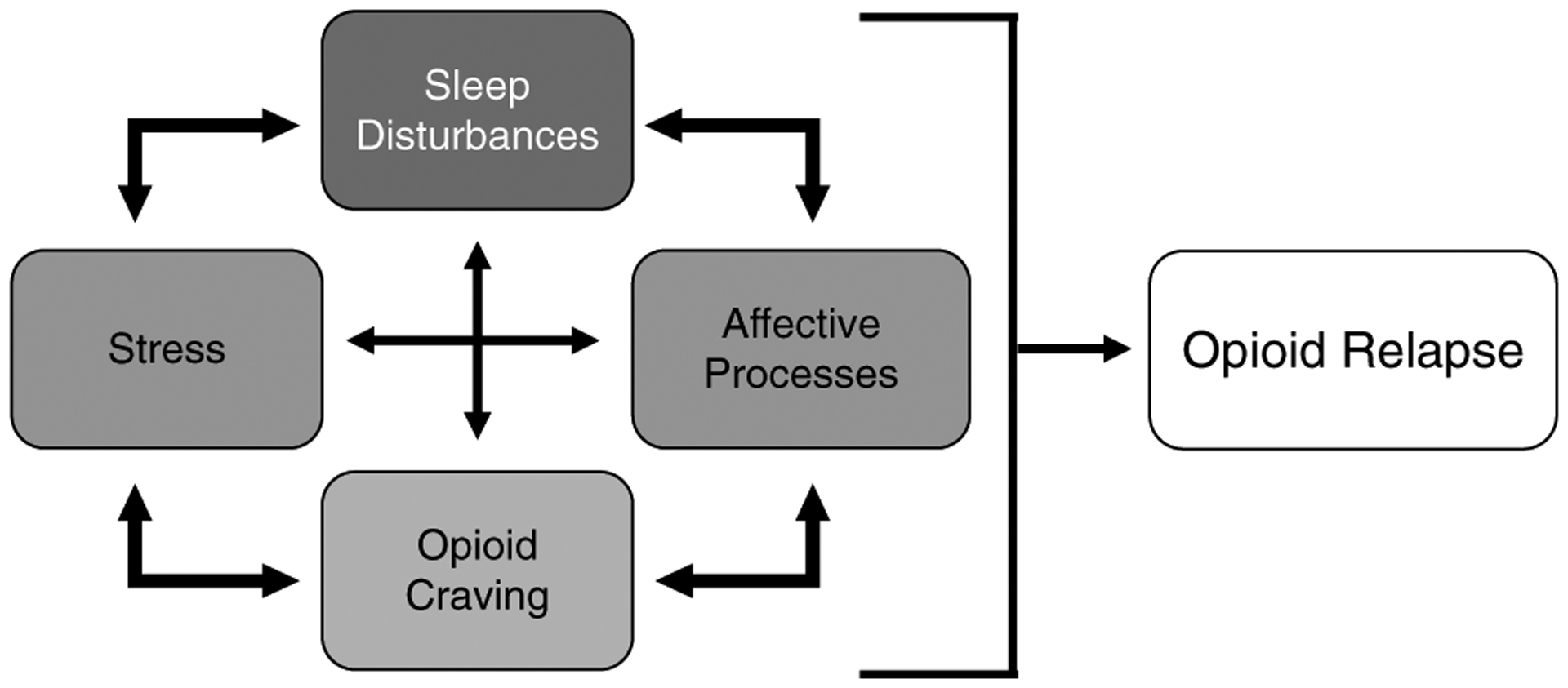
Note. All of these factors can increase the risk for relapse at any given time (i.e., state changes), and may be part of a persistent dysregulation of diurnal rhythms that decreases the likelihood of successful, long-term recovery.
* Affective processes refer to high negative and low positive affect.
We know of only four studies that have empirically examined methods for improving sleep in OUD patients. The first was a controlled trial of electrostimulation versus acute doses of methadone in persons with OUD who were undergoing supervised opioid withdrawal; the results of that study demonstrated that participants in the methadone group had greater total sleep time relative to individuals receiving electrostimulation, but neither group demonstrated adequate sleep during the study (Gossop & Bradley, 1984). The second study compared a 50–150 mg self-titrated dose of trazodone with placebo during methadone maintenance and demonstrated that while there was some initial improvement in total sleep time in the trazadone group, overall there were no significant group differences in sleep quantity or quality (Stein et al., 2012). A more recent study compared 30 mg mirtazapine, 10 mg zolpidem, 30 mg mirtazapine + 10 mg zolpidem, and placebo using a within-subjects, cross-over design in persons maintained on methadone; this study reported a significant improvement in sleep onset latency and total sleep time in the 30 mg mirtazapine group, which surpassed other treatment groups (Stein et al., 2020). Overall, mirtazapine improved sleep onset latency and total sleep time by ~23 min compared with placebo, which is clinically significant, but this pilot study had a relatively small sample size (N = 10), and larger trials are necessary to fully assess the efficacy of mirtazapine in OUD patients with insomnia. Finally, a between-subject, 12-week randomized-controlled trial of 10 mg melatonin versus placebo in methadone-maintained patients (N = 54) demonstrated that melatonin improves scores on the Pittsburgh Sleep Quality Index and depressive symptoms on the Beck Depressive Inventory relative to placebo (Ghaderi et al., 2019). These results are promising yet the trial had a small-to-moderate sample size and did not assess sleep quantity with objective measures. Nonetheless, the results of the study suggest that melatonin might be useful in addressing symptoms of insomnia in methadone-maintained patients.
Identifying Therapeutic Targets to Improve Sleep in Opioid Use Disorder Patients
There are several possible avenues for future treatment development targeting sleep in OUD populations. The results of the recent mirtazapine/zolpidem trial are important in (a) identifying mirtazapine as a potential medication to improve sleep in methadone-maintained patients and (b) demonstrating that zolpidem might not be optimal in this population despite its widespread use for insomnia (Stein et al., 2020). In general, the abuse liability of benzodiazepines and “Z” drugs such as zolpidem have precluded their adoption as sleep medications in persons seeking OUD treatment, especially given the potential for respiratory depression in coadministration of benzodiazepines and opioids (which is especially relevant for persons on OMT) (Jones & McAninch, 2015). Thus, while circadian rhythms of GABA signaling are relevant to both OUD and sleep disturbance (Vansteensel et al., 2003), GABA receptor agonists are unlikely to be widely used to improve sleep in this population.
