Abstract
Background:
Prior ARDS trials have identified hypo-inflammatory and hyper-inflammatory subphenotypes, with distinct differences in short-term outcomes. It is unknown if such differences extend beyond 90 days or are associated with physical, mental health, or cognitive outcomes.
Methods:
568 patients in the multicenter Statins for Acutely Injured Lungs from Sepsis (SAILS) trial of rosuvastatin versus placebo were included and assigned a subphenotype. Among 6- and 12-month survivors (N=232 and 219, respectively, representing 243 unique survivors), subphenotype status was evaluated for association with a range of patient-reported outcomes (e.g., mental health symptoms, quality of life). Patient subsets also were evaluated with performance-based tests of physical function (e.g., 6-minute walk test) and cognition.
Findings:
The hyper-inflammatory versus hypo-inflammatory subphenotype had lower overall 12-month cumulative survival (58% versus 72%, p<0.01); however, there was no significant difference in survival beyond 90 days (86% versus 89%, p=0.70). Most survivors had impairment across the range of outcomes, with little difference between subphenotypes at 6- and 12-month assessments. For instance, at 6 months, in comparing the hypo-inflammatory versus hyper-inflammatory subphenotypes, respectively, the median (IQR) patient-reported SF-36 mental health domain score was 47 (33, 56) versus 44 (35, 56) [p=0.99], and the percent predicted 6-minute walk distance was 66% (48%, 80%) versus 66% (49%, 79%) [p=0.76].
Interpretation:
Comparing the hyper-inflammatory versus hypo-inflammatory ARDS subphenotype, there was no significant difference in survival beyond 90 days and no consistent findings of important differences in 6- or 12-month physical, cognitive and mental health outcomes. These findings, when considered with prior results, suggest that inflammatory subphenotypes largely reflect the acute phase of illness and its short-term impact.
Keywords: ARDS, subphenotypes, critical illness, sepsis, cognitive dysfunction
INTRODUCTION
There is growing interest in understanding the heterogeneity among patients with acute respiratory distress syndrome (ARDS) [1]. This interest is attributed, in part, to the lack of benefit of many promising interventions, prompting calls for more precise targeting of interventions to subsets of patients most likely to benefit [2]. An existing body of research has evaluated inflammatory subphenotypes in ARDS based on clinical characteristics and chemical biomarkers [3–6], with two subphenotypes reported: hypo-inflammatory and hyper-inflammatory [7]. In a prior study evaluating patients from the Statins for Acutely Injured Lungs from Sepsis (SAILS) trial [8], a large multicenter randomized trial comparing rosuvastatin versus placebo in patients with sepsis-associated ARDS, there was significantly higher 90-day mortality in the hyper- versus hypo-inflammatory subphenotype (38% versus 21%, p<0.001), with no evidence of differential treatment response to rosuvastatin by subphenotype [6].
Similarly, analyses of other clinical trials showed worse short-term outcomes in the hyper-inflammatory subphenotype. For instance, in the “Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction–2” (HARP-2) study [9], a multi-center randomized trial of simvastatin versus placebo for ARDS, there was significantly higher 28-day mortality and a lower median ventilator-free days in the hyper- versus hypo-inflammatory subphenotype (39% versus 17%, p <0.0001, and 2 versus 18 days, p <0.0001, respectively) [3]. Moreover, in the ARDS Network Fluid and Catheter Treatment Trial (FACTT) [10], which compared a fluid-conservative versus fluid-liberal strategy in ARDS, 90-day mortality was significantly higher in the hyper- versus hypo-inflammatory subphenotype (45% versus 22%, p<0.0001) [11].
Despite consistent evidence of differences in short-term mortality between hyper- versus hypo-inflammatory subphenotypes, to our knowledge, no study has evaluated differences in longer-term survival or physical, cognitive and mental health outcomes. Notably, increased levels of inflammatory biomarkers (e.g., interleukin-6, interleukin-8, and C-reactive protein) during critical illness are associated with ICU-acquired weakness [12–14] that, in turn, is associated with impaired physical function after hospital discharge [15–17]. Similarly, systemic inflammation in critically ill patients is associated with short-term [18] and long-term cognitive impairment [19–22]. Finally, inflammatory biomarkers may be positively associated with psychological disorders (e.g., post-traumatic stress disorder (PTSD) and depression) in non-critically ill individuals [23,24]. Hence, our objective was to evaluate if subphenotype status was associated with differences in physical, mental health and cognitive outcomes, based on a prior prospective evaluation of SAILS patients over 6- and 12-month follow-up, conducted as part of the ARDSNet Long-Term Outcomes Study (ALTOS) [25,26].
METHODS
Patients enrolled in SAILS were randomized to receive either rosuvastatin or placebo until the earlier of 3 days after ICU discharge, study day 28, or death [8]. The study was terminated for futility after enrolling 745 of a 1000-patient sample size. The SAILS trial’s primary outcome was mortality before hospital discharge home or until study day 60 if the patient was still in a health care facility. As part of a secondary analysis of SAILS, patients were classified as a hypo-inflammatory or hyper-inflammatory subphenotype using latent class analysis (LCA). From the original LCA study, in which two separate modelling solutions were presented for the SAILS trial, we used the subphenotypes identified by the model using conventional class-defining variables. consistent with prior LCA studies [4,11]. The LCA model included a composite of 34 variables, including plasma protein biomarkers collected at the time of randomization, and clinical data (e.g. demographics, ARDS risk factors, systolic blood pressure, vasopressor use, routine laboratory values, and inflammatory markers) collected pre-enrollment. Details of all the class-defining variables used for the LCA model have been previously published [6].
A total of 35 of 37 SAILS study sites participated in 6- and 12-month follow-up via ALTOS. Of 568 SAILS patients enrolled while the ALTOS study was ongoing, 243 survived and were eligible for the ALTOS post-discharge follow-up assessments (Figure 1). The institutional review board at each participating site approved the study [25,26]. Because this was a secondary analysis of SAILS, no a priori sample size calculation was performed. Comparisons of 12-month survival, stratified by inflammatory subphenotype, were made for all 568 enrolled patients. In addition, at 6-and 12-month follow-up, eligible survivors were evaluated via telephone-based assessments conducted by trained research personnel who were blinded to treatment allocation. Patient-reported outcome measures included: age- and sex-adjusted physical function and mental health domain scores of the Medical Outcomes Study Short Form 36 (SF-36) instrument [27], health-related quality of life status using the utility score and the visual analogue scale score from the EQ-5D-3L instrument [28], physical functioning status from the Functional Performance Inventory-Short Form [29], anxiety and depression symptoms from the Hospital Anxiety and Depression Scale (HADS) subscale scores [30], and PTSD symptoms from the Impact of Events Scale-Revised (IES-R) [31].
