Abstract
Regulations limiting the sale of flavored e-cigarette products are controversial for their potential to interfere with e-cigarette use as a cessation aid in addition to curbing youth use. Limited research suggests that flavor might enhance the addictive potential of e-cigarettes; however, the acute effects of flavored aerosols on brain function among humans has not been assessed. The current study aimed to isolate and compare the neural substrates of flavored and unflavored e-cigarette aerosols on brain function among nine female daily smokers. Participants inhaled aerosolized e-liquid with 36 mg/ml of nicotine with and without a strawberry-vanilla flavor while undergoing functional magnetic resonance imaging. We used general linear modelling to compare whole-brain mean neural activation and seed-to-voxel task-based functional connectivity between the flavored and unflavored inhalation runs. Contrary to our hypothesis, the flavored aerosol was associated with weaker activation than the unflavored aerosol in the brain stem and bilateral parietal-temporal-occipital region of the cortex. Instead, the flavor engaged taste-related brain regions while suppressing activation of the neural circuits typically engaged during smoking and nicotine administration. Alternatively, functional connectivity between subcortical dopaminergic brain seeds and cortical brain regions involved in motivation and reward salience were stronger during the flavored compared to unflavored aerosol run. The findings suggest that fruity and dessert-flavored e-cigarettes may dampen the reward experience of aerosol inhalation for smokers who initiate e-cigarette use by inhibiting activation of dopaminergic brain circuits. These preliminary findings may have implications for understanding how regulations on flavored e-cigarettes might impact their use as cessation aids.
Keywords: Addiction, Brain, Electronic cigarette, Flavor, fMRI, Nicotine
Introduction
An estimated 8.1 million adults and 3.5 million youth in the United States currently use electronic cigarettes (e-cigarettes) (Creamer et al., 2019; Wang et al., 2020). Flavors are a primary reason why youth initiate e-cigarette use and have become increasingly popular among adults as well (Russell et al., 2018; Villanti et al., 2017). Approximately 75% of youth and 50% of adult e-cigarette users report using food-flavored e-cigarettes (Schneller et al., 2018). Long-term e-cigarette users migrate to fruity and dessert-like flavors and away from tobacco flavors over time (Du et al., 2020). More recently, federal and state regulations have focused on banning the sale of fruity and dessert-flavored e-cigarettes to help curb use among youth; however these regulations are controversial for their potential impact on smokers using e-cigarettes as a cessation aid (Yingst et al., 2020). Despite their widespread use, we still know little about how e-cigarettes influence the function of reward and cognitive control brain circuitry involved in addiction. We know even less about how flavor might further alter the brain’s reward and cognitive control systems when added to nicotine delivery systems like e-cigarettes, which may help inform regulations.
Addiction to tobacco products is primarily the result of nicotine binding to nicotinic acetylcholine receptors throughout cortical and subcortical brain circuitry (Volkow et al., 2019). This binding improves short-term attention and prompts the release of dopamine in the striatum which elevates mood and enhances sensitivity to rewards (Davis & Gould, 2008; Poorthuis et al., 2009). During the use of tobacco products, non-nicotine factors, such as taste, behavioral rituals, environmental contexts, and mood states become conditioned to the rewarding effects of nicotine (Davis & Gould, 2008; Hyman et al., 2006). This process of learned conditioning is driven by connections between mesolimbic (e.g., striatum, amygdala) and cortical (e.g., anterior cingulate, orbitofrontal cortex) regions involved in motivation, cognitive control, and behavioral planning (Everitt & Robbins, 2013; Hyman et al., 2006). Continued use of nicotine causes neuroadaptations in brain circuits that process reward stimuli, determine reward salience, and exert control over reward-seeking behavior (Volkow et al., 2019). Chronic smokers develop nicotine tolerance via increases in nicotinic acetylcholine receptor availability that contribute to aversive withdrawal symptoms during abstinence (Benowitz, 2010). The influence of conditioned stimuli and the desire to relieve withdrawal symptoms are believed to make nicotine a highly addictive drug despite relatively small increases in dopamine release compared to other drugs of abuse (Benowitz, 2010).
The development and maintenance of nicotine addiction differs by sex (The Motivational Impact of Nicotine and its Role in Tobacco Use, 2009). For females, conditioned non-nicotine factors are more reinforcing and more influential on smoking behavior than nicotine (The Motivational Impact of Nicotine and its Role in Tobacco Use, 2009). For example, when presented with cigarettes of varying but unknown nicotine doses, females are less likely to report differences in subjective liking or willingness to work for lower nicotine cigarettes compared to males (Perkins et al., 2002). Rather than nicotine dose, the taste and smell of a cigarette is an especially potent conditioned smoking stimuli for females, which can affect subjective experience and use behaviors (Perkins et al., 2001). In a laboratory experiment, blocking the smell and taste of a cigarette with a nose clip reduced subjective liking and satisfaction of a cigarette and the willingness to work for puffs more for females than males (Perkins et al., 2001). The sex-differences in nicotine and non-nicotine smoking reinforcement are thought to be one reason why female smokers have more persistent smoking and difficulty quitting compared to male smokers (The Motivational Impact of Nicotine and its Role in Tobacco Use, 2009). This preliminary research with smokers suggests that flavors may be an especially potent conditioned stimuli for female e-cigarette users even more so than male users.
To our knowledge, only one study has used functional magnetic resonance imaging (fMRI) to assess brain function during e-cigarette use among smokers (Wall et al., 2017). In that study, 10 adults who smoked at least semi-regularly completed fMRI during 10-minute cued and naturalistic puffing protocols. During the cued protocol, participants inhaled from the device when presented with 2-second visual cues. Participants were instructed to puff “naturally” during the naturalistic protocol. All participants used the same Njoy brand first-generation pen-style e-cigarette with a tobacco-flavored e-liquid that contained approximately 30mg/ml of nicotine. They found diffuse activation of subcortical and cortical circuits that mimicked neural responses to nicotine administration in other forms (Stein et al., 1998; Wall et al., 2017). Cortical activation was stronger during the cued compared to the naturalistic protocol. The study was the first to show the widespread neural effects of e-cigarettes. Their use of an e-cigarette device during scanning added real-world validity to their results. However, this study was not able to isolate the neural effects of the aerosol inhalation from the sensorimotor experiences that occurs while holding an e-cigarette and drawing deep inhalations. Additionally, this study did not assess the impact of dessert-like flavor on brain function or connectivity along addiction-relevant pathways.
Flavors, odors, and visual cues of high caloric foods engage dopaminergic brain circuitry similar to the effects of nicotine, suggesting that dessert-like flavors could have an additive effect on dopamine release in the striatum leading to more compulsive use of flavored e-cigarettes (Karunanayaka et al., 2015; Small, 2012; Stein et al., 1998; Tang et al., 2012; Wickham et al., 2018). In an experimental study, smokers used e-cigarettes containing varying combinations of sweetened and unsweetened e-liquids with and without nicotine for two days (Kroemer et al., 2018). After the use period, visual cues of the e-cigarette that contained sweetened and nicotinized e-liquid elicited more nucleus accumbens activity during fMRI than cues of the e-cigarettes containing unsweetened nicotinized e-liquid (Kroemer et al., 2018). Studies with mice have found that menthol flavorants can enhance nicotine-related reward by increasing dopamine neuron excitability in the ventral tegmental area (Cooper & Henderson, 2020). Green apple flavorants (i.e., farsnesol, farnesene) can upregulate nicotinic acetylcholine receptors and increase dopamine firing frequency in the ventral tegmental area independent of nicotine (Cooper & Henderson, 2020). To our knowledge, there are no studies assessing neural activation or functional connectivity during the acute administration of flavored nicotine e-cigarettes without a prior learning (i.e. use) period among humans. Understanding the neural effects of e-cigarettes and flavor additives can provide insight into their abuse liability and potentially inform strategies for e-cigarette addiction prevention and treatment.