Targeting the orexin neurotransmitter system is another promising approach for treating sleep disturbance in persons with OUD (Dunn et al., 2019; James et al., 2020; Zarrabian et al., 2020). Suvorexant has recently garnered attention as a potential treatment for sleep disturbance in OUD patients (James et al., 2020); suvorexant is a dual-orexin receptor antagonist (DORA) that is effective in improving sleep duration and continuity while also demonstrating a favorable safety profile in long-term (1 year) studies (Herring et al., 2012, 2016). Another DORA that has a higher affinity for the OX2 receptor, lemborexant, has also been recently approved by the FDA for the treatment of insomnia; lemborexant is also effective in improving sleep duration and continuity and has shown efficacy in older adults with insomnia (Murphy et al., 2017; Rosenberg et al., 2019). Given preclinical evidence that orexin receptor antagonists reduce opioid withdrawal and drug-seeking (Harris & Aston-Jones, 2006; Harris et al., 2005), these DORAs might improve other OUD outcomes beyond sleep disturbance. Still, both suvorexant and lemborexant are schedule IV controlled substances and should be rigorously evaluated in clinical OUD populations before widespread use in OUD treatment.
Cognitive-behavioral therapy for insomnia (CBT-I) is another frontline treatment with high efficacy for reducing insomnia symptoms, consolidating sleep, and extending sleep time (Mitchell et al., 2012; Morin et al., 2006; Okajima et al., 2011). We are not aware of any studies that have evaluated CBT-I for patients with OUD. However, several studies have demonstrated that CBT-I is effective in reducing insomnia symptoms in patients with insomnia and comorbid alcohol use disorder (Arnedt et al., 2011; Chakravorty et al., 2019). In studies to date, CBT-I has been more effective than pharmacotherapy in improving sleep quality among people with alcohol use disorder (Miller et al., 2017). However, alcohol use behavior is not substantially altered by CBT-I (Miller et al., 2017). There have been several randomized clinical trials of CBT-I in patients with insomnia and comorbid chronic pain, with results consistently showing robust improvements in insomnia symptoms (for reviews, see Finan et al., 2014; Koffel et al., 2015). Although CBT-I has been effective in improving sleep among patients with chronic pain, effects on pain have been less consistent (Finan et al., 2014). Together, these studies demonstrate that CBT-I is effective for insomnia in patients with comorbid disorders that profoundly influence behavior and function, suggesting that there is a rationale to evaluate CBT-I in patients with comorbid insomnia and OUD. However, based on studies in other comorbid disorders, it does not appear likely that CBT-I as a stand-alone treatment would substantially improve drug use outcomes. Also, it is not clear whether ongoing opioid use, either illicit or provided via OMT, would influence the sleep-related outcomes of CBT-I. These questions should be taken up in pilot trials to inform a larger-scale randomized clinical trial.
Conclusion
Sleep disturbance is a common issue in persons with OUD and an understudied aspect of OUD recovery. There is ample evidence from preclinical and clinical research that sleep is related to opioid withdrawal, craving, and relapse behaviors. However, there are very few studies in OUD populations that target sleep to improve these outcomes. Nonetheless, sleep health is part of a constellation of mental and physical health outcomes that should be the target of future research on OUD recovery, not only because it might improve “primary” OUD outcomes such as opioid withdrawal and relapse, but also because sleep disturbance is commonly reported by OUD patients as a persistent problem, and because the field should, in general, move beyond simply defining OUD treatment outcomes by drug use behaviors, and shift toward improving the overall health and well-being of persons in OUD recovery.
Public Significance Statement.
In light of the ongoing opioid crisis, there is a pressing need to improve treatment outcomes for opioid use disorder. Sleep disturbance is a common complaint among persons with opioid use disorder, and interventions that target sleep in this population could improve stress, affect, craving, and/or relapse outcomes.
Acknowledgments
Funding for this work was provided by the following: The National Center for Complementary and Integrative Health R61 AT010134 02 (Patrick H. Finan), the National Institute on Drug Abuse R01 DA048206 02 (Patrick H. Finan), and the National Heart, Lung and Blood Institute U01 HL150835 01 (Andrew S. Huhn/Patrick H. Finan).
Both Andrew S. Huhn and Patrick H. Finan were responsible for conceptualization, funding acquisition, investigation, project administration, supervision, writing of the original draft, reviewing, and editing.
Andrew S. Huhn receives research funding from Ashley Addiction Treatment through his university. Patrick H. Finan is on the Scientific Advisory Board of Ninnion Therapeutics.