Figure 1. Flow diagram.
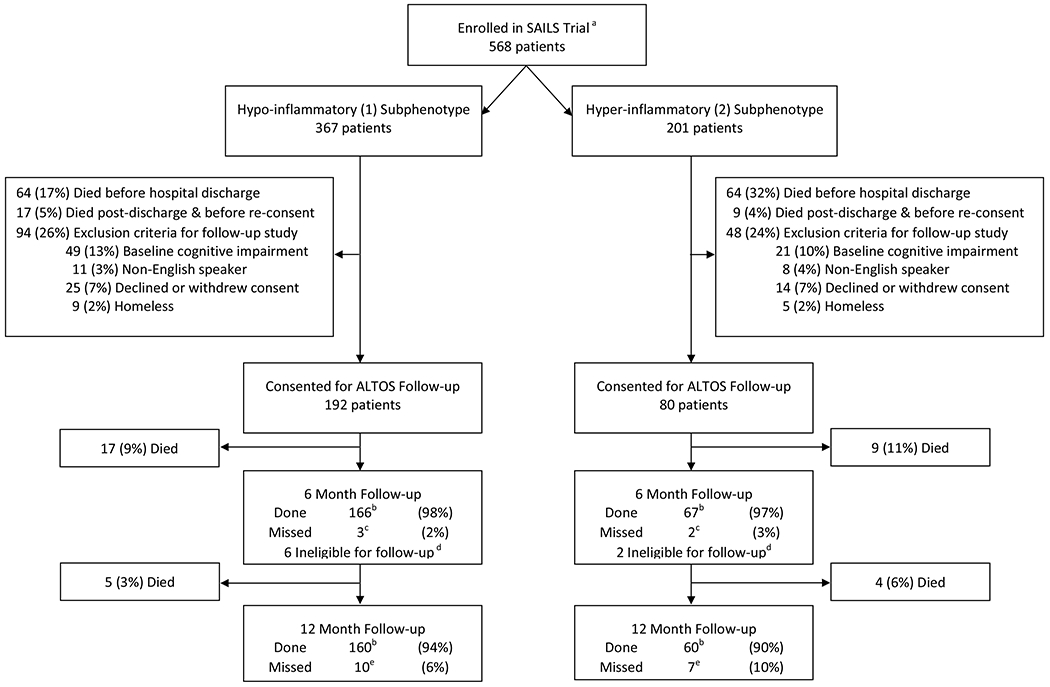
a Less than the 745-patient sample size of SAILS due to 2 sites not participating in this study and a temporary cessation of recruitment into this study due to funding-related issues.
b Details of the number of patients who underwent assessments for each of the outcomes are reported in Tables 3 and 4. One patient at each of 6- and 12-month follow-up periods did not complete all of the outcome measures and hence, they were excluded from the sample size reported in the manuscript and the data in Tables 3 and 4.
c Four of 5 patients missed were alive at 6 months, one missed patient in the hyper-inflammatory subphenotype had unknown mortality status at 6 months.
d Eight eligible patients were not eligible for follow-up due to temporary cessation of recruitment (due to funding-related issues) for many of the study sites.
e Ten of 10 patients and 6 of 7 patients were alive in the hypo- and hyper-inflammatory subphenotypes, respectively. One patient in the hyper-inflammatory subphenotype was censored for survival analysis.
In addition, patients enrolled from 12 of the 35 participating study sites underwent in-person assessments of physical performance at 6- and 12-month follow-up. These assessments included: 6-minute walk distance, 4-meter gait speed, hand grip, manual muscle testing using the Medical Research Council scale, and pulmonary function testing [25]. Cognitive function was evaluated during the index ICU stay via daily assessment of delirium status using the validated Confusion Assessment Method for the ICU (CAM-ICU) [26,32], and at 6- and 12-month follow-up, using a validated test battery that included assessment of executive function, language, verbal reasoning, and immediate and delayed memory, as previously described in detail [26].
Differences in each of the aforementioned outcomes related to physical, mental health and cognitive domains were compared between survivors with hypo- and hyper-inflammatory subphenotypes.
Statistical analyses
Descriptive statistics, using counts and percentages, or medians and interquartile ranges (IQR), were reported. Survival curves were plotted using the Kaplan-Meier method, and comparisons made using the log-rank test. Statistical testing for between-group differences among eligible survivors was conducted using the Wilcoxon Rank-Sum and Fisher’s exact tests for continuous and categorical variables, respectively. As described previously in the ALTOS parent study [26], missing data for delirium status was addressed via multiple imputation, with data summarized via Rubin’s method [33]. All analyses were performed using R Studio version 1.2.5033 (Boston, MA). A two-sided p<0.05 was considered statistically significant.
RESULTS
Of 568 eligible patients, 367 (65%) and 201 (35%) were in the hypo-inflammatory and hyper-inflammatory subphenotypes, respectively (Figure 1). Table 1 summarizes patient baseline and intensive care data for this entire cohort (N=568) and for those patients who survived their critical illness, met eligibility criteria for ALTOS, and completed post-discharge follow-up (N=243; Figure 1). Demographic data were similar between the two subphenotypes. However, as expected, most intensive care variables (e.g., shock at baseline, APACHE III score, and organ-failure-free days) were significantly worse in the hyper-inflammatory subphenotype (Table 1, Supplementary Table 1). Moreover, the hyper-inflammatory subphenotype had a greater burden of delirium in the ICU (Table 2).
Table 1.
