Abstract
Mesenchymal stem cells (MSCs) differentiation toward cardiovascular lineage prediction using the global methylome profile will highlight its prospective utility in regenerative medicine. We examined the propensity prediction to cardiovascular lineage using 5-Aza, a well-known cardiac lineage inducer. The customized 180 K microarray was performed and further analysis of global differentially methylated regions by Ingenuity pathway analysis (IPA) in both MSCs and 5-AC-treated MSCs. The cluster enrichment tools sorted differentially enriched genes and further annotated to construct the interactive networks. Prediction analysis revealed pathways pertaining to the cardiovascular lineage found active in the native MSCs, suggesting its higher propensity to undergo cardiac, smooth muscle cell, and endothelial lineages in vitro. Interestingly, gene interaction network also proposed majorly stemness gene network NANOG and KLF6, cardiac-specific transcription factors GATA4, NKX2.5, and TBX5 were upregulated in the native MSCs. Furthermore, the expression of cardiovascular lineage specific markers such as Brachury, CD105, CD90, CD31, KDR and various forms of ACTIN (cardiac, sarcomeric, smooth muscle) were validated in native MSCs using real time PCR and immunostaining and blotting analysis. In 5-AC-treated MSCs, mosaic interactive networks were observed to persuade towards osteogenesis and cardiac lineage, indicating that 5-AC treatment resulted in nonspecific lineage induction in MSCs, while MSCs by default have a higher propensity to undergo cardiovascular lineage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-03058-2.
Keywords: Methylation, Pathway, Prediction, Lineage, Mesenchymal stem cells
Introduction
Cardiovascular diseases (CVD), mainly Myocardial infarction, cardiomyopathies are the leading cause of global deaths due to the loss of functional myocardium. The human myocardium has underprivileged regenerative potential; hence, the damaged myocardium failed to repair and further cause dysrhythmia, ventricular fibrillation, acute coronary occlusion leading to death (Krishna et al. 2011; Thygesen et al. 2007). Many regenerative approaches are being considered for the successful regeneration of damaged myocardium, reducing the global mortality rate due to CVD. Hitherto, Mesenchymal stem cells (MSCs) are considered as the choice of the cell for cardiovascular regenerative studies pertaining to its unlimited availability and unrestricted ethical concerns (Madigan and Atoui 2018; Doppler et al. 2013). Numerous studies have demonstrated differentiation protocols using MSCs effectively triggered cardiomyocyte (CM) differentiation. However, a low success rate was achieved in the overall differentiation of MSCs into functional cardiomyocytes (Makino et al. 1999; Moscoso et al. 2005; Yang et al. 2012, 2017; Fukuda 2003; Qian et al. 2012). Therefore, it is mandated to study the upstream regulatory region of MSCs for any interventions, thus causing incompetent differentiation of MSCs. Recently, DNA methylation a well-studied modification of the covalent addition of methyl group at the fifth carbon moiety in cytosine nucleotide, has gained more attention in lineage differentiation studies (Razin and Shemer 1995; Lachner and Jenuwein 2002). Any aberrations in the DNA methylation pattern directly led to cancer, dysregulated signaling pathways, delayed lineage commitment, and loss of gene function (Russo et al. 1996; Avgustinova and Benitah 2016).