References
- Aghajanian GK, & Wang YY (1987). Common α 2- and opiate effector mechanisms in the locus coeruleus: Intracellular studies in brain slices. Neuropharmacology, 26(7B), 793–799. 10.1016/0028-3908(87)90054-2 [DOI] [PubMed] [Google Scholar]
- Al-Barazanji KA, Wilson S, Baker J, Jessop DS, & Harbuz MS (2001). Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. Journal of Neuroendocrinology, 13(5), 421–424. 10.1046/j.1365-2826.2001.00655.x [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Armitage R, & Brower KJ (2011). Cognitive-behavioral therapy for insomnia in alcohol dependent patients: A randomized controlled pilot trial. Behaviour Research and Therapy, 49(4), 227–233. 10.1016/j.brat.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson G, & Davenne D (2007). Relationships between sleep, physical activity and human health. Physiology & Behavior, 90(2–3), 229–235. 10.1016/j.physbeh.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, & Tracy SL (2012). The treatment of central sleep apnea syndromes in adults: Practice parameters with an evidence-based literature review and meta-analyses. Sleep (Basel), 35(1), 17–40. 10.5665/sleep.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester E, Badia JR, Hernández L, Carrasco E, de Pablo J, Fornas C, Rodriguez-Roisin R, & Montserrat JM (1999). Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. American Journal of Respiratory and Critical Care Medicine, 159(2), 495–501. 10.1164/ajrccm.159.2.9804061 [DOI] [PubMed] [Google Scholar]
- Barth KS, Maria MM, Lawson K, Shaftman S, Brady KT, & Back SE (2013). Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence. The American Journal on Addictions, 22(5), 486–491. 10.1111/j.1521-0391.2013.12038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, & Bassetti CL (2005). Hypocretins (orexins) and sleep-wake disorders. Lancet Neurology, 4(10), 673–682. 10.1016/S1474-4422(05)70196-4 [DOI] [PubMed] [Google Scholar]
- Bertz JW, Epstein DH, Reamer D, Kowalczyk WJ, Phillips KA, Kennedy AP, Jobes ML, Ward G, Piltnick BA, Figueiro MG, Rea MS, & Preston KL (2019). Sleep reductions associated with illicit opioid use and clinic-hour changes during opioid agonist treatment for opioid dependence: Measurement by electronic diary and actigraphy. Journal of Substance Abuse Treatment, 106, 43–57. 10.1016/j.jsat.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, & de Lecea L (2005). Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19168–19173. 10.1073/pnas.0507480102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT (2001). Comorbid posttraumatic stress disorder and substance use disorders. Psychiatric Annals, 31(5), 313–319. 10.3928/0048-5713-20010501-09 [DOI] [Google Scholar]
- Briand LA, & Blendy JA (2010). Molecular and genetic substrates linking stress and addiction. Brain Research, 1314, 219–234. 10.1016/j.brainres.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce SC, Harris JD, Bixler EO, Taylor M, Muelly E, Deneke E, Thompson KW, & Meyer RE (2015). Possible evidence for re-regulation of HPA axis and brain reward systems over time in treatment in prescription opioid-dependent patients. Journal of Addiction Medicine, 9(1), 53–60. 10.1097/ADM.0000000000000087 [DOI] [PubMed] [Google Scholar]
- Burke CK, Peirce JM, Kidorf MS, Neubauer D, Punjabi NM, Stoller KB, Hursh S, & Brooner RK (2008). Sleep problems reported by patients entering opioid agonist treatment. Journal of Substance Abuse Treatment, 35(3), 328–333. 10.1016/j.jsat.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Morales KH, Arnedt JT, Perlis ML, Oslin DW, Findley JC, & Kranzler HR (2019). Cognitive behavioral therapy for insomnia in alcohol-dependent veterans: A randomized, controlled pilot study. Alcoholism, Clinical and Experimental Research, 43(6), 1244–1253. 10.1111/acer.14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier A, Bisac S, Poirot I, Vignau J, & Cottencin O (2010). Sleep quality and apnea in stable methadone maintenance treatment. Substance Use & Misuse, 45(9), 1431–1434. 10.3109/10826081003682255 [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, & Wand GS (2006). The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology, 31(1), 204–211. 10.1038/sj.npp.1300856 [DOI] [PubMed] [Google Scholar]
- Colasanti B, Kirchman A, & Khazan N (1975). Changes in the electroencephalogram and REM sleep time during morphine abstinence in pellet-implanted rats. Research Communications in Chemical Pathology and Pharmacology, 12(1), 163–172. [PubMed] [Google Scholar]
- Collins FS, Koroshetz WJ, & Volkow ND (2018). Helping to end addiction over the long-term: The research plan for the NIH HEAL initiative. Journal of the American Medical Association, 320(2), 129–130. 10.1001/jama.2018.8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23(2), 63–75. 10.1097/HRP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- Covington HE III, & Miczek KA (2001). Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges.” Psychopharmacology, 158(4), 388–398. 10.1007/s002130100858 [DOI] [PubMed] [Google Scholar]
- Crivello A, Barsocchi P, Girolami M, & Palumbo F (2019). The meaning of sleep quality: A survey of available technologies. IEEE Access: Practical Innovations, Open Solutions, 7, 167374–167390. 10.1109/ACCESS.2019.2953835 [DOI] [Google Scholar]
- Cronin A, Keifer JC, Baghdoyan HA, & Lydic R (1995). Opioid inhibition of rapid eye movement sleep by a specific mu receptor agonist. British Journal of Anaesthesia, 74(2), 188–192. 10.1093/bja/74.2.188 [DOI] [PubMed] [Google Scholar]
- Cutrufello NJ, Ianus VD, & Rowley JA (2020). Opioids and sleep. Current Opinion in Pulmonary Medicine, 26(6), 634–641. 10.1097/MCP.0000000000000733 [DOI] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, & Moreira TS (2014). Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 307(1), R57–R67. 10.1152/ajpregu.00024.2014 [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, & Nakazato M (1999). Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proceedings of the National Academy of Sciences of the United States of America, 96(2), 748–753. 10.1073/pnas.96.2.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVido J, Connery H, & Hill KP (2015). Sleep-disordered breathing in patients with opioid use disorders in long-term maintenance on buprenorphine-naloxone: A case series. Journal of Opioid Management, 11(4), 363–366. 10.5055/jom.2015.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, & Wallace MS (2007). The effect of opioids on sleep architecture. Journal of Clinical Sleep Medicine, 3(1), 33–36. [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Andrew Tompkins D, & Strain EC (2018). Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addictive Behaviors, 76, 8–14. 10.1016/j.addbeh.