The current study aimed to isolate and compare the neural substrates of the acute administration of nicotine e-cigarette aerosol with and without a dessert-like flavor among female smokers. Females, who experience more reinforcement from non-nicotine factors in tobacco products, may be especially responsive to flavors in e-cigarettes compared to men. Towards this aim, female regular smokers inhaled strawberry-vanilla flavored and unflavored e-cigarette aerosols both containing 36 mg/ml nicotine while undergoing fMRI, in a single-session study. First, we hypothesized that both aerosols would be associated with significant blood-oxygen level-dependent (BOLD) signals along cortico-thalamo-striatal brain pathways previously shown to be engaged during nicotine administration and e-cigarette use (Stein et al., 1998; Wall et al., 2017). Second, we expected flavored aerosol to be associated with stronger BOLD signals in regions linked with reward, flavor perception, reactivity to food cues, learning, and emotion, including the nucleus accumbens, orbitofrontal cortex (OFC), insula, amygdala, and prefrontal cortex compared to the unflavored aerosol (Rolls, 2016; Small, 2012; Soudry et al., 2011; Tang et al., 2012). Third, we expected nicotine dependence, smoking urge, and subjective ratings of the aerosol to be correlated with BOLD signals during aerosol inhalation. Fourth, we hypothesized that compared to unflavored, flavored aerosol would result in stronger functional connectivity of subcortical seed regions implicated in associative learning and the development of nicotine addiction, including the striatum, hippocampus, and amygdala.
Materials and methods
Participants.
Eleven adult smokers were recruited from a database of smokers who had previously participated in tobacco research at the Pennsylvania State University College of Medicine and agreed to be contacted for future research. Eligible participants were those that smoked ≥ 5 cigarettes per day for ≥1 year and were able to read and write in English and understand study procedures. Over 75% of people who smoke 5 cigarettes per day meet DSM-5 criteria for Tobacco Use Disorder, rising to over 90% of those smoking >10 CPD (Oliver & Foulds, 2020). Subjects were excluded if they had used e-cigarettes in the past month, were currently involved in a smoking quit attempt, had a significant or uncontrolled medical condition (eg, COPD, kidney failure), were allergic to the e-liquid ingredients propylene glycol (PG) or vegetable glycerin (VG), had smell dysfunction based on the standardized Burghart Sniffin’ Sticks smell threshold test, or had any safety contraindications for MRI (e.g., implanted metal clips, claustrophobia). Two participants that completed the protocol were excluded from analysis for excessive head motion and weakened BOLD signals activation due to stopping and restarting the protocol. The final analysis had nine female participants.
Procedures.
After completing a telephone screen to assess eligibility, participants completed one in-person laboratory visit that included a standardized smell threshold test using the Burghart Sniffin Sticks, an exhaled carbon monoxide measurement, semi-structured interview, computerized survey, and functional MRI scan. Participants were not required to abstain from smoking prior to the visit to reduce the confounding effects of nicotine withdrawal on brain function. Exhaled carbon monoxide was collected with a Bedfont microTM Smokerlyzer® (coVita, LLC, Santa Barbara, CA) upon arrival. The semi-structured interview included the NIDA Quick Screen to assess substance use and questions developed by our research team on MRI safety, tobacco use, subjective withdrawal and physical experiences, and medical history. The computerized survey included questions developed by our team on demographics and tobacco and nicotine use characteristics the Fagerström Test for Cigarette Dependence [FTND (Heatherton et al., 1991)], Hooked on Nicotine Checklist [HONC (Wellman et al., 2005)], Questionnaire on Smoking Urges-Brief [QSU-Brief (Toll et al., 2006)], Center for Epidemiological Studies Depression Scale (Radloff, 1977), Perceived Stress Scale (Cohen et al., 1983), and the Kessler 6 Distress Scale (Kessler et al., 2002). Participant gender and biological sex were assessed with the question, “What is your gender identity” with the response options: male (male assigned at birth); female (female assigned at birth), transmale/ transman/ female to male (female assigned at birth); transfemale/ transwoman/ male to female (male assigned at birth); Other identity. The semi-structured interview and computerized survey lasted approximately one hour and was directly followed by the MRI scans. After the MRI scans, participants rated the aerosol flavor on a 5-point likert scale of none, a little bit, somewhat, very much, and extremely on the following descriptors: sweet, pleasant, satisfying, tastes good, and familiar. Participants rated how pleasant the amount of nicotine they received from the aerosol was on a scale from 0- very unpleasant to 10- very pleasant. All procedures were approved by the Investigational Review Board at the Pennsylvania State University College of Medicine (00010904) and participants provided informed consent prior to any procedures.
fMRI Paradigm.
Aerosol was delivered to participants during the MRI scan with the MRI Electronic Aerosol Delivery System (MEADS) (Hobkirk et al., 2020). The MEADS works in coordination with a computerized olfactometer to deliver aerosol from breath-actuated e-cigs directly into a spacer (plastic holding container) where the aerosol is stored temporarily for inhalation. Participants inhaled the aerosol directly from the spacer through a plastic tube fitted with a mouthpiece. Although some e-cigarette devices are safe for use during MRI (Wall et al., 2017), the MEADS allowed for the delivery of two different aerosols (i.e., flavored and unflavored) during the same scanning session. Each fMRI run lasted 6.5 minutes separated by approximately 60 seconds. Each fMRI run included eight 10s deliveries of an aerosol with 30s between each delivery followed by 60s of fresh air delivery. After viewing a 12s crosshair, participants saw the word “BREATHE” displayed on an LCD screen viewed with a mirror during the entire aerosol delivery phase (320s) and “REST” during the 60s fresh air delivery. Participants were instructed to use their mouth to inhale and exhale at a natural pace through the tubing during the entire scanning session. Therefore, participants were inhaling aerosol into their mouth during the entire 320s aerosol delivery phase and fresh air during the 60s rest period from the same tubing. Participants were specifically asked not to “puff” on the tubing as one would during a typical smoking experience. This was done to isolate the effects of the aerosol specifically without the confounding BOLD activation caused by deep inhalation and breath holding that accompanies puffing (Thomason et al., 2005; Wang et al., 2014).
For each fMRI inhalation run, participants inhaled aerosol generated from e-liquid containing 36 mg/ml nicotine concentration. One run was conducted with a strawberry-vanilla flavor added to the e-liquid and the other run with an unflavored e-liquid. We previously found that this inhalation protocol with flavored and unflavored 36 mg/ml e-liquids delivered an aerosol containing 3.5 mg/ml nicotine, resulting in a plasma nicotine level of 8.5 ng/ml approximately 25 minutes after the nicotine delivery [see (Hobkirk et al., 2020) for details]. For the current study, the flavored and unflavored inhalation runs were delivered on the same visit and fmri session, and the order of flavored and unflavored runs was randomized (5 received flavored first and 4 unflavored first). The participants, but not the researchers, were blinded to the order of the runs (flavored or unflavored) prior to the scan. E-liquid freebase nicotine, flavors, and solvents, propylene glycol (PG) and vegetable glycerin (VG), were purchased from nicvape.com. All e-liquids were mixed in-house and composed of a 70:30 (PG:VG) base which was chosen specifically to reduce the size of the aerosol plumes. The strawberry-vanilla flavor included 5% strawberry and 10% vanilla E-Flavor™ concentrates. We chose a strawberry-vanilla flavor to balance real-world implications and safety. Cigarette flavor bans in the United States are primarily on fruity and dessert-like flavored e-liquids because these flavors are the most common during youth e-cigarette initiation (Rose et al., 2020). To ensure that our flavor was informative for these policies, we chose a strawberry-vanilla flavor to fit into both the fruity and dessert-like categories. We chose the specific flavorants for our experiment for their relatively low production of free radicals (Bitzer et al., 2018). We were particularly concerned about safety since we were planning this study during an outbreak of e-cigarette and vaping-associated lung injury in the United States (Werner et al., 2020).