Entire cohort and survivors’ baseline variables, stratified by subphenotype *
| Hypo-inflammatory | Hyper-inflammatory | |||
|---|---|---|---|---|
| Variable | Entire cohort N=367 |
Survivors N=173 |
Entire cohort N=201 |
Survivors N=70 |
| Baseline patient data | ||||
| Age, years | 53 (40,65) | 52 (40,60) | 56 (45,67) | 52 (43,61) |
| Female, N (%) | 178 (49) | 90 (52) | 106 (53) | 40 (57) |
| White, N (%) | 297 (81) | 147 (85) | 154 (77) | 57 (81) |
| Diabetes, N (%) | 71 (19) | 31 (18) | 56 (28) | 24 (34) |
| BMI, kg/m2 | 29 (24,35) | 29 (25,37) | 29 (23,34) | 31 (26,36) |
| Living at home independently, N (%) | 294 (80) | 153 (88) | 152 (76) | 61 (87) |
| Baseline intensive care data | ||||
| Medical ICU, N (%) | 233 (63) | 104 (60) | 123 (61) | 39 (56) |
| APACHE III | 80 (65,97) | 78 (61,94) | 111 (92,127) | 108 (87,125) |
| PaO2:FiO2 | 161 (122,212) | 152 (113,202) | 164 (122,211) | 154 (130,205) |
| Shock at baseline, N (%) | 111 (30) | 53 (31) | 139 (69) | 45 (64) |
| Primary lung injury risk factor, N (%) | ||||
| Pneumonia | 283 (77) | 129 (75) | 121 (60) | 45 (64) |
| Non-pulmonary infection | 55 (15) | 29 (17) | 54 (27) | 20 (29) |
| Aspiration | 22 (6) | 12 (7) | 17 (8) | 3 (4) |
| Trauma | 4 (1) | 2 (1) | 0 (0) | 0 (0) |
| Organ-failure-free days to day 14 ¶ | ||||
| Cardiovascular | 11 (8,13) | 12 (10,13) | 7 (2,11) | 10 (6,12) |
| Coagulation | 14 (14,14) | 14 (14,14) | 11 (4,14) | 14 (10,14) |
| Liver | 14 (14,14) | 14 (14,14) | 13 (4,14) | 14 (5,14) |
| Renal | 14 (13,14) | 14 (14,14) | 11 (3,14) | 14 (5,14) |
| Ventilator-free days to day 28** | 22 (8,25) | 24 (20,26) | 15 (0,22) | 20 (15,23) |
| ICU LOS, days | 10 (7,15) | 9 (7,14) | 13 (8,19) | 14 (9,19) |
| Hospital LOS, days | 16 (11,23) | 15 (10,22) | 20 (13,30) | 22 (14,34) |
APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; FiO2, fraction of inspired oxygen; ICU, intensive care unit; LOS, length of stay; PaO2, partial pressure of arterial oxygen.
Median values (IQR) are presented unless otherwise indicated. Survivors represent the patients who survived to hospital discharge and underwent 6 and 12-month follow-up assessments.
These variables represent the number of days without organ/system failure (as defined herein) during the first 14 days after randomization. Organs/systems were considered failure-free after patients were discharged from the hospital. Cardiovascular failure was defined as a systolic blood pressure <= 90 mmHg or use of vasopressor; coagulation failure defined as platelet count <= 80,000 per cubic millimeter; liver failure defined as serum bilirubin level of >= 2.0 mg per deciliter (34 μmol per liter); renal failure defined as serum creatinine >= 2.0 mg per deciliter (180 μmol per liter).
This variable represents the number of days without ventilator assistance during the first 28 days after randomization.
Table 2.
Delirium data *
| Total population (N=272) |
Hypo-inflammatory (N=176) |
Hyper-inflammatory (N=96) |
P‡ | |
|---|---|---|---|---|
| Delirium prevalence, % (95% CI) ¶ | ||||
| total population | 79 (74 – 84) | 74 (67 – 81) | 88 (81 – 95) | <0.01 |
| placebo group | 80 (72 – 87) | 75 (65 – 85) | 88 (78 – 98) | 0.06 |
| statin group | 78 (71 – 86) | 73 (64 – 83) | 88 (78 – 98) | 0.04 |
| Number of ICU days with delirium, mean (95% CI) | ||||
| total population | 3.9 (3.4 – 4.4) | 3.3 (2.7 – 3.8) | 5.0 (4.0 – 5.9) | <0.01 |
| placebo group | 3.4 (2.8 – 4.1) | 2.8 (2.1 – 3.5) | 4.5 (3.4 – 5.6) | 0.01 |
| statin group | 4.3 (3.5 – 5.1) | 3.7 (2.8 – 4.5) | 5.5 (4.0 – 7.1) | 0.04 |
| Proportion of ICU days with delirium, % (95% CI) ** | ||||
| total population | 40 (34 – 46) | 38 (30 – 45) | 44 (34 – 54) | 0.13 |
| placebo group | 38 (30 – 47) | 37 (27 – 48) | 40 (26 – 54) | 0.65 |
| statin group | 41 (33 – 50) | 38 (28 – 48) | 48 (33 – 63) | 0.09 |
Missing sedation (RASS) and delirium (CAM-ICU) data were imputed, as previously described in the parent study [26], with pooling over the imputed datasets conducted using Rubin’s method [33].
Calculated using two-sample test for proportions or means.
Delirium prevalence is the percentage of patients who had at least one positive CAM-ICU result among those assessed for delirium (patient days with a RASS score of −4 or −5 were not eligible for CAM-ICU assessment).
Calculated as the percentage of days in which patients were delirious out of all ICU days in which delirium was assessed (as defined above).
Survival in the hypo-inflammatory versus hyper-inflammatory subphenotype (Figure 2) was significantly greater during the first 90 days after randomization (78% versus 63%; log rank test: p<0.01), but not thereafter (89% versus 86% of patients surviving beyond 90 days survived through 12-month follow-up, p=0.70).
Figure 2. Survival analyses in the entire cohort (N=568) during follow-up*.