DNA methylation is concomitantly coupled with gene expression, cell identity, the cell's inherent differentiation potential, and the cell's homeostasis to carry out the specified function (Cimmino 2017; Berdasco and Esteller 2011; Cakouros and Gronthos 2019; Jaenisch and Bird 2003). Mammalian genome globally has consistently methylated regions known as CpG islands, susceptible to methylation changes during the lineage-specific differentiation. Pertaining to pluripotency, global hypomethylation in ESCs and iPSCs ensures the self-renewal property and vast differentiation potential. However, hypomethylation at the enhancer’s regions, high expression of DNMTs, and TET enzymes are interrelated with the exit of pluripotency characteristics in ESCs or iPSCs. Therefore, it is evident that DNA methylation is crucial in establishing the epigenetic memory and regulates the cell type specific expression pattern, thus facilitating differentiation (Lee et al. 2014). Consequently, methylation microarrays have recently gained enormous attention in analyzing the various aspects of lineage differentiation. Representing a technical tool that predicts the normal gene expression landscapes and their interacting domains participating while lineage commitment (Sørensen et al. 2010; Franzen et al. 2017). This advanced robust predictive technical platform is very crucial for target gene identification in lineage induction studies and enabling us to understand the molecular mechanism occurring at the upstream level of DNA. Previous studies reported the expression of Brachury in the common cardiovascular precursor marker coincides with the lineage divergence between cardiomyocyte and endothelial/ hematopoietic lineage, respectively (Kattman et al. 2006; Moretti et al. 2006; Wu et al. 2006). Downstream lineage differentiation into various cell types depend on the expression of Isl/Flk/NKX2.5 for CM lineage and Flk/CD45−/CD31+/CD05+ in case of endothelial cells (Shafiee et al. 2018). Therefore, global microarray analysis in stem cells would benefit us to understand the lineage differentiation potential of progenitor population and identify the appropriate cell type for regenerative purposes.
Cardiovascular engineering approaches are highly dependent on identifying a suitable cell type that supports the differentiation and restores the damaged heart for regeneration. Therefore, the choice of cells and the suitable cues are the rate-limiting step in determining successive cardiac tissue engineering through regeneration (Ptaszek et al. 2012). Unlike ESCs and iPSCs, MSCs are the apparent choice of cells in the conduit considered for cardiac regeneration due to its immune modulation property. However, the occurrence of MSCs reported in a wide variety of adult tissue paradoxically, the differentiation potential of MSCs varies with the source and age of the source. Cardiovascular lineage differentiation is a multifaceted process through which a stem cell gains its fate upon induction with specific cues. The lineage commitment of pluripotent and multipotent cells is the direct cumulative consequence of various intrinsic and extrinsic factors leading to the induction, progression, and terminal differentiation of cells into cardiomyocytes (Li et al. 2009; Takahashi and Yamanaka 2006; Le et al. 2016; Miroshnikova et al. 2017; Maalouf et al. 2009; Thal et al. 2012; Ramesh et al. 2021; Guo et al. 2018). The notable intrinsic factors include cross talk between signaling pathways, expression of lineage-specific genes, and the suppression of irrelevant genes for a specific lineage, etc. (Li et al. 2009; Takahashi and Yamanaka 2006). On the other hand, the extrinsic factors that regulate the differentiation process are epigenetic cues, such as growth factors, mechanical cues, chemical cues, such as small molecule inhibitors, etc. (Makino et al. 1999; Le et al. 2016; Miroshnikova et al. 2017; Maalouf et al. 2009; Thal et al. 2012; Ramesh et al. 2021; Guo et al. 2018). “One such molecule is 5-Azacytidine (5-AC), a non-specific DNA methyl transferase inhibitor and a well-known cardiac inducing cues that has been widely used in the differentiation protocols (Ramesh et al. 2021). In current study, we analyzed the global upstream methylome profiling of MSCs and its efficiency to undergo cardiovascular lineage. We performed gene ontology analysis to identify common crosstalk lineage intersection markers in MSCs and 5-AC-treated MSCs with respect to cardiovascular lineages.”
Therefore, our current study provides the insights of DNA methylation profiling and its predictive interacting networks in MSCs derived from umbilical cord tissue and further validating the markers specific for cardiovascular lineage.