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Huhn AS, Bergeria CL, Gipson CD, & Weerts EM (2019). Non-opioid neurotransmitter systems that contribute to the opioid withdrawal syndrome: A review of preclinical and human evidence. The Journal of Pharmacology and Experimental Therapeutics, 371(2), 422–452. 10.1124/jpet.119.258004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Huhn AS, & Strain EC (2019). Differential adoption of opioid agonist treatments in detoxification and outpatient settings. Journal of Substance Abuse Treatment, 107, 24–28. 10.1016/j.jsat.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Weerts EM, Huhn AS, Schroeder JR, Tompkins DA, Bigelow GE, & Strain EC (2020). Preliminary evidence of different and clinically meaningful opioid withdrawal phenotypes. Addiction Biology, 25(1), Article e12680. 10.1111/adb.12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacret D, Veasey SC, & Blendy JA (2020). Bidirectional relationship between opioids and disrupted sleep: Putative mechanisms. Molecular Pharmacology, 98(4), 445–453. 10.1124/mol.119.119107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, & Preston KL (2009). Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry, 66(1), 88–94. 10.1001/archgenpsychiatry.2008.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farney RJ, McDonald AM, Boyle KM, Snow GL, Nuttall RT, Coudreaut MF, Wander TJ, & Walker JM (2013). Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. The European Respiratory Journal, 42(2), 394–403. 10.1183/09031936.00120012 [DOI] [PubMed] [Google Scholar]
- Fathi H, Yoonessi A, Ardani AR, Majdzadeh R, & Rezaeitalab F (2020). Effects of abstinence from opioids on self-reported craving and sleep. Cogent Psychology, 7(1), Article 1713440. 10.1080/23311908.2020.1713440 [DOI] [Google Scholar]
- Fell J, Röschke J, Mann K, & Schäffner C (1996). Discrimination of sleep stages: A comparison between spectral and nonlinear EEG measures. Electroencephalography and Clinical Neurophysiology, 98(5), 401–410. 10.1016/0013-4694(96)95636-9 [DOI] [PubMed] [Google Scholar]
- Finan PH, Buenaver LF, Coryell VT, & Smith MT (2014). Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Medicine Clinics, 9(2), 261–274. 10.1016/j.jsmc.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Mun CJ, Epstein DH, Kowalczyk WJ, Phillips KA, Agage D, Smith MT, & Preston KL (2020). Multimodal assessment of sleep in men and women during treatment for opioid use disorder. Drug and Alcohol Dependence, 207, Article 107698. 10.1016/j.drugalcdep.2019.107698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. (2018). Public meeting on patient-focused drug development for chronic pain. https://www.fda.gov/drugs/news-events-human-drugs/public-meeting-patient-focused-drug-development-chronic-pain
- Fortuna LR, Cook B, Porche MV, Wang Y, Amaris AM, & Alegria M (2018). Sleep disturbance as a predictor of time to drug and alcohol use treatment in primary care. Sleep Medicine, 42, 31–37. 10.1016/j.sleep.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Sheaves B, Goodwin GM, Yu LM, Nickless A, Harrison PJ, Emsley R, Luik A, Foster RG, Wadekar V, Hinds C, Gumley A, Jones R, Lightman S, Jones S, Bentall R, Kinderman P, Rowse G, Brugha T, … Espie CA (2017). The effects of improving sleep on mental health (OASIS): A randomised controlled trial with mediation analysis. The Lancet. Psychiatry, 4(10), 749–758. 10.1016/S2215-0366(17)30328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier EA, Guzick SE, Brummett CM, Baghdoyan HA, & Lydic R (2011). Buprenorphine disrupts sleep and decreases adenosine concentrations in sleep-regulating brain regions of Sprague Dawley rat. Anesthesiology: The Journal of the American Society of Anesthesiologists, 115(4), 743–753. 10.1097/ALN.0b013e31822e9f85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, & DiLeone RJ (2003). Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(8), 3106–3111. 10.1523/JNEUROSCI.23-08-03106.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Ceresini S, Esposito A, Zaimovic A, Moi G, Bussandri M, Raggi MA, & Molina E (2003). Neuroendocrine and behavioural responses to opioid receptor-antagonist during heroin detoxification: Relationship with personality traits. International Clinical Psychopharmacology, 18(5), 261–269. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Soulière F, Chouvet G, & Luppi PH (1998). Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. The European Journal of Neuroscience, 10(3), 964–970. 10.1046/j.1460-9568.1998.00106.x [DOI] [PubMed] [Google Scholar]
- Ghaderi A, Banafshe HR, Mirhosseini N, Motmaen M, Mehrzad F, Bahmani F, Aghadavod E, Mansournia MA, Reiter RJ, Karimi MA, & Asemi Z (2019). The effects of melatonin supplementation on mental health, metabolic and genetic profiles in patients under methadone maintenance treatment. Addiction Biology, 24(4), 754–764. 10.1111/adb.12650 [DOI] [PubMed] [Google Scholar]
- Goeders NE (2003). The impact of stress on addiction. European Neuropsychopharmacology, 13(6), 435–441. 10.1016/j.euroneuro.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry, 159(10), 1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, & Bradley B (1984). Insomnia among addicts during supervised withdrawal from opiates: A comparison of oral methadone and electrostimulation. Drug and Alcohol Dependence, 13(2), 191–198. [DOI] [PubMed] [Google Scholar]
- Gottesmann C (2002). GABA mechanisms and sleep. Neuroscience, 111(2), 231–239. 10.1016/S0306-4522(02)00034-9 [DOI] [PubMed] [Google Scholar]
- Gros DF, Milanak ME, Brady KT, & Back SE (2013). Frequency and severity of comorbid mood and anxiety disorders in prescription opioid dependence. The American Journal on Addictions, 22(3), 261–265. 10.1111/j.1521-0391.2012.12008.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, & Nino-Murcia G (1988). Determinants of daytime sleepiness in obstructive sleep apnea. Chest, 94(1), 32–37. 10.1378/chest.94.1.32 [DOI] [PubMed] [Google Scholar]