Image acquisition.
Using a Siemens 3T Prisma system (Siemens Healthineers, Erlangen, Germany) and a 64 channel head-neck coil, images were acquired using 34-slice oblique-axial series (3 × 3 × 4 mm voxels) for 196 volumes using an echo planar imaging pulse sequence with the following parameters: repetition time = 2000 ms; echo time = 30 ms; field of view = 24 × 24 cm2; flip angle = 90°; in-plane matrix size 80× 80. A high-resolution structural image (1 × 1× 1 mm3 spatial resolution) was acquired using an MPRAGE sequence.
Image preprocessing.
Image preprocessing and statistical analysis was completed using FEAT (FMRI Expert Analysis Tool, v.6.00 (Woolrich et al., 2004; Woolrich et al., 2001) included in the FSL (FMRI of the Brain Software Library) package, v.5.0.10 (Jenkinson et al., 2012). Images were brain extracted using the brain extraction tool [BET2 (Jenkinson et al., 2005)] and preprocessed using the standard features including slice timing correction, high pass filtering with a cut-off of 60s, motion correction using FMRIB’s Linear Image Registration Tool (MCFLIRT), and spatial smoothing at FWHM of 5mm. Six volumes (12s) were deleted from the beginning of each run to allow for image stabilization. Functional images were registered to the high-resolution T1-weighted anatomical image and a Montreal Neurological Institute (MNI-152) 2mm standard using linear registration.
Image analysis.
At the first level of analysis, the onset of the aerosol delivery with a 20s duration was convolved with a double-gamma hemodynamic response function. We used a 20s duration to capture the 10s of aerosol delivery while the spacer was filling with aerosol and the following 10s when the spacer still contained a large quantity of aerosol. Temporal derivatives were included in the model to improve fit. FSL motion outliers was used to calculate the root mean square intensity difference of each volume to the reference volume (refrms), which were controlled for to account for large changes in motion during each run. At the second level of analysis, voxel-wise general linear models (one-sample t-tests) were conducted to identify regions of significant group mean activation during the flavored and unflavored runs using whole-brain analyses. A whole-brain voxel-wise paired t-test contrasted BOLD signals during the flavored vs. unflavored runs. Voxel-wise whole-brain general linear models identified brain regions showing significant associations between BOLD signals during each run with self-reported levels of nicotine dependence on the HONC and FTCD, smoking urges on the QSU, and self-reported ratings of the pleasantness of the nicotine level in the aerosol. Voxel-wise whole-brain general linear models were conducted to identify brain regions showing significant associations between BOLD signals during the flavored run with the subjective ratings of the aerosol (i.e., sweet, pleasant, satisfying, tastes good, and familiar). Post-hoc voxel-wise whole-brain t-tests were conducted to examine the potential confounding effects of run order (paired; run 1 vs. run 2) and cigarette flavor preference (two-sample; menthol vs. tobacco flavor). Anatomical regions of significance were identified with the Harvard-Oxford Cortical and Subcortical Atlases.
For the mean activation, paired t-test between inhalation runs, self-report associations, and post-hoc t-tests, voxels were thresholded at p≤ .01 and cluster-extent thresholded at p≤ .05. We used liberal significance thresholds to ensure that we did not overlook relevant brain activation for these preliminary whole-brain analyses. We used the standard cluster thresholding option in FSL’s FEAT software to identify significant contiguous clusters of voxels (Woolrich et al., 2004). The cluster thresholding uses Gaussian Random Field (GRF) theory to compare the estimated cluster-level significance to a cluster probability threshold. A gray matter mask was applied for all second-level analyses. The order of the runs and number of respirations (inhalation frequency) during each run were included as covariates. We did not have the respiration count for one participant’s flavored inhalation run due to a software failure. Since the respiration counts for the flavored and unflavored inhalation runs were highly correlated for the sample (r=0.80), we imputed the missing value with the participant’s respiration count for the unflavored inhalation run.
Functional connectivity.
The CONN Functional Connectivity Toolbox (v.20.a (Whitfield-Gabrieli & Nieto-Castanon, 2012)) using Matlab (v.R2019a) and SPM12 (Wellcome, Department of Imaging Neurosicence, University College of London) was used to assess statistical differences in whole-brain functional connectivity with subcortical reward circuitry during the flavored vs. unflavored inhalation runs. The functional and structural images were preprocessed using the default pipeline. This included functional image realignment and unwarping, slice timing correction, ART-based outlier identification and scrubbing, skull-stripping, grey/white/CSF segmentation, normalization to MNI standard space, and smoothing. Structural images were skull-stripped, grey/white/CSF segmented, and normalized to MNI standard space. Seed-to-voxel based bivariate correlations were calculated using hemodynamic response function weighting for assessing condition-specific connectivity. Seeds included the left and right nucleus accumbens, putamen, pallidum, caudate, hippocampus, and amygdala. Significant differences in network connectivity between the flavored and unflavored inhalation runs were examined using voxel-wise paired t-tests while controlling for run order and respiration counts. Voxels were thresholded at p≤ .001 uncorrected. Cluster-level p-values were then calculated by comparing the significant cluster sizes against probability estimated cluster sizes. Cluster p-values higher than the False Discovery Rate-corrected threshold were considered significant.
Results
Participants.
The final sample included nine women with a mean age of 50 years (SD=12.1). Seven participants identified as White, one African American/ Black, and one biracial. Participants smoked a mean of 16.3 (SD=3.8) cigarettes per day for 27 years (SD=13.4) on average. Six participants smoked menthol cigarettes. All exhaled carbon monoxide measurements on the day of the scan were above the recommended cut-off (>5–9 ppm) for distinguishing a tobacco user from a non-user (range 16 – 42 ppm) (Benowitz et al., 2020). Eight participants denied any prior use of e-cigarettes and one participant reported using an e-cigarette 4 months prior to the study visit.
Self-report questionnaires and aerosol ratings.
The mean FTCD score was 4.1 (SD=0.9: range 3–6), which is consistent with low to moderate dependence. The mean HONC score was 7.7 (SD=1.6: range 5–9) and all participants endorsed at least one item indicating loss of autonomy over their cigarette use. Participants reported a mean smoking urge rating of 33 (SD=8.7; range 16–43). After the MRI scan, participants rated the mean pleasantness of the nicotine level a 6.8 on a 0–10 scale (SD= 2.7; range 2–10). Average aerosol ratings on the 0–4 Likert scale were: sweet, M= 2.4, SD= 0.7; pleasant, M= 2.8, SD= 1.1; satisfying, M=2.7, SD=1.1; tastes good, M= 2.7, SD= 1.2; and familiar, M= 2.2, SD= 1.3.
Mean BOLD signal.
Results of the fMRI analyses are included in Table 1 and Fig. 1. The flavored aerosol run was associated with a significant BOLD signal increase in five clusters encompassing the right orbitofrontal and insular cortices, bilateral frontal operculum and frontal pole, left superior parietal lobule, precentral, postcentral and supramarginal gyri, and the left cerebellum and occipital fusiform gyrus. The unflavored aerosol was associated with a significant BOLD signal increase in six clusters encompassing the bilateral thalamus, pallidum, amygdala, hippocampus and OFC, insular cortex, supramarginal gyrus, precentral gyrus, postcentral gyrus, precuneus, inferior temporal gyrus, and inferior lateral occipital cortex.
Table 1.