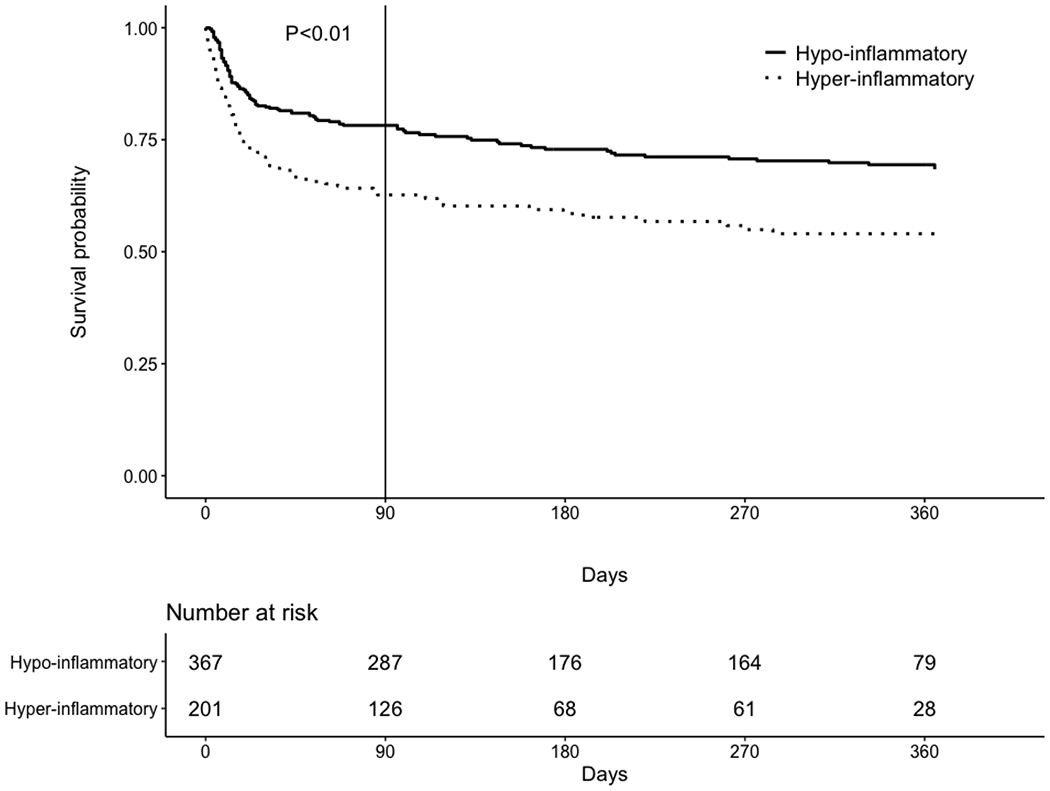
*Survival data are censored at 365 days of follow-up. For the hypo-inflammatory versus hyper-inflammatory subphenotype, there was no significant difference in survival beyond 90 days (89% versus 86% of patients surviving beyond 90 days survived through 12-month follow-up, respectively, p=0.70).
Functional outcomes at 6 and 12 months in the entire cohort
For 6-month follow-up assessments, data on functional outcomes were available for 165 (98%) of 169 and 67 (97%) of 69 eligible survivors in the hypo-inflammatory and hyper-inflammatory subphenotypes, respectively (Table 3). Among eligible survivors at 12-month follow-up, 160 (94%) in the hypo-inflammatory subphenotype and 59 (90%) in the hyper-inflammatory subphenotype underwent assessment (Table 4).
Table 3.
6-month outcomes, by subphenotype *
| Patient-reported outcomes† | Total N=232** |
Hypo-inflammatory N=165 |
Hyper-inflammatory N=67 |
P Value‡ |
|---|---|---|---|---|
| Health-related quality of life: SF-36 (normalized scores) | ||||
| Physical Functioning | 36 (23,48) | 36 (23,49) | 34 (21,47) | 0.12 |
| Physical Component Summary | 35 (28,47) | 37 (27,48) | 33 (29,43) | 0.29 |
| Mental Health | 47 (33,56) | 47 (33,56) | 44 (35,56) | 0.99 |
| Mental Component Summary | 47 (34,57) | 48 (33,58) | 44 (36,56) | 0.56 |
| Health-related quality of life: EQ-5D-3L | ||||
| Utility score | 0.77 (0.51,0.84) | 0.78 (0.56,0.84) | 0.71 (0.46,0.83) | 0.31 |
| Visual analogue scale | 72 (50,80) | 70 (50,80) | 74 (50,85) | 0.92 |
| Physical functioning | ||||
| Functional Performance Inventory-Short Form | 1.9 (1.3,2.5) | 2.0 (1.4,2.6) | 1.7 (1.2,2.4) | 0.14 |
| Mental health symptoms | ||||
| Anxiety subscale of HADS | 7 (3,11) | 6 (3,10) | 8 (4,12) | 0.14 |
| Depression subscale of HADS | 5 (2,10) | 5 (2,10) | 5 (2,10) | 0.81 |
| Post-traumatic stress disorder IES-R | 0.8 (0.2,1.7) | 0.7 (0.2,1.5) | 1.2 (0.3,1.9) | 0.11 |
| In-patient healthcare utilization | ||||
| Physical rehabilitation, N (%) ¶ | 51 (22) | 33 (20) | 18 (27) | 0.29 |
| Skilled nursing, N (%) ¶ | 18 (8) | 10 (6) | 8 (12) | 0.17 |
| Hospital re-admission, N (%) | 68 (29) | 49 (30) | 19 (28) | 1.00 |
| No. admissions to hospital, among those with admission | 1.0 (1.0,2.2) | 1.0 (1.0,2.0) | 1.0 (1.0,3.0) | 0.66 |
| Performance-based physical outcomes | N=65 | N=44 | N=21 | |
| 6-minute walk distance, % predicted | 66 (48,80) | 66 (48,80) | 66 (49,79) | 0.76 |
| 4-meter gait speed, m/s | 0.9 (0.8,1.1) | 0.9 (0.8,1.2) | 0.9 (0.8,1.0) | 0.48 |
| Hand Grip, % predicted§ | 78 (63,105) | 84 (65,107) | 77 (62,90) | 0.28 |
| Muscle strength, % maximum | 95 (92,98) | 96 (93,100) | 95 (90,98) | 0.26 |
| Maximal Inspiratory Pressure, % predicted | 91 (58,110) | 93 (60,113) | 82 (56,100) | 0.19 |
| FEV1 % predicted | 80 (62,91) | 80 (64,91) | 78 (58,89) | 0.63 |
| FVC % predicted | 77 (62,94) | 77 (63,95) | 77 (60,89) | 0.46 |
| Cognitive outcomes¥ | N=127 | N=89 | N=38 | |
| Executive function | 5 (3,6) | 5 (3,6) | 5 (2,6) | 0.78 |
| Language | 32 (23,39) | 33 (24,39) | 29 (23,38) | 0.48 |
| Verbal reasoning & concept formation | 10 (7,12) | 10 (7,12) | 11 (7,12) | 0.91 |
| Working memory & attention | 9 (8,11) | 9 (8,11) | 9 (7,11) | 0.42 |
| Immediate memory (Logical Memory I) | 9 (6,11) | 9 (7,11) | 7 (5,11) | 0.27 |
| Delayed memory (Logical Memory II) | 9 (6,11) | 9 (7,11) | 8 (6,11) | 0.33 |
FEV1, forced expiratory volume first second; FPI-Short Form overall score and subscale scores (for each, range: 0–3); FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; IES-R, Impact of Event Scale-revised; SF-36, Medical Outcomes Study Short Form 36 V.2.