- Harris GC, & Aston-Jones G (2006). Arousal and reward: A dichotomy in orexin function. Trends in Neurosciences, 29(10), 571–577. 10.1016/j.tins.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437(7058), 556–559. 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- Hartwell EE, Pfeifer JG, McCauley JL, Moran-Santa Maria M, & Back SE (2014). Sleep disturbances and pain among individuals with prescription opioid dependence. Addictive Behaviors, 39(10), 1537–1542. 10.1016/j.addbeh.2014.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay KR, Huhn AS, Tompkins DA, & Dunn KE (2019). Recovery goals and long-term treatment preference in persons who engage in nonmedical opioid use. Journal of Addiction Medicine, 13(4), 300–305. 10.1097/ADM.0000000000000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez I, He M, Brooks MM, & Zhang Y (2018). Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in medicare part D beneficiaries. JAMA Network Open, 1(2), Article e180919. 10.1001/jamanetworkopen.2018.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, Krystal AD, Walsh JK, Benca RM, Rosenberg R, Sangal RB, Budd K, Hutzelmann J, Leibensperger H, Froman S, Lines C, Roth T, & Michelson D (2016). Suvorexant in patients with insomnia: Results from two 3-month randomized controlled clinical trials. Biological Psychiatry, 79(2), 136–148. 10.1016/j.biopsych.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, Lines C, Roth T, & Michelson D (2012). Orexin receptor antagonism for treatment of insomnia A randomized clinical trial of suvorexant. Neurology, 79(23), 2265–2274. 10.1212/WNL.0b013e31827688ee [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Delucchi KL, & Olfson M (2017). Outpatient, combined use of opioid and benzodiazepine medications in the United States, 1993–2014. Preventive Medicine Reports, 9, 49–54. 10.1016/j.pmedr.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe RC, Hegge FW, & Phillips JL (1980). Acute heroin abstinence in man: I. Changes in behavior and sleep. Drug and Alcohol Dependence, 5(5), 341–356. 10.1016/0376-8716(80)90160-X [DOI] [PubMed] [Google Scholar]
- Huhn AS, Brooner RK, Sweeney MM, Antoine D, Hammond AS, Ayaz H, & Dunn KE (2020). The association of prefrontal cortex response during a natural reward cue-reactivity paradigm, anhedonia, and demoralization in persons maintained on methadone. Addictive Behaviors, 113, Article 106673. 10.1016/j.addbeh.2020.106673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Harris J, Cleveland HH, Lydon DM, Stankoski D, Cleveland MJ, Deneke E, & Bunce SC (2016). Ecological momentary assessment of affect and craving in patients in treatment for prescription opioid dependence. Brain Research Bulletin, 123, 94–101. 10.1016/j.brainresbull.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Hobelmann JG, Strickland JC, Oyler GA, Bergeria CL, Umbricht A, & Dunn KE (2020). Differences in availability and use of medications for opioid use disorder in residential treatment settings in the united states. JAMA Network Open, 3(2), Article e1920843. 10.1001/jamanetworkopen.2019.20843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn AS, Sweeney MM, Brooner RK, Kidorf MS, Tompkins DA, Ayaz H, & Dunn KE (2019). Prefrontal cortex response to drug cues, craving, and current depressive symptoms are associated with treatment outcomes in methadone-maintained patients. Neuropsychopharmacology, 44(4), 826–833. 10.1038/s41386-018-0252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, & Sinha R (2007). Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology, 15(2), 134–143. 10.1037/1064-1297.15.2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, & Aston-Jones G (2020). Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: Why sleep on this any longer? Neuropsychopharmacology, 45(5), 717–719. 10.1038/s41386-020-0619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaoude P, Lal A, Vermont L, Porhomayon J, & El-Solh AA (2016). Pain intensity and opioid utilization in response to CPAP therapy in veterans with obstructive sleep apnea on chronic opioid treatment. Journal of Clinical Sleep Medicine, 12(8), 1105–1111. 10.5664/jcsm.6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, & McAninch JK (2015). Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. American Journal of Preventive Medicine, 49(4), 493–501. 10.1016/j.amepre.2015.03.040 [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, & Comer SD (2012). Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug and Alcohol Dependence, 125(1–2), 8–18. 10.1016/j.drugalcdep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, George O, & de Guglielmo G (2020). Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proceedings of the National Academy of Sciences of the United States of America, 117(4), 2140–2148. 10.1073/pnas.1915143117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, & Moore SD (2015). Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. The Journal of Pharmacology and Experimental Therapeutics, 355(2), 206–211. 10.1124/jpet.115.225870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor S, Mochizuki T, Janisiewicz AM, Clark E, Nishino S, & Scammell TE (2009). Orexin neurons are necessary for the circadian control of REM sleep. Sleep, 32(9), 1127–1134. 10.1093/sleep/32.9.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DC (1975a). Human sleep and EEG through a cycle of methadone dependence. Electroencephalography and Clinical Neurophysiology, 38(1), 35–43. 10.1016/0013-4694(75)90208-4 [DOI] [PubMed] [Google Scholar]
- Kay DC (1975b). Human sleep during chronic morphine intoxication. Psychopharmacology, 44(2), 117–124. 10.1007/BF00420997 [DOI] [PubMed] [Google Scholar]
- Kay DC, Eisenstein RB, & Jasinski DR (1969). Morphine effects on human REM state, waking state and NREM sleep. Psychopharmacology, 14(5), 404–416. 10.1007/BF00403581 [DOI] [PubMed] [Google Scholar]
- Kendzerska T, Gershon AS, Hawker G, Leung RS, & Tomlinson G (2014). Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: A decade-long historical cohort study. PLOS Medicine, 11(2), Article e1001599. 10.1371/journal.pmed.1001599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzerska T, Gomes T, Gershon AS, Hogan M, McIsaac DI, Talarico R, McKim D, Sandoz J, Dales R, & Tanuseputro P (2020). Opioid use and initiation of positive airway pressure treatment in adults referred for sleep disorder assessment: An explanatory population-based study. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine, 4(3), 194–204. 10.1080/24745332.2019.1684856 [DOI] [Google Scholar]
- Khazan N, & Colasanti B (1972). Protracted rebound in rapid movement sleep time and electroencephalogram voltage output in morphine-dependent rats upon withdrawal. The Journal of Pharmacology and Experimental Therapeutics, 183(1), 23–30. [PubMed] [Google Scholar]
- Koffel EA, Koffel JB, & Gehrman PR (2015). A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Medicine Reviews, 19, 6–16. 10.1016/j.smrv.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frölich M, & Pijl H (2002). Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. The Journal of Clinical Endocrinology and Metabolism, 87(2), 805–809. 10.1210/jcem.87.2.8246 [DOI] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron, 59(1), 11–34. 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013). Negative reinforcement in drug addiction: The darkness within. Current Opinion in Neurobiology, 23(4), 559–563. 10.1016/j.conb.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitdield TW, & George O (2014). Addiction as a stress surfeit disorder. Neuropharmacology, 76, 370–382. 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, Bunney BS, & Aghajanian GK (1974). Noradrenergic neurons: Morphine inhibition of spontaneous activity. European Journal of Pharmacology, 25(2), 165–169. 10.1016/0014-2999(74)90045-4 [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Ragunath J, & Kleber HD (1986). Cortisol levels during chronic naltrexone maintenance treatment in ex-opiate addicts. Biological Psychiatry, 21(2), 217–220. 10.1016/0006-3223(86)90150-2 [DOI] [PubMed] [Google Scholar]