Clusters of significant blood-oxygen-level-dependent signals for each contrast
| Peak Anatomical region | Max z-score | MNI coordinates (x, y, z) at peak | Number of Voxels | Other anatomical regions cluster |
|---|---|---|---|---|
| During flavored aerosol run | ||||
| R Frontal pole | 4.4 | 46, 44, 10 | 1189 | R frontal operculum cortex |
| L Superior parietal lobule | 3.81 | −48, −42, 60 | 1013 | L precentral gyrus, L postcentral gyrus, L supramarginal gyrus (anterior division) |
| L Cerebellum | 4.15 | −28, −58, −28 | 589 | L occipital fusiform gyrus |
| L Frontal operculum cortex | 4.16 | −32, 28, 10 | 454 | L frontal pole |
| R Frontal orbital cortex | 4.16 | 24, 8, −16 | 313 | R insular cortex, R amygdala, R putamen |
| During unflavored aerosol run | ||||
| L Brain stem | 4.69 | −16, −58, −54 | 11701 | B thalamus, B pallidum, B amygdala, B hippocampus, B inferior temporal gyrus, B temporal occipital fusiform cortex |
| L Postcentral gyrus | 5.3 | −60, −18, 24 | 2290 | L precentral gyrus, L supramarginal gyrus, L inferior frontal gyrus, L insula, L central operculum cortex, L parietal operculum cortex |
| R Supramarginal gyrus (anterior division) | 4.98 | 60, −20, 24 | 1521 | R precentral gyrus, R postcentral gyrus, R inferior frontal gyrus, R insula, R central operculum cortex, R parietal operculum cortex |
| L Lateral occipital cortex (superior division) | 4.22 | −12, −78, 46 | 688 | B lateral occipital cortex, B precuneus cortex |
| R Lateral occipital cortex (inferior division) | 4.27 | 58, −64, 0 | 524 | R middle temporal gyrus, R angular gyrus |
| L Lateral occipital cortex (inferior division) | 4.21 | −48, −74, 10 | 519 | L middle temporal gyrus, L inferior temporal gyrus, L angular gyrus |
| Significantly stronger BOLD signal during unflavored vs. flavored aerosol runs | ||||
| L Brain stem | 3.81 | −12, −38, −30 | 585 | N/A |
| L Middle temporal gyrus | 3.74 | −54, −52, 0 | 452 | L supramarginal gyrus, L angular gyrus , L lateral occipital cortex |
| R brain stem | 3.51 | 4, −56, −50 | 316 | N/A |
| R Lateral occipital cortex (inferior division) | 3.86 | 56, −66, −8 | 234 | R temporal occipital fusiform cortex, R occipital fusiform gyrus |
| Association between BOLD signal during unflavored aerosol run and FTCD score | ||||
| R Angular gyrus | 4.00 | 44, −52, 56 | 388 | R supramarginal gyrus, R lateral occipital cortex |
| R frontal pole | 4.03 | 44, 36, 22 | 264 | R middle frontal gyrus |
| Association between BOLD signal during unflavored aerosol run and nicotine pleasantness | ||||
| L Postcentral gyrus | 5.50 | −50, −30, 52 | 2634 | L precentral gyrus, L supramarginal gyrus, L parietal operculum cortex, L planum termporale |
| R supramarginal gyrus | 5.31 | 62, −26, 36 | 2576 | R precentral gyrus, R postcentral gyrus, R superior parietal lobule, R parietal operculum cortex, R central opercular cortex, R preceuneus, R posterior cingulate, R planum temporale |
| L superior frontal gyrus | 4.88 | −22, 12, 64 | 1301 | L precentral gyrus, L middle frontal gyrus |
| R superior frontal gyrus | 4.43 | 14, 2, 62 | 1270 | R juxtapositional lobule cortex, R paracingulate |
| L frontal pole | 4.31 | −44, 38, 8 | 589 | L middle frontal gyrus |
| R lingual gyrus | 3.62 | 16, −54, −8 | 305 | N/A |
| R frontal operculum cortex | 4.15 | 34, 22, 8 | 294 | R insular cortex, R orbitofrontal cortex |
| L posterior cingulate gyrus | 4.2 | −12, −16, 36 | 231 | L precentral gyrus |
| L lateral occipital cortex | 3.76 | −16, −66, 56 | 224 | N/A |
| R middle temporal gyrus | 3.27 | 64, −54, 4 | 214 | R lateral occipital cortex |
| Association between BOLD signal during unflavored aerosol run and smoking urges | ||||
| L supramarginal gyrus | 6.25 | −60, −28, 44 | 2678 | L precentral gyrus, L postcentral gyrus, L parietal operculum |
| R supramarginal gyrus | 5.34 | 66, −34, 28 | 1923 | R precentral gyrus, R postcentral gyrus, R parietal operculum, R central operculum, R planum temporale |
| L middle frontal gyrus | 4.71 | −38, 36, 36 | 989 | L inferior frontal gyrus, L frontal pole |
| L cerebellum | 3.76 | −22, −66, −30 | 762 | N/A |
| L orbitofrontal cortex | 4.55 | −46, 16, −8 | 444 | L insular cortex, L temporal pole |
| L superior frontal gyrus | 4.77 | −22, 12, 64 | 432 | N/A |
| R cerebellum | 3.78 | 24, −64, −22 | 411 | N/A |
| R cerebellum | 3.78 | 24, −64, −22 | 411 | N/A |
| R superior frontal gyrus | 3.93 | 16, 4, 64 | 384 | R juxtapositional lobule cortex, R precentral gyrus |
| R inferior temporal gyrus | 3.83 | 64, −52, −12 | 278 | R middle temporal gyrus, R lateral occipital cortex |
| R posterior cingulate gyrus | 4.15 | 14, −30, 38 | 267 | cortex anterior cingulate gyrus |
| R frontal pole | 5.83 | 42, 36, 36 | 239 | N/A |
| Significantly stronger BOLD signal during first run vs. second run | ||||
| R Frontal pole | 3.66 | 30, 52, 34 | 1180 | N/A |
| L Caudate | 3.84 | −10, 10, 10 | 824 | B caudate, B thalamus, R putamen, R pallidum |
| R Occipital pole | 3.79 | 10, −96, −4 | 795 | R lingual gyrus, |
| R lateral occipital cortex | 3.77 | −32, 34, 46 | 656 | R occipital fusiform gyrus |
| R postcentral gyrus | 3.8 | 2, −34, 74 | 401 | R precentral gyrus, precuneus, superior parietal lobule |
| R supramarginal gyrus | 3.53 | 58, −38, 20 | 346 | R angular gyrus, lateral occipital cortex |
| L cerebellum | 3.54 | −48, −58, −36 | 291 | N/A |
| L inferior temporal gyrus | 3.47 | −46, −2, −36 | 287 | L middle temporal gyrus, L temporal fusiform gyrus, |
| Comparison of menthol vs. tobacco cigarette preference during flavored aerosol run | ||||
| L middle frontal gyrus | 3.97 | −32, 8, 64 | 530 | L superior frontal gyrus, L frontal pole |
| R occipital pole | 3.85 | 20, −88, 34 | 440 | R lateral occipital cortex, R cuneal cortex, R precuneus |
| L lateral occipital cortex | 4.16 | −54, −66, 30 | 332 | L angular gyrus |
| L frontal pole | 4.18 | −20, 56, 8 | 278 | N/A |
| Comparison of tobacco vs. menthol cigarette preference during unflavored aerosol run | ||||
| L cerebellum | 4.31 | −36, −52, −44 | 543 | N/A |
Note: L=left, R=right, B=bilateral, FTCD= Fagerström Test for Nicotine Dependence.
Figure 1.