Materials and methods
Isolation & 5-Aza induction of MSCs
All necessary permissions and approvals for conducting this study using human medical waste umbilical cord has been obtained from the Institute Stem Cells Ethical Committee (IEC) of Indian Institute of Technology Madras, India (IEC/2016/01/RSV/-4/20). Human umbilical cords were transported within 1–2 h of the delivery from the hospital to the laboratory after obtaining consent from the patient. Isolation of MSCs from human Wharton’s Jelly region of Umbilical cord tissue was done as per the protocol described earlier (Seshareddy et al. 2008). Adherent spindle shaped cells after seeding were maintained in complete medium consists of α-MEM with 1X Non-Essential Amino acids, 1X sodium pyruvate, 1X L-Glutamine, 10% fetal bovine serum. Then, cultures were incubated at 37 °C in a humidified CO2 incubator. The culture medium was changed at every 2 days interval until 80% confluency was reached. After reaching confluency, cells were harvested using accutase enzyme and propagated at a ratio of 1:3 until 5th passage (P5) for the further experimental purpose. MSCs were characterized by FACS analysis for MSCs specific markers. WJ-MSCs at P3 were induced to cardiomyogenic lineage with 10 µM concentration of 5-AC in α-MEM medium supplemented with 2% Fetal Bovine Serum (FBS) for 48–74 h by following the modified protocol (Smits et al. 2009). After treatment, the culture was maintained in M199 medium with 10% horse serum and 5% FBS up to 30 days with intermittent media change in weekly twice.
DNA isolation
Furthermore, the DNA from MSCs, 5-Aza-treated MSCs and human auricular tissue (hCT) samples were extracted using Zymo quick gDNA extraction kit (ZYMO Corporation, USA), and genomic DNA integrity was assessed by running it on a 0.8% agarose gel during electrophoresis. Here, human auricular tissue obtained from human after consent serves the reference positive control for cardiovascular lineage.
Customized functional genomic distribution of 180 k DNA microarray chip
This study used a customized human 180 k DNA microarray chip (Agilent human methylation 180 k bead chip) consisting of 173,195 cytosine positions (180 k CpGs) selected from different regions of genes from the whole human genome. The selected CpGs from 22 pairs of autosomal chromosomes and a pair of sex chromosomes were further analyzed. Out of all 22 pairs, chromosome 1 contains the highest number of CpGs, which corresponds to 8%, and chromosome Y contains fewer CpGs comprising 0.4% (Govarthanan et al. 2020). Furthermore, DNA Methylation-specific microarray was performed, and the data were analyzed for gene interactome.
Pathway analysis of differentially methylated genes
Individual log ratios of MSCs, 5-AC-treated MSCs, and Cardiac tissue were included. Fold change was calculated by hMSC log-ratio/hCT. Genes with fold change ≥ 4 were categorized as hypermethylated, and fold change ≤ 4 were categorized as hypomethylated. The rest of the genes were considered as unaltered. To identify the canonical pathways and predict the upstream regulators, we used Ingenuity pathway analysis software (IPA®, Qiagen [CA, USA]) www.qiagenbioinformatics.com/products/ingenuitypathway-analysis).
To identify the genes, which are differentially regulated by methylation and the relevant biological processes, we first compared the MSC probes with the 5-AC-treated MSCs and then MSCs with hCT (human cardiac tissue). Comparing these two, we got the major pathways involved during functional cardiac tissue development. The upregulated genes with fold change ≤ 1.5 were selected and analyzed for functional enrichment using Enrichr (https://maayanlab.cloud/Enrichr). In WIKI pathway analysis result of Enrichr for upregulated or open CpGs unmethylated, pathways are depicted in Fig. 6. In the ontology analysis of Enrichr, we got the biological pathways involved as GO function and represented as Fig. S1.
Fig. 6.