Median values (IQR) are presented unless otherwise indicated. Higher scores are better, except for mental health outcomes.
One patient had a 6-month follow-up assessment, but did not complete all of the outcome measures and hence, is excluded from the sample size reported in this table.
Wilcoxon rank-sum test was used for continuous variables and Fisher’s exact test for proportions.
Explanation of scoring: SF-36 physical function and mental health normalized scores (mean=50; SD =10; range: 0–100); SF-36 physical and mental health summary normalized scores (mean=50; SD=10); EQ-5D utility score (range: −0.11 to 1.0) and visual analogue scale score (range: 0–100); FPI-Short Form overall score and subscale scores (for each, range: 0–3); HADS subscale scores for anxiety and depression (for each, range: 0–21, with scores ≥8 indicating substantial symptoms); IES-R (range: 0–4, with scores ≥1.6 indicating substantial symptoms); manual muscle testing score (range: 0–60, with scores <48 indicating ‘ICU-acquired weakness’).
Includes number of patients discharged from hospital to these facilities or admitted to these facilities in the course of follow-up.
Greater handgrip of right and left hands.
Explanation of scoring: executive function was evaluated with the Hayling Sentence Completion Test scaled score (range 1–10); language was evaluated with the Controlled oral word association total score; verbal reasoning and concept formation was evaluated with the Similarities age-adjusted scaled score (range 1–19); attention and working memory was evaluated with the Digit Span age-adjusted scaled score (range 1–19); and immediate and delayed memory was evaluated with the Logical Memory I and II age-adjusted scaled scores (range 1–19).
Table 4.
12-month outcomes, by subphenotype*
| Patient-reported outcomes† | Total N=219** |
Hypo-inflammatory N=160 |
Hyper-inflammatory N=59 |
P Value‡ |
|---|---|---|---|---|
| Health-related quality of life: SF-36 (normalized scores) | ||||
| Physical Functioning | 36 (25,51) | 36 (23,51) | 37 (28,50) | 0.48 |
| Physical Component Summary | 38 (28,51) | 37 (27,51) | 39 (31,50) | 0.73 |
| Mental Health | 47 (33,56) | 44 (33,56) | 53 (39,58) | 0.09 |
| Mental Component Summary | 47 (34,58) | 45 (33,57) | 55 (42,59) | 0.03 |
| Health-related quality of life: EQ-5D-3L | ||||
| Utility score | 0.77 (0.55,0.84) | 0.77 (0.52,0.84) | 0.78 (0.62,0.84) | 0.50 |
| Visual analogue scale | 75 (51,88) | 75 (50,90) | 75 (66,85) | 0.65 |
| Physical functioning | ||||
| Functional Performance Inventory-Short Form | 2.0 (1.4,2.6) | 2.0 (1.4,2.6) | 2.1 (1.4,2.6) | 0.87 |
| Mental health symptoms | ||||
| Anxiety subscale of HADS | 7 (2,10) | 7 (3,11) | 6 (2,10) | 0.61 |
| Depression subscale of HADS | 5 (1,10) | 6 (2,10) | 4 (1,9) | 0.05 |
| Post-traumatic stress disorder score- IES-R | 0.7 (0.2,1.7) | 0.7 (0.2,1.7) | 0.6 (0.2,1.5) | 0.62 |
| In-patient healthcare utilization | ||||
| Physical rehabilitation, N (%) ¶ | 8 (4%) | 5 (3%) | 3 (5%) | 0.69 |
| Skilled nursing, N (%) ¶ | 6 (3%) | 5 (3%) | 1 (2%) | 1.00 |
| Hospital re-admission, N (%) | 59 (27%) | 42 (26%) | 17 (29%) | 0.86 |
| No. admissions to hospital, among those with admission | 1.0 (1.0,2.0) | 1.0 (1.0,2.0) | 1.0 (1.0,2.0) | 0.70 |
| Performance-based physical outcomes | N=53 | N=37 | N=16 | |
| 6-Minute walk distance, % predicted | 74 (56,85) | 74 (61,84) | 71 (55,92) | 0.82 |
| 4-meter gait speed, m/s | 1.0 (0.8,1.2) | 1.0 (0.9,1.2) | 1.0 (0.8,1.1) | 0.67 |
| Hand grip, % predicted§ | 91 (69,109) | 93 (75,113) | 81 (67,101) | 0.18 |
| Muscle strength, % maximum | 97 (93,100) | 97 (93,100) | 97 (95,98) | 1.00 |
| Maximal inspiratory pressure, % predicted | 109 (77,120) | 110 (77,120) | 90 (70,122) | 0.41 |
| FEV1 % predicted | 80 (65,95) | 81 (69,95) | 78 (58,94) | 0.52 |
| FVC % predicted | 81 (63,93) | 82 (71,93) | 77 (58,91) | 0.34 |
| Cognitive outcomes¥ | N=141 | N=101 | N=40 | |
| Executive function | 6 (4,6) | 6 (5,6) | 6 (4,6) | 0.73 |
| Language | 32 (23,40) | 33 (24,41) | 27 (22,35) | 0.14 |
| Verbal reasoning & concept formation | 10 (8,12) | 10 (8,13) | 10 (8,12) | 0.91 |
| Working memory & attention | 9 (8,11) | 9 (8,11) | 9 (7,10) | 0.37 |
| Immediate memory | 9 (7,11) | 9 (7,11) | 8 (7,11) | 0.36 |
| Delayed memory | 9 (7,11) | 9 (7,11) | 8 (7,10) | 0.50 |
FEV1, forced expiratory volume first second; FPI-Short Form overall score and subscale scores (for each, range: 0–3); FVC, forced vital capacity; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; IES-R, Impact of Event Scale-revised; SF-36, Medical Outcomes Study Short Form 36 V.2.
Median values (IQR) are presented unless otherwise indicated. Higher scores are better, except for mental health outcomes.