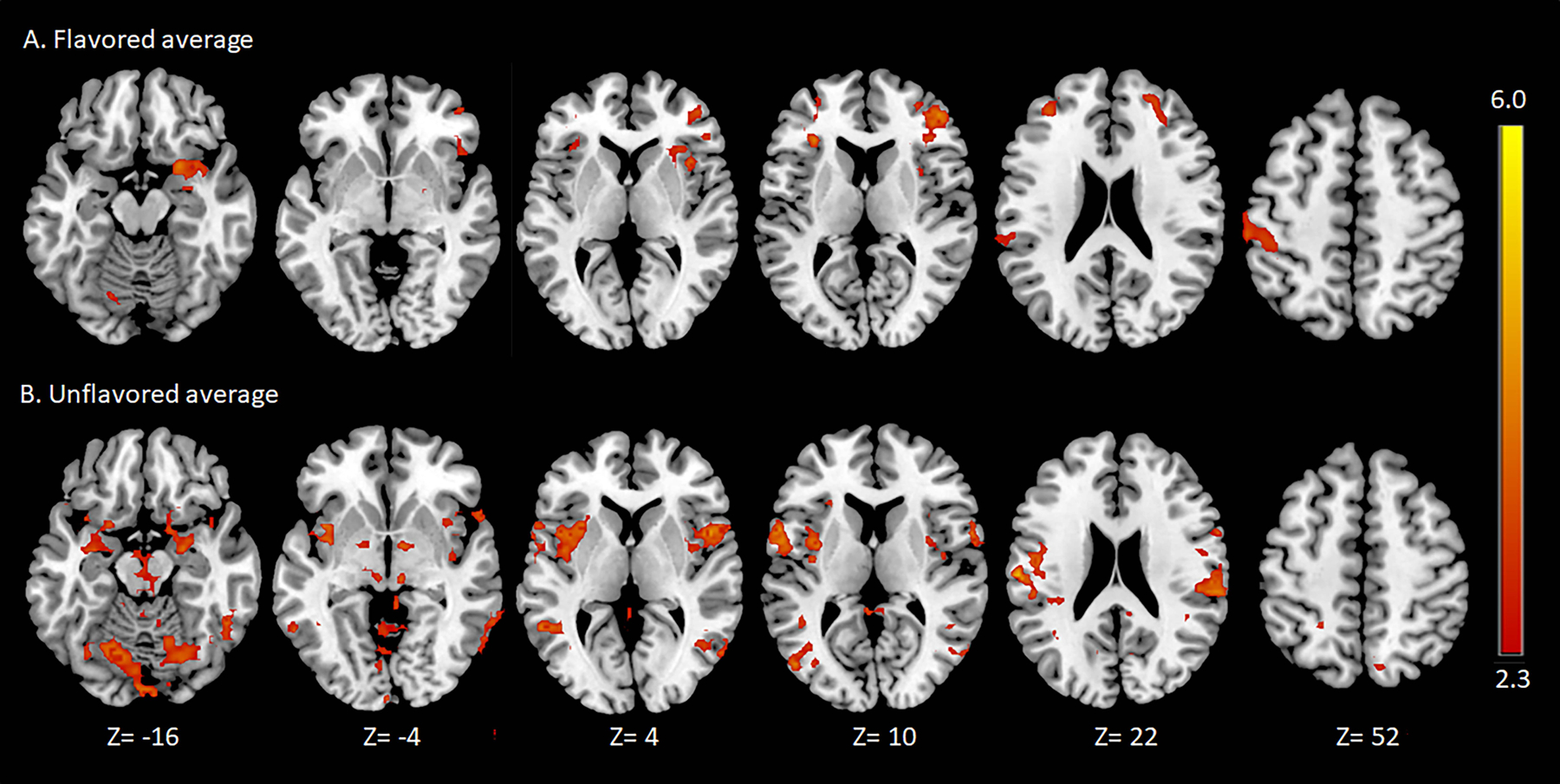
Significant mean cluster-thresholded (p < .05) blood-oxygen-level-dependent (BOLD) signal (z > 2.3) during the flavored (A) and unflavored (B) aerosol runs for sample of smokers (n=9). Axial slices labeled in MNI-152 standard space. Images displayed in neurological convention (right=right). Images were created using MRIcron v.1.0.20190902.
Flavored vs. unflavored BOLD signal.
The unflavored aerosol was associated with significantly stronger BOLD activation compared to the flavored aerosol in the brain stem, left middle temporal gyrus, and right lateral occipital cortex. No brain regions had stronger BOLD signals during the flavored compared to unflavored aerosol run.
BOLD signal correlations with smoking characteristics and aerosol ratings.
BOLD signals during the unflavored aerosol run were significantly correlated with: 1) dependence scores on the FTCD in the right angular gyrus and frontal pole; 2) ratings of nicotine pleasantness in 10 clusters encompassing regions of the anterior and posterior cingulate, precentral, postcentral, and supramarginal gyri, frontal operculum, and posterior parietal cortex; 3) smoking urges on the QSU in 12 clusters in the anterior and posterior cingulate, lateral prefrontal cortex, precentral, postcentral, and supramarginal gyri, temporal cortex, and cerebellum. Unflavored aerosol BOLD signal was not correlated with scores on the HONC and there were no significant correlations between BOLD signals during the flavored aerosol run and smoking characteristics or aerosol ratings (i.e., sweet, pleasant, satisfying, tastes good, and familiar).
Run order.
A paired sample t-test revealed stronger activation in the right frontal pole, bilateral caudate and thalamus, right dorsal striatum, and right occipital cortex during the first run compared to the second run. There were no regions of stronger activation during the second run.
Cigarette flavor preference.
Two-sample t-tests revealed stronger activation in the left middle frontal gyrus, right frontal pole, left lateral occipital cortex, and right occipital pole for menthol compared to tobacco flavor cigarette smokers during the flavored aerosol run. Tobacco flavor cigarette smokers had stronger activation in the left cerebellum during the unflavored aerosol run compared to menthol smokers. Tobacco smokers had no regions of increased activation during the flavored run and menthol smokers had no regions of increased activation during the unflavored run.
Functional Connectivity.
During the flavored compared to the unflavored run, functional connectivity was significantly stronger between the left caudate seed with the left anterior cingulate cortex (ACC) (49 voxels, p =.002), OFC and insula (26 voxels, p=.040); left putamen seed with the left OFC and insula (51 voxels, p<.001); right putamen seed with the left frontal pole (84 voxels, p<.001), right lateral occipital cortex (61 voxels, p<.001), and right cerebellum (26 voxels, p=.018); left amygdala seed with right cerebellum (33 voxels, p=.017); right nucleus accumbens seed with left frontal pole (32 voxels, p=.030); left nucleus accumbens seed and left cerebellum (32 voxels, p=.021). Functional connectivity was significantly stronger during the unflavored compared to the flavored run between the right nucleus accumbens and left precentral gyrus (28 voxels, p=.030) and the left nucleus accumbens and the left brain stem (27 voxels, p=.026).
Discussion
For the current study, we were able to isolate and compare the neural substrates of inhaling dessert-like flavor in nicotine e-cigarette aerosol among 9 female daily smokers who were not current e-cigarette users. As expected, inhaling e-cigarette aerosols with and without flavor were both associated with widespread brain activation throughout cortical and subcortical brain regions as seen previously during e-cigarette use and cigarette smoking (Stein et al., 1998; Wall et al., 2017). The unflavored aerosol was associated with BOLD signal increases in dopaminergic subcortical brain regions as well as a diffuse range of cortical structures throughout the prefrontal, insular, and parietal cortices. The flavored aerosol was associated with activation in brain regions typically linked with flavor perception, including the primary olfactory and gustatory cortices located in the amygdala, anterior insula, frontal operculum, and the orbitofrontal cortex (Veldhuizen et al., 2011). These results are consistent with the only other study assessing the neural effect of the acute administration of nicotine e-cigarette aerosols on brain function among smokers, which also found diffuse activation throughout cortical and subcortical brain regions of the cortico-thalamo-striatal pathway (Wall et al., 2017). The findings confirm that nicotine e-cigarette use has the potential to influence a wide range of neural circuits involved in learning, cognition, and behavior.