- Kraus ML, Alford DP, Kotz MM, Levounis P, Mandell TW, Meyer M, Salsitz EA, Wetterau N, Wyatt SA, & the American Society Of Addiction Medicine. (2011). Statement of the American Society of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addiction. Journal of Addiction Medicine, 5(4), 254–263. 10.1097/ADM.0b013e3182312983 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, & LaForge KS (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience, 8(11), 1450–1457. 10.1038/nn1583 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Ragunath J, Plevy S, Hamer D, Schneider B, & Hartman N (1984). ACTH, cortisol and β-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides, 5(1–3), 277–278. 10.1016/0143-4179(84)90081-7 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Wardlaw SL, Hartman N, Raghunath J, Friedman J, Schneider B, & Frantz AG (1983). Circadian rhythms and levels of β-endorphin, ACTH, and cortisol during chronic methadone maintenance treatment in humans. Life Sciences, 33(Suppl 1), 409–411. 10.1016/0024-3205(83)90529-5 [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, & Silverman BL (2011). Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet, 377(9776), 1506–1513. 10.1016/S0140-6736(11)60358-9 [DOI] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, & Yamashita H (2000). Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport, 11(9), 1977–1980. 10.1097/00001756-200006260-00034 [DOI] [PubMed] [Google Scholar]
- Lal C, Strange C, & Bachman D (2012). Neurocognitive impairment in obstructive sleep apnea. Chest, 141(6), 1601–1610. 10.1378/chest.11-2214 [DOI] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, Milanés MV, & Núñez C (2012). Hypothalamic orexin—A neurons are involved in the response of the brain stress system to morphine withdrawal. PLOS ONE, 7(5), Article e36871. 10.1371/journal.pone.0036871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif ZE, Šaltyte Benth J, Solli KK, Opheim A, Kunoe N, Krajci P, Sharma-Haase K, & Tanum L (2019). Anxiety, depression, and insomnia among adults with opioid dependence treated with extended-release naltrexone vs buprenorphine-naloxone: A randomized clinical trial and follow-up study. JAMA Psychiatry, 76(2), 127–134. 10.1001/jamapsychiatry.2018.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley DM, Cleveland HH, Huhn AS, Cleveland MJ, Harris J, Stankoski D, Deneke E, Meyer RE, & Bunce SC (2017). Daily sleep quality affects drug craving, partially through indirect associations with positive affect, in patients in treatment for nonmedical use of prescription drugs. Addictive Behaviors, 65, 275–282. 10.1016/j.addbeh.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, & Weiss F (2010). Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Research, 1314, 145–161. 10.1016/j.brainres.2009.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, & Williams JT (2014). Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron, 82(6), 1346–1356. 10.1016/j.neuron.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtry V, Nizamie SH, Parvez N, & Pradhan N (2014). Sleep profile in opioid dependence: A polysomnographic case-control study. Journal of Clinical Neurophysiology, 31(6), 517–522. 10.1097/WNP.0000000000000117 [DOI] [PubMed] [Google Scholar]
- Meier A, Lambert-Harris C, McGovern MP, Xie H, An M, & McLeman B (2014). Co-occurring prescription opioid use problems and posttraumatic stress disorder symptom severity. The American Journal of Drug and Alcohol Abuse, 40(4), 304–311. 10.3109/00952990.2014.910519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Meyer RE, Ellingboe J, Mirin SM, & McDougle M (1975). Effects of heroin and methadone on plasma cortisol and testosterone. The Journal of Pharmacology and Experimental Therapeutics, 195(2), 296–302. [PubMed] [Google Scholar]
- Miller MB, Donahue ML, Carey KB, & Scott-Sheldon LAJ (2017). Insomnia treatment in the context of alcohol use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence, 181, 200–207. 10.1016/j.drugalcdep.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Ross J, & Darke S (2007). The impact of posttraumatic stress disorder on treatment outcomes for heroin dependence. Addiction, 102(3), 447–454. 10.1111/j.1360-0443.2006.01711.x [DOI] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, Simpson NS, & Dinges DF (2012). Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion, 12(5), 1015–1020. 10.1037/a0026871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Gehrman P, Perlis M, & Umscheid CA (2012). Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Family Practice, 13(1), Article 40. 10.1186/1471-2296-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogri M, Desai H, Webster L, Grant BJ, & Mador MJ (2009). Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep and Breathing, 13(1), 49–57. 10.1007/s11325-008-0208-4 [DOI] [PubMed] [Google Scholar]
- Montserrat JM, Ferrer M, Hernandez L, Farré R, Vilagut G, Navajas D, Badia JR, Carrasco E, De Pablo J, & Ballester E (2001). Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: A randomized controlled study with an optimized placebo. American Journal of Respiratory and Critical Care Medicine, 164(4), 608–613. 10.1164/ajrccm.164.4.2006034 [DOI] [PubMed] [Google Scholar]
- Moore RY, & Speh JC (1993). GABA is the principal neurotransmitter of the circadian system. Neuroscience Letters, 150(1), 112–116. 10.1016/0304-3940(93)90120-A [DOI] [PubMed] [Google Scholar]
- Moreton JE, Roehrs T, & Khazan N (1976). Drug self-administration and sleep-awake activity in rats dependent on morphine, methadone, or l-α-acetylmethadol. Psychopharmacology, 47(3), 237–241. 10.1007/BF00427607 [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004). Sleep, 29(11), 1398–1414. 10.1093/sleep/29.11.1398 [DOI] [PubMed] [Google Scholar]
- Mun CJ, Finan PH, Epstein DH, Kowalczyk WJ, Agage D, Letzen JE, Phillips KA, & Preston KL (2020). Craving mediates the association between momentary pain and illicit opioid use during treatment for opioid-use disorder: An ecological momentary assessment study. Addiction. Advance online publication. 10.1111/add.15344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P, Moline M, Mayleben D, Rosenberg R, Zammit G, Pinner K, Dhadda S, Hong Q, Giorgi L, & Satlin A (2017). Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: Results from a bayesian, adaptive, randomized, double-blind, placebo-controlled study. Journal of Clinical Sleep Medicine, 13(11), 1289–1299. 10.5664/jcsm.6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2018). Overdose death rates. Retrieved 20 January 2019, from https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- Nordmann S, Lions C, Vilotitch A, Michel L, Mora M, Spire B, Maradan G, Morel A, Roux P, Carrieri MP, & the ANRS Methaville study group. (2016). A prospective, longitudinal study of sleep disturbance and comorbidity in opiate dependence (the ANRS Methaville study). Psychopharmacology, 233(7), 1203–1213. 10.1007/s00213-016-4202-4 [DOI] [PubMed] [Google Scholar]