One patient had a 12-month follow-up assessment, but did not complete all of the outcome measures and hence, is excluded from the sample size reported in this table.
Wilcoxon rank-sum test was used for continuous variables and Fisher’s exact test for proportions
Explanation of scoring: SF-36 physical function and mental health normalized scores (mean=50; SD =10; range: 0–100); SF-36 physical and mental health summary normalized scores (mean=50; SD=10); EQ-5D utility score (range: −0.11 to 1.0) and visual analogue scale score (range: 0–100); FPI-Short Form overall score and subscale scores (for each, range: 0–3); HADS subscale scores for anxiety and depression (for each, range: 0–21, with scores ≥8 indicating substantial symptoms); IES-R (range: 0–4, with scores ≥1.6 indicating substantial symptoms); manual muscle testing score (range: 0–60, with scores <48 indicating ‘ICU-acquired weakness’).
Includes number of patients discharged from hospital to these facilities or admitted to these facilities in the course of follow-up.
Greater handgrip of right and left hands.
Explanation of scoring: executive function was evaluated with the Hayling Sentence Completion Test scaled score (range 1–10); language was evaluated with the Controlled oral word association total score; verbal reasoning and concept formation was evaluated with the Similarities age-adjusted scaled score (range 1–19); attention and working memory was evaluated with the Digit Span age-adjusted scaled score (range 1–19); and immediate and delayed memory was evaluated with the Logical Memory I and II age-adjusted scaled scores (range 1–19).
For patient-reported outcomes, both subphenotypes had significant impairments in SF-36 physical and mental health domains when compared against age- and sex-matched population norms (Tables 3 and 4). For example, in the entire cohort, the median (IQR) value for the standardized (mean=50, standard deviation=10; range 0-100, with higher score indicating better function) SF-36 physical component summary scores at 6 and 12 month-follow-up were 35 (28, 47) and 38 (28, 51), respectively. Survivors also demonstrated impairment across the range of performance-based physical outcome measures, and cognitive assessments (Tables 3 and 4).
Comparison of functional outcomes between the two subphenotypes
Patient-reported outcomes were similar between hypo- and hyper-inflammatory subphenotypes at 6- and 12-month follow-up. For instance, the Functional Performance Inventory-Short Form median (IQR) score was 2.0 (1.4, 2.6) versus 1.7 (1.2, 2.4) at 6 months (p=0.14), and 2.0 (1.4, 2.6) versus 2.1 (1.4, 2.6) at 12 months (p=0.87) for the hypo- versus hyper-inflammatory subphenotype, respectively. Among performance-based physical outcomes, the median (IQR) value for hand grip percent predicted was 84% (65%, 107%) versus 77% (62%, 90%) at 6 months (p=0.28) and 93% (75%, 113%) versus 81% (67%, 101%) at 12 months (p=0.18) for hypo- versus hyper-inflammatory subphenotype, respectively. Similarly, there was no significant differences in cognitive function (Tables 3 and 4).
Comparison of changes over time from the 6-month to 12-month assessment demonstrated that hyper-inflammatory versus hypo-inflammatory subphenotype patients reported significantly better improvement in physical functioning and mental health symptoms (Supplementary Table 2). However, there was no significant improvement in performance-based physical outcomes or cognitive function (Supplementary Table 2).
When stratifying the analyses by treatment group (i.e., rosuvastatin versus placebo), there was no significant difference in outcomes between hypo-inflammatory and hyper-inflammatory subphenotypes (Supplementary Tables 3 and 4). Moreover, all patients enrolled from 5 of the 12 ARDSNet study sites (N=98) underwent additional evaluation, after discharge from hospital, that included retrospective assessment of their baseline (i.e., pre-ARDS) health-related quality of life and physical functioning. In this subset of patients, comparison between the hypo- (N=65) and hyper-inflammatory (N=33) subphenotypes showed no significant difference in any of these patient-reported outcome measures at baseline, 6 months, or 12 months (Supplementary Table 5). There also was no significant changes in outcomes over the duration of follow-up (Supplementary Table 6).
DISCUSSION
This analysis compared 6- and 12-month patient outcomes for the hypo-inflammatory versus hyper-inflammatory subphenotype among survivors of sepsis-associated ARDS from the multicenter SAILS randomized trial of rosuvastatin versus placebo. Prior studies demonstrated significant differences in short-term mortality between these two subphenotype groups, but have not evaluated longer-term survival or functional outcomes [3,4,6,11]. Comparing hypo- versus hyper-inflammatory patients in the current analyses demonstrated no significant difference in survival beyond the first 90 days, consistent with prior research evaluating other severity of illness measures [34]. Moreover, comparing hypo-inflammatory versus hyper-inflammatory patients, there were no consistent findings of important differences in physical, cognitive or mental health outcomes at 6- and 12-month follow-up. These findings can have important implications for clinicians caring for ARDS patients both in the ICU and after discharge. Moreover, in considering methods to enrich patient recruitment for ARDS randomized trials [35], the inflammatory subphenotype may have the greatest value for trials focused on achieving differences in short-term mortality.
Consistent with prior studies, our analyses demonstrated that hypo-inflammatory versus hyper-inflammatory patients demonstrated significantly better ICU and hospital length of stay, ventilator-free days, and short-term survival [3,4,11]. The greater burden of delirium in the hyper-inflammatory subphenotype is consistent with increased inflammation being part of the pathophysiology of delirium in the ICU setting [36].
Similar to other studies, our results also showed impairments in physical, cognitive and mental health outcomes in survivors at 6- and 12-month follow-up [37]. However, despite survivors’ differences in severity of illness and length of stay, when comparing the hyper- versus hypo-inflammatory subphenotype survivors, there were no consistent findings of clinically important differences in their 6- and 12-month physical, cognitive, or mental health outcomes. This finding may be best understood when considering several related issues. First, pre-ARDS baseline status is an important predictor of post-discharge status [38–42]. Our analyses demonstrated that, in an a priori subset of survivors, baseline status was similar between the hypo-inflammatory versus hyper-inflammatory subphenotype groups (Supplementary Table 5). Second, prior studies of ARDS survivors have demonstrated that severity of illness is not associated with mental health outcomes [41,43,44]. Third, a recent multicenter prospective cohort study demonstrated no association between individual inflammatory markers, measured early during critical illness, and cognitive outcomes and physical disability at 3 and 12 month follow-up [45]. Fourth, physical recovery after discharge is a dynamic process in ARDS survivors [17,46]. Differences in short-term rates of recovery (with potentially a greater rate of improvement in the hyper-inflammatory versus hypo-inflammatory subphenotype) may have impacted subsequent outcomes assessed at 6- and 12-month follow-up. Finally, the small improvements in patient-reported outcomes, but not performance-based tests, from 6 to 12-month follow-up in the hyper-inflammatory versus hypo-inflammatory subphenotype may reflect inherent differences between patient-reported and performance-based outcome measures [47], or be due to multiple statistical comparisons. Hence, this finding should be prospectively evaluated in future studies.