The brain activation we observed was likely elicited by both nicotine and non-nicotine factors during aerosol inhalation. We did not expect to see strong pharmacological effects of nicotine administration in this study since participants were not required to abstain from smoking and our preliminary testing found that our paradigm administers a relatively small (~4 ng/ml) nicotine boost compared to a combustible cigarette (15–21 ng/ml) (Hobkirk et al., 2020; Williams et al., 2010). However, participants went at least one hour without smoking prior to the scan, and therefore were not completely nicotine satiated during the first run. Indeed, we found more BOLD signals in the caudate and thalamus during the first run compared to the second run, suggesting that participants may have been nicotine deprived during the first run and satiated during the second run. Some of the run order effects we observed may have also been related to other factors, such as comfort in the scanner as evidenced by the increased activation observed in the dorsomedial prefrontal cortex during the first run, which aids in regulating fear and emotion.
Contrary to our hypothesis that flavored aerosol would elicit stronger activation across flavor and reward-associated brain regions, the flavored aerosol was associated with weaker BOLD signals compared to the unflavored aerosol in the brain stem and bilateral parietal-temporal-occipital region of the cortex. Instead, the dessert-like flavor appeared to engage taste-related brain regions while suppressing activation of the reward-associated neural circuits typically engaged during smoking and nicotine administration (Stein et al., 1998; Wall et al., 2017). Since both aerosols contained the same nicotine content, we suspect that the differences we observed between the unflavored and flavored aerosol may have been related to either direct or conditioned responses to the sensory non-nicotine stimuli in the aerosol.
The unflavored aerosol may have provided non-nicotine factors, such as the “throat hit” of a cigarette, while the flavored aerosol may have masked this sensation. Nicotine stimulates the trigeminal neural system located in the brainstem and somatosensory brain regions (Albrecht et al., 2009). The sensation, while unpleasant at first, may actually serve as a nicotine cue that enhances the development of addiction. The tobacco industry has long understood that trigeminal stimulation enhances the addictive properties of tobacco products (Megerdichian et al., 2007). During testing, we received anectodal reports from participants that 36 mg/ml aerosol irritated their throat, which was likely more salient while inhaling the unflavored aerosol (Hobkirk et al., 2020). Alternatively, reactions to the strawberry-vanilla flavored aerosol varied across participants and some rated it as unpleasant. Thus the insula activation during the flavored aerosol run could have been due to disgust reactions to the flavoring (Britton et al., 2006).
In addition to potentially masking the throat hit of the aerosol or serving as an aversive stimuli for some, the dessert-like flavor may have simply created a sensory experience that was too different from the typical smoking experience to elicit conditioned neural reactivity. Indeed, our pattern of brain activation during the unflavored aerosol inhalation in the current study was very similar to the activation seen in response to smoking cues, particularly throughout the dorsal striatum, sensorimotor cortex, and frontoparietal network (Engelmann et al., 2012). Smokers report more satisfaction from e-cigarettes than other nicotine delivery products because of the non-nicotine factors that more readily approximate the smoking experience (Steinberg et al., 2014). We also found that menthol smokers were more responsive to the flavored aerosol throughout cortical brain regions than smokers who prefer tobacco flavored cigarettes. Thus, some of the activation we observed during the flavored aerosol run may have been conditioned responses to flavored nicotine inhalation. Although the unflavored aerosol was not the same as the tobacco or menthol flavor participants typically smoke, it likely approximated the normal smoking experience more than the strawberry-vanilla flavor. Since both aerosols contained the same amount of nicotine, conditioned responses to non-nicotine factors are a likely reason for the observed differences, however future research using aerosols that do not contain nicotine will be important for parsing out the role of conditioned reactivity and potential interactions between nicotine and non-nicotine factors.
Of note however, is the lack of significant activity in the cingulate cortex during the unflavored aerosol inhalation in the current study. The anterior and posterior cingulate are robust regions of smoking cue-reactivity involved in the modulation of craving (Brody et al., 2007; Lin et al., 2020). More generally, the ACC is involved in conflict monitoring and decision making (Botvinick, 2007). Wall and colleagues found ACC activation during cued e-cigarette use (i.e., using timed visual prompts), but not during naturalistic smoking (Wall et al., 2017). Despite the lack of significant ACC reactivity during aerosol inhalation, we did find that the inhalation-associated activity increased with higher self-reported smoking urge prior to the scan. The findings suggest that the ACC may only be involved in acute reactivity to non-nicotine smoking factors during states of withdrawal. Replicating this study with smokers in a state of abstinence will help to clarify the role of the ACC.
As further support that the flavor changed the e-cigarette inhalation from a smoking to gustatory experience, BOLD signals during the flavored run were not correlated with smoking dependence, urges, or perceived pleasantness of the nicotine level. These smoking-related factors were, however, correlated with BOLD signals during the unflavored aerosol run throughout cortical brain regions, including the ACC and insula, which have been consistently linked with smoking urge and craving (Volkow et al., 2019). There was a robust correlation between the perceived pleasantness of the nicotine level with activation in the cingulo-insular and fronto-parietal brain regions that are involved in cognitive control and interoceptive experience (Laird et al., 2011). Given the strong activation in the gustatory cortex during the flavored aerosol run, we were surprised that BOLD signals were not significantly correlated with taste and sweetness ratings; however, this may have been the result of limited variability in the aerosol ratings for our small sample.
Chronic smoking is associated with reduced subjective and neurological reactivity to non-smoking rewards, which may also contribute to the surprisingly limited neural reactivity to the dessert-like flavor in the current study. A recent meta-analysis found that smokers, compared to healthy control participants, have reduced responding to non-smoking rewards in the left striatum, but increased responding in the right insula and inferior frontal gyrus (Lin et al., 2020). Although we did not have a healthy control comparison in the current study, our findings are consistent with the meta-analysis in that the insula and other regions of the prefrontal cortex were reactive to the dessert-like flavor while the striatum was not. Therefore, the reward-related neuroadaptations of chronic nicotine use may further exacerbate the neural suppression of reward-related brain circuitry during flavored e-cigarette use.
Our results could explain the initial reduced liking and wanting of sweetened and nicotine e-cigarettes observed among smokers in prior laboratory research (Kroemer et al., 2018). It is possible that if participants used the dessert-flavored e-cigarette for two days as was done by Kroemer and colleagues, we would have seen similar brain reactivity in the striatum during inhalation of the flavored aerosol (Kroemer et al., 2018). Although smokers’ rates of e-cigarette initiation with dessert-like flavors is increasing, the majority still prefer to initiate use with traditional tobacco or menthol flavors over dessert-like flavors (Russell et al., 2018). However, with continued e-cigarette use, current and former smokers tend to switch to dessert-like flavors, especially younger smokers and those who become exclusive e-cigarette users (Du et al., 2020). This might suggest that in the beginning, dessert flavors suppress the rewarding neural effects of the conditioned smoking experience, but over time, also become conditioned nicotine cues. Conditioning in addiction occurs via dopaminergic projections between subcortical and cortical brain regions that are engaged with each drug administration (Hyman et al., 2006). The unflavored aerosol run was associated with stronger connectivity between brain regions previously shown to have increased connectivity for smokers compared to non-smokers (Janes et al., 2012). Acute nicotine administration also elicits activation of these brain regions in human and animal studies studies (Bruijnzeel et al., 2014; Stein et al., 1998). The flavored aerosol run, however, was associated with stronger connectivity throughout mesocorticolimbic circuitry shown to drive reward valuation, seeking, and craving, such as the ACC, OFC, and insula (Hyman et al., 2006; Volkow et al., 2019). Strengthening these dopaminergic projections may facilitate associative learning and the development of new conditioned e-cigarette cues.
The current study had several limitations. For this preliminary study, we only conducted one laboratory visit and randomized the order of the runs. To limit the potential effects of run order on our flavor comparisons, participants did not abstain from smoking to reduce nicotine withdrawal effects on brain reactivity and we randomized and controlled for run order in all analyses. However, there were still run-order effects and potentially carry-over effects between runs. Since more participants in our experiment completed the flavored run first, we may have observed an even larger difference between the flavored and unflavored runs if we had not controlled for run order. Replicating these results in a crossover design with separate laboratory visits for the flavored and unflavored runs would add support for the findings. In addition, we may have been underpowered to detect small effects for the run comparison and correlations between flavor ratings and BOLD signal. In light of our potential lack of statistical power, the results suggest that the independent effect of dessert-like flavors in e-cigarette aerosol may be even stronger than what we observed in the current study.