- Ohayon MM, & Vecchierini MF (2002). Daytime sleepiness and cognitive impairment in the elderly population. Archives of Internal Medicine, 162(2), 201–208. 10.1001/archinte.162.2.201 [DOI] [PubMed] [Google Scholar]
- Okajima I, Komada Y, & Inoue Y (2011). A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms, 9(1), 24–34. 10.1111/j.1479-8425.2010.00481.x [DOI] [Google Scholar]
- Ong AD, Kim S, Young S, & Steptoe A (2017). Positive affect and sleep: A systematic review. Sleep Medicine Reviews, 35, 21–32. 10.1016/j.smrv.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, & Ghodse H (1997). Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug and Alcohol Dependence, 48(1), 9–16. 10.1016/S0376-8716(97)00097-5 [DOI] [PubMed] [Google Scholar]
- Pačesová D, Novotný J, & Bendová Z (2016). The effect of chronic morphine or methadone exposure and withdrawal on clock gene expression in the rat suprachiasmatic nucleus and AA-NAT activity in the pineal gland. Physiological Research, 65(3), 517–525. 10.33549/physiolres.933183 [DOI] [PubMed] [Google Scholar]
- Panin F, & Peana AT (2019). Sleep and the pharmacotherapy of alcohol use disorder: Unfortunate bedfellows. A systematic review with meta-analysis. Frontiers in Pharmacology, 10, Article 1164. 10.3389/fphar.2019.01164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Schreiber S, & Adelson M (2006). Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug and Alcohol Dependence, 82(2), 103–110. 10.1016/j.drugalcdep.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Hamburger RB, & Adelson M (2011). No change of sleep after 6 and 12 months of methadone maintenance treatment. Journal of Addiction Medicine, 5(2), 141–147. 10.1097/ADM.0b013e3181e8b6c4 [DOI] [PubMed] [Google Scholar]
- Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, & Punjabi NM (2010). Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine, 182(2), 269–277. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault PF, Schuster CR, Heinrich RL, & Van der Kolk B (1972). Altered plasma cortisol response in patients on methadone maintenance. Clinical Pharmacology and Therapeutics, 13(2), 269–273. 10.1002/cpt1972132269 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, & Nissen C (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Robert C, Stinus L, & Limoge A (1999). Sleep impairments in rats implanted with morphine pellets. Neuropsychobiology, 40(4), 214–217. 10.1159/000026622 [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Murphy P, Zammit G, Mayleben D, Kumar D, Dhadda S, Filippov G, LoPresti A, & Moline M (2019). Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: A phase 3 randomized clinical trial. JAMA Network Open, 2(12), Article e1918254. 10.1001/jamanetworkopen.2019.18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Dakin CL, Abbott CR, Morgan DG, Ghatei MA, & Bloom SR (2001). The central effects of orexin-A in the hypothalamic-pituitary-adrenal axis in vivo and in vitro in male rats. Journal of Neuroendocrinology, 13(6), 561–566. 10.1046/j.1365-2826.2001.00672.x [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, … Yanagisawa M (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92(4), 573–585. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, & Lu J (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature, 437(7063), 1257–1263. 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- Scammell TE, & Saper CB (2007). Orexins: Looking forward to sleep, back at addiction. Nature Medicine, 13(2), 126–128. 10.1038/nm0207-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer A, Aichinger-Hinterhofer M, Maier C, Vollert J, & Walther JW (2015). Sleep-disordered breathing decreases after opioid withdrawal: Results of a prospective controlled trial. Pain, 156(11), 2167–2174. 10.1097/j.pain.0000000000000279 [DOI] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Banys P, & Hall SM (2000). Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. Journal of the American Medical Association, 283(10), 1303–1310. 10.1001/jama.283.10.1303 [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, & Stein MD (2010). Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug and Alcohol Dependence, 108(1–2), 77–83. 10.1016/j.drugalcdep.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, & Stein MD (2011). Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug and Alcohol Dependence, 113(2–3), 245–248. 10.1016/j.drugalcdep.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Corso RP, Brower KJ, Millman RP, & Stein MD (2009). Home polysomnography in methadone maintenance patients with subjective sleep complaints. The American Journal of Drug and Alcohol Abuse, 35(3), 178–182. 10.1080/00952990902839786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY, Kosten TR, & Lu L (2009). Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. The American Journal of Drug and Alcohol Abuse, 35(5), 267–272. 10.1080/00952990902933878 [DOI] [PubMed] [Google Scholar]
- Sinha R (2007). The role of stress in addiction relapse. Current Psychiatry Reports, 9(5), 388–395. 10.1007/s11920-007-0050-6 [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141(1), 105–130. 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatrud JB, & Dempsey JA (1983). Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. Journal of Applied Physiology, 55(3), 813–822. 10.1152/jappl.1983.55.3.813 [DOI] [PubMed] [Google Scholar]
- Staedt J, Wassmuth F, Stoppe G, Hajak G, Rodenbeck A, Poser W, & Rüther E (1996). Effects of chronic treatment with methadone and naltrexone on sleep in addicts. European Archives of Psychiatry and Clinical Neuroscience, 246(6), 305–309. 10.1007/BF02189023 [DOI] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, & Anderson BJ (2004). Sleep disturbances among methadone maintained patients. Journal of Substance Abuse Treatment, 26(3), 175–180. 10.1016/S0740-5472(03)00191-0 [DOI] [PubMed] [Google Scholar]