Our study has strengths, including its prospective and multicenter design, and low rates of loss to follow-up for the 6- and 12-month assessments. However, there are potential limitations. First, the study population was recruited from a national randomized trial with specific eligibility criteria focused on sepsis-associated ARDS, potentially limiting generalizability to broader patient populations. However, the same phenotypes were validated in other ARDS populations that were not specifically focused on sepsis as the primary risk factor [4,11]. Second, the sample size was based upon the parent trial, SAILS, and these analyses could be underpowered for assessing differences in functional outcomes. However, many of the observed differences between the two subphenotypes during 6- and 12-month assessments were small and may not be clinically important. Third, comparisons of functional outcomes can be conducted only among survivors, an analysis which may be subject to bias especially given the high mortality rate in ARDS patients [48]. Notably, there was little difference across survivors and non-survivors within subphenotype group; however, there could be unmeasured variables related to both survival and the functional outcomes that introduce bias in these comparisons. Forth, validation of our results is needed in future studies. Finally, pre-hospitalization status cannot be obtained prospectively given the acute and unpredictable onset of ARDS; thus, limiting the ability to fully understand the extent to which 6- and 12-month impairments were pre-existing. However, in a subset of patients, baseline status was estimated via retrospective assessment of relevant patient-reported outcome measures, and decrease from baseline was reported, consistent with prior findings [49]. Moreover, the vast majority of participants in ALTOS were living at home independently prior to admission as a crude measure of baseline function [25], and data from other studies with prospective baseline assessment demonstrate that new impairments occur among ICU survivors [50–52]. Nonetheless, assessment of premorbid status was limited and we cannot rule out the possibility of important unmeasured baseline differences between the subphenotypes.
CONCLUSION
In this multicenter evaluation of hypo-inflammatory versus hyper-inflammatory subphenotype status and 6- and 12-month outcomes among 243 survivors of sepsis-associated ARDS, there was no significant survival difference beyond 90 days. Despite inflammation being hypothesized as a mechanism for functional impairments after critical illness, patients in both inflammatory subphenotypes had no consistent findings of important differences in physical, mental health or cognitive outcomes at 6- and 12-month follow-up. These findings can inform clinicians caring for ARDS patients in the ICU and during follow-up. Additionally, in considering methods to enrich patient recruitment for ARDS trials, the inflammatory subphenotype may have its greatest value for trials focused on short-term mortality and related outcome measures, rather than longer-term functional outcomes.
Supplementary Material
KEY MESSAGES.
What is the key question?
In sepsis-associated ARDS, are there clinically important differences in 6 and 12-month physical, mental health or cognitive outcomes in patients with hyper-inflammatory versus hypo-inflammatory subphenotype?
What is the bottom line?
Despite significantly worse short-term mortality for the hyper-inflammatory versus hypo-inflammatory ARDS subphenotype, there was no significant difference in survival beyond 90 days, nor any consistent findings of important differences in 6- and 12-month physical, cognitive and mental health outcomes.
Why read on?
This study represents the first evaluation of a broad range of 6- and 12-month functional outcomes in survivors of sepsis-associated ARDS, stratified by inflammatory subphenotype during the acute phase of critical illness. The results of the study can be valuable for clinicians caring for ARDS patients both in the ICU and post-discharge, and for enriching patient recruitment for ARDS trials, where this inflammatory subphenotype may have its greatest value for trials focused on short-term mortality and related outcome measures, rather than longer-term functional outcomes.
Funding support:
National Heart, Lung and Blood Institute funded this follow-up study (N01HR56170, R01HL091760 and 3R01HL091760-02S1) and the SAILS trial (contracts HHSN268200536165C to HHSN268200536176C and HHSN268200536179C), along with the Johns Hopkins Institute for Clinical and Translational Research (ICTR) (UL1 TR 000424-06). Additionally, the SAILS trial was supported by the Investigator-Sponsored Study Program of AstraZeneca. CSC was supported by HL140026.