While the current analysis provides insight into the addictive potential of e-cigarettes for female daily smokers, the results may not generalize to non-smokers or dual users. An important and crucial next step is identifying the neural substrates of dessert-like flavors for daily e-cigarette users or adolescents and young adults who are naïve to nicotine. Administering nicotine to youth and non-tobacco users presents ethical challenges. Other methodological factors may also limit generalizability of the current results. We asked participants to breathe naturally rather than puff on the aerosol as they would during real-world e-cigarette use. We did this to limit the BOLD signals related to deep inhalation in order to isolate the neural effects of the aerosol specifically. In addition, we used e-liquids with a nicotine concentration that was lower than some popular e-cigarette brands currently on the market (36 mg/ml vs. 59 mg/ml, respectively) (Hobkirk et al., 2020). It is possible that we would have seen increased brain activation during the flavored aerosol run if we had used an e-liquid with a higher nicotine concentration or nicotine salts that provide faster systemic delivery to the bloodstream than freebase nicotine (O’Connell et al., 2019). Finally, our study assessed the effects of only one flavor, we did not control alcohol, caffeine, or food consumption prior to the scan, and the sample was comprised of all female smokers with relatively low levels of nicotine dependence on the FTCD. Smoking behavior is more dependent on olfactory cues for female than male smokers (Perkins et al., 2001). Thus, females are an important population for research on flavored e-cigarette use; however, our results may not generalize to male smokers. This effect may look different for men who may be less responsive to the dessert-type flavors and more responsive to the nicotine in the aerosol. Further, results may also vary by flavor and by menstrual cycle phase, which can influence brain reactivity to reward stimuli (Dreher et al., 2007).
In summary, our results confirm that e-cigarette use engages a diffuse set of brain regions involved in addictive behaviors and provides the first evidence that adding dessert-like flavors to e-cigarette aerosols may significantly alter the neural circuitry subserving e-cigarette use for smokers. More specifically, the current study found that a dessert-like flavor suppressed brain activation in regions typically reactive to nicotine administration and conditioned smoking cues for daily female smokers who do not use e-cigarettes. This effect may explain why smokers tend to prefer e-cigarettes with traditional cigarette flavors (e.g., tobacco and menthol) when switching for harm reduction (Du et al., 2020). The results add to our understanding of how sensory stimuli can modulate the neural effects of substance use. This preliminary finding is informative for recommendations on the use of e-cigarettes as a cigarette substitute for smokers and for policy regulations on flavored tobacco products. Future research should assess how brain reactivity during e-cigarette use may change with conditioning over time and the effect of e-cigarette flavors for nicotine naïve smokers, such as youth initiating e-cigarette use for the first time, or long-time e-cigarette users who may have conditioned associations between nicotine and dessert-like flavors. This research will help to understand the influence of flavors on neural engagement and downstream addictive e-cigarette behaviors.
Public Health Relevance:
The current study provides objective evidence from neuroimaging that dessert-like flavors in e-cigarettes may interfere with satisfaction for daily female smokers by suppressing activation of addiction-related neural circuits during use. In addition, e-cigarette flavors may enhance the development of addiction by strengthening connections between dopaminergic and cortical brain circuitry during use. The results suggest little added benefit of flavored e-cigarettes for female smokers switching for harm reduction.
Disclosures and Acknowledgements
This project was supported by the Penn State CTSI Grant (UL-TR000127 & UL1-TR002014) from the National Center for Advancing Translational Sciences, National Institutes of Health and the Penn State University Highmark Gift Fund. ALH is supported by a National Institute on Drug Abuse career development award (K23-DA045081). This project was facilitated by the Penn State College of Medicine MRI Core Facility, Penn State Center for Tobacco and Health, and is funded in part under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The Department of Health specifically disclaims responsibility for any analysis, interpretations or conclusions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ALH, ZB, RG, PK, JF, JW, JPR, RJE and QXY were involved in the development of the study concept and design. KRH, BH, and JY assisted with data collection. ALH, AF, CB, and DM assisted with data analysis. ALH wrote the initial draft of the manuscript and KRH, CB, DM, JF, PK, JW, and QXY provided critical revisions. All authors critically reviewed content and approved the final version for publication.
JF has received grants from the NIH; has previously received a grant, personal fees, and nonfinancial support (e.g. study drug) from Pfizer Inc., unrelated to the submitted work; in the past (>3 years ago), has done paid consulting for pharmaceutical companies involved in manufacturing smoking-cessation medications (e.g., GlaxoSmithKline, Johnson & Johnson); and has acted as a deposed and compensated expert witness on behalf of plaintiffs suing cigarette manufacturers. The authors have no other conflicts of interest to disclose.
Footnotes
Aspects of this study were presented at the 2020 Annual Meeting for the Society for Research on Nicotine and Tobacco and the 2021 Annual Meeting of the Society of Behavioral Medicine.
References
- Albrecht J, Kopietz R, Linn J, Sakar V, Anzinger A, Schreder T, Pollatos O, Bruckmann H, Kobal G, & Wiesmann M (2009). Activation of olfactory and trigeminal cortical areas following stimulation of the nasal mucosa with low concentrations of S(−)-nicotine vapor--an fMRI study on chemosensory perception. Human Brain Mapping, 30(3), 699–710. 10.1002/hbm.20535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2010). Nicotine addiction. N Engl J Med, 362(24), 2295–2303. 10.1056/NEJMra0809890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, & Piper ME (2020). Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob Res, 22(7), 1086–1097. 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, & Richie JP Jr. (2018). Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med, 120, 72–79. 10.1016/j.freeradbiomed.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM (2007). Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci, 7(4), 356–366. 10.3758/cabn.7.4.356 [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, & Liberzon I (2006). Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage, 31(1), 397–409. 10.1016/j.neuroimage.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, & Cohen MS (2007). Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry, 62(6), 642–651. 10.1016/j.biopsych.2006.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Alexander JC, Perez PD, Bauzo-Rodriguez R, Hall G, Klausner R, Guerra V, Zeng H, Igari M, & Febo M (2014). Acute nicotine administration increases BOLD fMRI signal in brain regions involved in reward signaling and compulsive drug intake in rats. Int J Neuropsychopharmacol, 18(2). 10.1093/ijnp/pyu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Cooper SY, & Henderson BJ (2020). The Impact of Electronic Nicotine Delivery System (ENDS) Flavors on Nicotinic Acetylcholine Receptors and Nicotine Addiction-Related Behaviors. Molecules, 25(18). 10.3390/molecules25184223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, & Neff L (2019). Tobacco Product Use and Cessation Indicators Among Adults - United States, 2018. MMWR Morb Mortal Wkly Rep, 68(45), 1013–1019. 10.15585/mmwr.mm6845a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, & Gould TJ (2008). Associative learning, the hippocampus, and nicotine addiction. Curr Drug Abuse Rev, 1(1), 9–19. 10.2174/1874473710801010009 [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, & Berman KF (2007). Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A, 104(7), 2465–2470. 10.1073/pnas.0605569104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Bascom R, Fan T, Sinharoy A, Yingst J, Mondal P, & Foulds J (2020). Changes in Flavor Preference in a Cohort of Long-Term Electronic Cigarette Users. Ann Am Thorac Soc, 17(5), 573–581. 10.1513/AnnalsATS.201906-472OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, & Cinciripini PM (2012). Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage, 60(1), 252–262. 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2013). From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev, 37(9 Pt A), 1946–1954. 10.1016/j.neubiorev.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hobkirk AL, Bitzer Z, Goel R, Sica CT, Livelsberger C, Yingst J, Houser KR, Rupprecht S, Trushin N, Karunanayaka P, Foulds J, Richie JP, Spreen L, Hoglen B, Wang J, Elias RJ, & Yang QX (2020). An Electronic Aerosol Delivery System for Functional Magnetic Resonance Imaging. Subst Abuse, 14, 1178221820904140. 10.1177/1178221820904140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, & Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci, 29, 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, & Kaufman MJ (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend, 125(3), 252–259. 10.1016/j.drugalcdep.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). FSL. Neuroimage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, & Smith S (2005). BET2: MR-based estimation of brain, skull and scalp surfaces. In. Eleventh Annual Meeting of the Organization for Human Brain Mapping. [Google Scholar]