- Stein MD, Kurth ME, Anderson BJ, & Blevins CE (2020). A pilot crossover trial of sleep medications for sleep-disturbed methadone maintenance patients. Journal of Addiction Medicine, 14(2), 126–131. 10.1097/ADM.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Kurth ME, Sharkey KM, Anderson BJ, Corso RP, & Millman RP (2012). Trazodone for sleep disturbance during methadone maintenance: A double-blind, placebo-controlled trial. Drug and Alcohol Dependence, 120(1–3), 65–73. 10.1016/j.drugalcdep.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, & Bigelow GE (1993). Dose-response effects of methadone in the treatment of opioid dependence. Annals of Internal Medicine, 119(1), 23–27. 10.7326/0003-4819-119-1-199307010-00004 [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Wang D, Cunnington D, Quinnell T, Tran H, Kronborg I, & Drummer OH (2005). Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest, 128(3), 1339–1347. 10.1378/chest.128.3.1339 [DOI] [PubMed] [Google Scholar]
- Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, & Sands SA (2015). Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. The European Respiratory Journal, 45(2), 408–418. 10.1183/09031936.00062914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, John J, Shan L, Swaab DF, Wu MF, Ramanathan L, McGregor R, Chew KT, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, & Siegel JM (2018). Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Science Translational Medicine, 10(447), Article eaao4953. 10.1126/scitranslmed.aao4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Minkel J, & Humphreys K (2006). Opioid substitution treatment reduces substance use equivalently in patients with and without posttraumatic stress disorder. Journal of Studies on Alcohol, 67(2), 228–235. 10.15288/jsa.2006.67.228 [DOI] [PubMed] [Google Scholar]
- Troitino A, Labedi N, Kufel T, & El-Solh AA (2014). Positive airway pressure therapy in patients with opioid-related central sleep apnea. Sleep and Breathing, 18(2), 367–373. 10.1007/s11325-013-0894-4 [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, & Ritchie K (2005). Sleep and depression. The Journal of Clinical Psychiatry, 66(10), 1254–1269. 10.4088/JCP.v66n1008 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Reyes BA, & Valentino RJ (2010). The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Research, 1314, 162–174. 10.1016/j.brainres.2009.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryswyk E, & Antic NA (2016). Opioids and sleep-disordered breathing. Chest, 150(4), 934–944. 10.1016/j.chest.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Deboer T, Dahan A, & Meijer JH (2003). Differential responses of circadian activity onset and offset following GABA-ergic and opioid receptor activation. Journal of Biological Rhythms, 18(4), 297–306. 10.1177/0748730403254283 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, & Cha SS (2014). Medication-assisted therapies—Tackling the opioid-overdose epidemic. The New England Journal of Medicine, 370(22), 2063–2066. 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Li TK (2004). Drug addiction: The neurobiology of behaviour gone awry. Nature Reviews Neuroscience, 5(12), 963–970. 10.1038/nrn1539 [DOI] [PubMed] [Google Scholar]
- Walter M, Wiesbeck GA, Bloch N, Aeschbach S, Olbrich HM, Seifritz E, & Dürsteler-MacFarland KM (2008). Psychobiological responses to drug cues before and after methadone intake in heroin-dependent patients: A pilot study. European Neuropsychopharmacology, 18(5), 390–393. 10.1016/j.euroneuro.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, & Kronborg I (2005). Central sleep apnea in stable methadone maintenance treatment patients. Chest, 128(3), 1348–1356. 10.1378/chest.128.3.1348 [DOI] [PubMed] [Google Scholar]
- Wasef S, Mir S, Ryan C, Waseem R, Bellingham G, Kashgari A, Wong J, & Chung F (2020). Treatment for patients with sleep apnea on opioids for chronic pain: Results of the OpSafe trial. Journal of Clinical Sleep Medicine. Advance online publication. 10.5664/jcsm.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, & Baghdoyan HA (2007). Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience, 144(1), 375–386. 10.1016/j.neuroscience.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Choi Y, Desai H, Webster L, & Grant BJ (2008). Sleep-disordered breathing and chronic opioid therapy. Pain Medicine, 9(4), 425–432. 10.1111/j.1526-4637.2007.00343.x [DOI] [PubMed] [Google Scholar]
- Wicher D, & Marion-Poll F (2018). Function and regulation of chemoreceptors. Frontiers in Cellular Neuroscience, 12, Article 496. 10.3389/fncel.2018.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, & de Lecea L (2004). Interaction between the corticotropin-releasing factor system and hypocretins (orexins): A novel circuit mediating stress response. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(50), 11439–11448. 10.1523/JNEUROSCI.3459-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Montes KS, Schwebel FJ, & Tucker JA (2020). What is recovery? Alcohol Research: Current Reviews, 40(3), Article 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, & Stein EA (2002). GABAergic mechanisms of opiate reinforcement. Alcohol and Alcoholism (Oxford, Oxfordshire), 37(5), 485–494. 10.1093/alcalc/37.5.485 [DOI] [PubMed] [Google Scholar]
- Xiao L, Tang YL, Smith AK, Xiang YT, Sheng LX, Chi Y, Du W, Guo S, Jiang Z, Zhang G, & Luo XN (2010). Nocturnal sleep architecture disturbances in early methadone treatment patients. Psychiatry Research, 179(1), 91–95. 10.1016/j.psychres.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Xu GP, Van Bockstaele E, Reyes B, Bethea T, & Valentino RJ (2004). Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(38), 8193–8197. 10.1523/JNEUROSCI.1657-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, & Mohsenin V (2005). Obstructive sleep apnea as a risk factor for stroke and death. The New England Journal of Medicine, 353(19), 2034–2041. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- Young GA, Moreton JE, Meltzer L, & Khazan N (1975). REM sleep distributions in post-addict rats relapsing to morphine self-administration: Effects of naloxone subcutaneous pellets. Research Communications in Chemical Pathology and Pharmacology, 11(3), 355–363. [PubMed] [Google Scholar]
- Zarrabian S, Riahi E, Karimi S, Razavi Y, & Haghparast A (2020). The potential role of the orexin reward system in future treatments for opioid drug abuse. Brain Research, 1731, Article 146028. 10.1016/j.brainres.2018.11.023 [DOI] [PubMed] [Google Scholar]
- Zhang GF, Ren YP, Sheng LX, Chi Y, Du WJ, Guo S, Jiang ZN, Xiao L, Luo XN, Tang YL, Smith AK, Liu ZQ, & Zhang HX (2008). Dysfunction of the hypothalamic-pituitary-adrenal axis in opioid dependent subjects: Effects of acute and protracted abstinence. The American Journal of Drug and Alcohol Abuse, 34(6), 760–768. 10.1080/00952990802385781 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, & Kreek MJ (2006). Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. The Journal of Endocrinology, 191(1), 137–145. 10.1677/joe.1.06960 [DOI] [PubMed] [Google Scholar]