References
- 1.Matthay MA, Arabi YM, Siegel ER, et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med 2020;46:2136–52. doi: 10.1007/s00134-020-06296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffey JG, Kavanagh BP. Negative trials in critical care: why most research is probably wrong. Lancet Respir Med 2018;6:659–60. doi: 10.1016/S2213-2600(18)30279-0 [DOI] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018;6:691–8. doi: 10.1016/S2213-2600(18)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611–20. doi: 10.1016/S2213-2600(14)70097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delucchi K, Famous KR, Ware LB, et al. Stability of ARDS subphenotypes over time in two randomised controlled trials. Thorax 2018;73:439–45. doi: 10.1136/thoraxjnl-2017-211090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med 2018;44:1859–69. doi: 10.1007/s00134-018-5378-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JG, Calfee CS. ARDS Subphenotypes: Understanding a Heterogeneous Syndrome. Crit Care 2020;24:102. doi: 10.1186/s13054-020-2778-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network, Truwit JD, GR Bernard, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014;370:2191–200. doi: 10.1056/NEJMoa1401520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin to reduce pulmonary dysfunction in patients with acute respiratory distress syndrome: the HARP-2 RCT. Southampton (UK): : NIHR Journals Library; 2018. http://www.ncbi.nlm.nih.gov/books/NBK481405/ (accessed 2 Apr 2020). [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–75. doi: 10.1056/NEJMoa062200 [DOI] [PubMed] [Google Scholar]
- 11.Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195:331–8. doi: 10.1164/rccm.201603-0645OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witteveen E, Wieske L, van der Poll T, et al. Increased Early Systemic Inflammation in ICU-Acquired Weakness; A Prospective Observational Cohort Study. Crit Care Med 2017;45:972–9. doi: 10.1097/CCM.0000000000002408 [DOI] [PubMed] [Google Scholar]
- 13.Weber-Carstens S, Deja M, Koch S, et al. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care 2010;14:R119. doi: 10.1186/cc9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batt J, Herridge M, Dos Santos C. Mechanism of ICU-acquired weakness: skeletal muscle loss in critical illness. Intensive Care Med 2017;43:1844–6. doi: 10.1007/s00134-017-4758-4 [DOI] [PubMed] [Google Scholar]
- 15.Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care 2015;19:196. doi: 10.1186/s13054-015-0937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304. doi: 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 17.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42:849–59. doi: 10.1097/CCM.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander SA, Ren D, Gunn SR, et al. Interleukin 6 and apolipoprotein E as predictors of acute brain dysfunction and survival in critical care patients. Am J Crit Care 2014;23:49–57. doi: 10.4037/ajcc2014578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care 2012;16:R233. doi: 10.1186/cc11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Boogaard M, Kox M, Quinn KL, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care 2011;15:R297. doi: 10.1186/cc10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciel M, Benedet SR, Lunardelli EB, et al. Predicting Long-term Cognitive Dysfunction in Survivors of Critical Illness with Plasma Inflammatory Markers: a Retrospective Cohort Study. Mol Neurobiol 2019;56:763–7. doi: 10.1007/s12035-018-1166-x [DOI] [PubMed] [Google Scholar]
- 22.Hughes CG, Patel MB, Brummel NE, et al. Relationships between markers of neurologic and endothelial injury during critical illness and long-term cognitive impairment and disability. Intensive Care Med 2018;44:345–55. doi: 10.1007/s00134-018-5120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passos IC, Vasconcelos-Moreno MP, Costa LG, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015;2:1002–12. doi: 10.1016/S2215-0366(15)00309-0 [DOI] [PubMed] [Google Scholar]
- 24.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinglas VD, Hopkins RO, Wozniak AW, et al. One-year outcomes of rosuvastatin versus placebo in sepsis-associated acute respiratory distress syndrome: prospective follow-up of SAILS randomised trial. Thorax 2016;71:401–10. doi: 10.1136/thoraxjnl-2015-208017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med 2016;4:203–12. doi: 10.1016/S2213-2600(16)00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware J, Kosinski M, Dewey J. How to Score Version 2 of the SF-36® Health Survey. Lincoln, QualityMetric Incorporated; 2000. [Google Scholar]
- 28.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–20. doi: 10.1097/00005650-200503000-00003 [DOI] [PubMed] [Google Scholar]
- 29.Leidy NK. Psychometric properties of the functional performance inventory in patients with chronic obstructive pulmonary disease. Nurs Res 1999;48:20–8. doi: 10.1097/00006199-199901000-00004 [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 31.Weiss DS. The Impact of Event Scale: Revised. In: Wilson JP, Tang CS, eds. Cross-Cultural Assessment of Psychological Trauma and PTSD. Boston, MA: : Springer US; 2007. 219–38. doi: 10.1007/978-0-387-70990-1_10 [DOI] [Google Scholar]
- 32.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: : Wiley; 1987. [Google Scholar]
- 34.Wang CY, Calfee CS, Paul DW, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 2014;40:388–96. doi: 10.1007/s00134-013-3186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care 2019;25:12–20. doi: 10.1097/MCC.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers 2020;6:90. doi: 10.1038/s41572-020-00223-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012;40:502–9. doi: 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 38.Geense WW, van den Boogaard M, Peters MAA, et al. Physical, Mental, and Cognitive Health Status of ICU Survivors Before ICU Admission: A Cohort Study. Crit Care Med Published Online First: 16 June 2020. doi: 10.1097/CCM.0000000000004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffith DM, Salisbury LG, Lee RJ, et al. Determinants of Health-Related Quality of Life After ICU: Importance of Patient Demographics, Previous Comorbidity, and Severity of Illness. Crit Care Med 2018;46:594–601. doi: 10.1097/CCM.0000000000002952 [DOI] [PubMed] [Google Scholar]
- 40.Bienvenu OJ, Friedman LA, Colantuoni E, et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med 2018;44:38–47. doi: 10.1007/s00134-017-5009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabiee A, Nikayin S, Hashem MD, et al. Depressive Symptoms After Critical Illness: A Systematic Review and Meta-Analysis. Crit Care Med 2016;44:1744–53. doi: 10.1097/CCM.0000000000001811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry 2008;30:421–34. doi: 10.1016/j.genhosppsych.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016;43:23–9. doi: 10.1016/j.genhosppsych.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker AM, Sricharoenchai T, Raparla S, et al. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med 2015;43:1121–9. doi: 10.1097/CCM.0000000000000882 [DOI] [PubMed] [Google Scholar]
- 45.Brummel NE, Hughes CG, Thompson JL, et al. Inflammation and Coagulation during Critical Illness and Long-Term Cognitive Impairment and Disability. Am J Respir Crit Care Med Published Online First: 8 October 2020. doi: 10.1164/rccm.201912-2449OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfoh ER, Wozniak AW, Colantuoni E, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med 2016;42:1557–66. doi: 10.1007/s00134-016-4530-1 [DOI] [PubMed] [Google Scholar]
- 47.Iwashyna TJ, Walsh TS. Interplay of physiology, social, familial and behavioural adaptation in the long-term outcome of ARDS. Thorax 2017;72:872–3. doi: 10.1136/thoraxjnl-2016-209859 [DOI] [PubMed] [Google Scholar]
- 48.Colantuoni E, Scharfstein DO, Wang C, et al. Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ 2018;360:j5748. doi: 10.1136/bmj.j5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown SM, Wilson EL, Presson AP, et al. Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax 2017;72:1094–103. doi: 10.1136/thoraxjnl-2017-210337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson ME, Barwise A, Heise KJ, et al. Long-Term Return to Functional Baseline After Mechanical Ventilation in the ICU. Crit Care Med 2018;46:562–9. doi: 10.1097/CCM.0000000000002927 [DOI] [PubMed] [Google Scholar]
- 51.Barnato AE, Albert SM, Angus DC, et al. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med 2011;183:1037–42. doi: 10.1164/rccm.201002-0301OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sareen J, Olafson K, Kredentser MS, et al. The 5-Year Incidence of Mental Disorders in a Population-Based ICU Survivor Cohort. Crit Care Med Published Online First: 15 June 2020. doi: 10.1097/CCM.0000000000004413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