- Karunanayaka PR, Wilson DA, Vasavada M, Wang J, Martinez B, Tobia MJ, Kong L, Eslinger P, & Yang QX (2015). Rapidly acquired multisensory association in the olfactory cortex. Brain Behav, 5(11), e00390. 10.1002/brb3.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, & Zaslavsky AM (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med, 32(6), 959–976. 10.1017/s0033291702006074 [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Veldhuizen MG, Delvy R, Patel BP, O’Malley SS, & Small DM (2018). Sweet taste potentiates the reinforcing effects of e-cigarettes. Eur Neuropsychopharmacol, 28(10), 1089–1102. 10.1016/j.euroneuro.2018.07.102 [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, & Fox PT (2011). Behavioral interpretations of instinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Deng J, Shi L, Wang Q, Li P, Li H, Liu J, Que J, Chang S, Bao Y, Shi J, Weinberger DR, Wu P, & Lu L (2020). Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl Psychiatry, 10(1), 97. 10.1038/s41398-020-0775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerdichian CL, Rees VW, Wayne GF, & Connolly GN (2007). Internal tobacco industry research on olfactory and trigeminal nerve response to nicotine and other smoke components. Nicotine Tob Res, 9(11), 1119–1129. 10.1080/14622200701648458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell G, Pritchard JD, Prue C, Thompson J, Verron T, Graff D, & Walele T (2019). A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern Emerg Med, 14(6), 853–861. 10.1007/s11739-019-02025-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, & Foulds J (2020). Association Between Cigarette Smoking Frequency and Tobacco Use Disorder in U.S. Adults. Am J Prev Med. 10.1016/j.amepre.2020.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, & Hutchison S (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research, 3(2), 141–150. 10.1080/14622200110043059 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, & Caggiula AR (2002). Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl), 163(2), 194–201. 10.1007/s00213-002-1168-1 [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, & Mansvelder HD (2009). Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol, 78(7), 668–676. 10.1016/j.bcp.2009.04.031 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale:A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rolls ET (2016). Reward Systems in the Brain and Nutrition. Annu Rev Nutr, 36, 435–470. 10.1146/annurev-nutr-071715-050725 [DOI] [PubMed] [Google Scholar]
- Rose SW, Johnson AL, Glasser AM, Villanti AC, Ambrose BK, Conway K, Cummings KM, Stanton CA, Delnevo C, Wackowski OA, Edwards KC, Feirman SP, Bansal-Travers M, Bernat J, Holder-Hayes E, Green V, Silveira ML, Zhou Y, Abudayyeh H, & Hyland A (2020). Flavour types used by youth and adult tobacco users in wave 2 of the Population Assessment of Tobacco and Health (PATH) Study 2014–2015. Tob Control, 29(4), 432–446. 10.1136/tobaccocontrol-2018-054852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C, McKeganey N, Dickson T, & Nides M (2018). Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduct J, 15(1), 33. 10.1186/s12954-018-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, & O’Connor RJ (2018). Use of flavored electronic cigarette refill liquids among adults and youth in the US-Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015). PLoS One, 13(8), e0202744. 10.1371/journal.pone.0202744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM (2012). Flavor is in the brain. Physiol Behav, 107(4), 540–552. 10.1016/j.physbeh.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Soudry Y, Lemogne C, Malinvaud D, Consoli SM, & Bonfils P (2011). Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis, 128(1), 18–23. 10.1016/j.anorl.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, & Bloom AS (1998). Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry, 155(8), 1009–1015. 10.1176/ajp.155.8.1009 [DOI] [PubMed] [Google Scholar]
- Steinberg MB, Zimmermann MH, Delnevo CD, Lewis MJ, Shukla P, Coups EJ, & Foulds J (2014). E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices. J Gen Intern Med, 29(11), 1444–1450. 10.1007/s11606-014-2889-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, & Dagher A (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav, 106(3), 317–324. 10.1016/j.physbeh.2012.03.009 [DOI] [PubMed] [Google Scholar]
- The Motivational Impact of Nicotine and its Role in Tobacco Use. (2009). (Bevins RA & Caggiula AR, Eds. 1 ed.). Springer-Verlag; New York. 10.1007/978-0-387-78748-0 [DOI] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JD, & Glover GH (2005). Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage, 25(3), 824–837. 10.1016/j.neuroimage.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Toll BA, Katulak NA, & McKee SA (2006). Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief). Addict Behav, 31(7), 1231–1239. 10.1016/j.addbeh.2005.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, & Lundström JN (2011). Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp, 32(12), 2256–2266. 10.1002/hbm.21188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Feirman SP, Tworek C, Glasser AM, Pearson JL, Cohn AM, Conway KP, Niaura RS, Bansal-Travers M, & Hyland A (2017). Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am J Prev Med, 53(2), 139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, & Baler R (2019). The Neuroscience of Drug Reward and Addiction. Physiol Rev, 99(4), 2115–2140. 10.1152/physrev.00014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MB, Mentink A, Lyons G, Kowalczyk OS, Demetriou L, & Newbould RD (2017). Investigating the neural correlates of smoking: Feasibility and results of combining electronic cigarettes with fMRI. Sci Rep, 7(1), 11352. 10.1038/s41598-017-11872-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun X, & Yang QX (2014). Methods for olfactory fMRI studies: Implication of respiration. Hum Brain Mapp, 35(8), 3616–3624. 10.1002/hbm.22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, & King BA (2020). E-cigarette Use Among Middle and High School Students - United States, 2020. MMWR Morb Mortal Wkly Rep, 69(37), 1310–1312. 10.15585/mmwr.mm6937e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Savageau JA, Godiwala S, Friedman K, & Hazelton J (2005). Measuring adults’ loss of autonomy over nicotine use: the Hooked on Nicotine Checklist. Nicotine Tob Res, 7(1), 157–161. 10.1080/14622200412331328394 [DOI] [PubMed] [Google Scholar]
- Werner AK, Koumans EH, Chatham-Stephens K, Salvatore PP, Armatas C, Byers P, Clark CR, Ghinai I, Holzbauer SM, Navarette KA, Danielson ML, Ellington S, Moritz ED, Petersen EE, Kiernan EA, Baldwin GT, Briss P, Jones CM, King BA, Krishnasamy V, Rose DA, & Reagan-Steiner S (2020). Hospitalizations and Deaths Associated with EVALI. N Engl J Med, 382(17), 1589–1598. 10.1056/NEJMoa1915314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wickham RJ, Nunes EJ, Hughley S, Silva P, Walton SN, Park J, & Addy NA (2018). Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology, 128, 33–42. 10.1016/j.neuropharm.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, Kipen H, & Benowitz NL (2010). Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res, 12(8), 855–859. 10.1093/ntr/ntq102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, & Smith SM (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage, 21(4), 1732–1747. 10.1016/j.neuroimage.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage, 14(6), 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Yingst JM, Bordner CR, Hobkirk AL, Hoglen B, Allen SI, Krebs NM, Houser KR, Livelsberger C, & Foulds J (2020). Response to Flavored Cartridge/Pod-Based Product Ban among Adult JUUL Users: “You Get Nicotine However You Can Get It”. Int J Environ Res Public Health, 18(1). 10.3390/ijerph18010207 [DOI] [PMC free article] [PubMed] [Google Scholar]