IPA pathway analysis of genes enriched in hCT. A Bar graph representing the active status of signaling pathway obtained in the enriched set of genes in hCT, B Gene interactive networks active in the hCT and its domains of interaction
RNA extraction and cDNA synthesis
Total RNA was extracted from native MSCs, 5-AC-treated MSCs and cardiac tissue using Trizol method (Sigma Aldrich, Merck, USA) according to the manufacturer's recommended protocol. Mono nuclear fraction (MNCs) from umbilical cord rich in hematopoietic population was used as normalization control to quantify the expression profiles of various markers in cardiovascular lineages. Approximately, 2 µg of total RNA was reverse-transcribed into cDNA in a 20-µL reaction volume using MMLV-RT enzyme (Thermo Fisher Scientific, USA) and oligo-dT primers (New England Biolabs, USA). Semi-quantitative Real Time-PCR (RT-PCR) was performed with corresponding primers of specific cell types listed in Table S1. Briefly, a two-step cycling protocol was performed consisting of a single 10 min cycle at 95 °C, followed by 40 cycles of 10 s. at 95 °C, and 30 s. at 60 °C. The melting curve analysis was performed after amplification to ensure the specificity of products. The relative mRNA expression was quantified by normalizing with housekeeping gene β-actin and then, fold change was calculated by ΔΔct method.
Immunocytochemistry
Pathway analysis predicted the expression of protein markers cardiovascular lineage specific lineage markers were abundant in native MSCs. Further validation was performed by ICC analysis. Native MSCs were fixed with 2% paraformaldehyde solution for 40 min at room temperature and permeabilized with 0.25% Triton X-100 in 1 × PBS solution for 15 min. The fixed cells were washed with DPBS three times and incubated with blocking buffer containing 5% normal goat serum for 1 h at room temperature. Furthermore, cells were incubated with primary antibody for overnight at 4 °C with gentle shaking (The detailed antibody description with its dilutions is listed in Table S2). Then, cells were washed with DPBS and incubated again for next 2 h with appropriate secondary antibody (IgG Alexa flor-594/IgG-FITC with 1:1000 dilutions) at room temperature. Nuclei were stained with Hoechst 33,258 dye (Sigma Aldrich, Merck, USA). The stained cells were observed under fluorescence microscope (Nikon Tie, Japan) at 10 × magnification.
Protein extraction
1 × 106 cells (both MSCs and 5-Aza-treated MSCs) were trypsinized and harvested for whole cell protein isolation. Cell pellet was washed in Phosphate Buffer Saline (PBS) and suspended in lysis buffer (8 M Urea, 4% CHAPS, 40 mM Trizma base) added along protease inhibitor cocktail (ThermoFisher). The mixture was allowed to spin in low rpm for 1 h and centrifuged at 14,000 × g for 15 min at 4 °C. Supernatant was collected and sample-solubilizing buffer was added.
Immunoblotting
30 µg of sample was loaded and electrophoresed in Sodium Dodecyl Sulfate Poly-Acrylamide Gel Electrophoresis (SDS-PAGE). Nitrocellulose membrane was used to transfer the protein on a wet BIORAD transfer apparatus at 100v for 2 h in cold. The membrane was blocked using 5% Skimmed milk in TBS (10 mM Tris, 150 mM NaCl) for 1 h at room temperature. Primary antibodies (mentioned in supplementary material, 1:1000 diluted in 5% BSA containing TBST) were incubated at 4 °C, overnight. Blots were washed once in TBST and incubated in secondary antibody (mentioned in supplementary material, 1:10,000 diluted in 5% BSA containing TBST) for 1 h at room temperature followed by three washes in TBST. Protein bands were detected using enhanced chemiluminescence system (Thermo Scientific Pierce ECL) in BIORAD Versa Doc TM MP 4000 system. Band intensity was calculated using Image J software.
Statistical analysis
All experiments were performed in biological triplicates (n = 3). The statistical analysis was performed by two way ANOVA test multiple comparison test using graph pad prism 6.0 software. For multiple comparisons, Sidak multiple comparison test was used with 95% confidence interval. In all statistical analyses, p < 0.05 value was considered as significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Microscopic findings
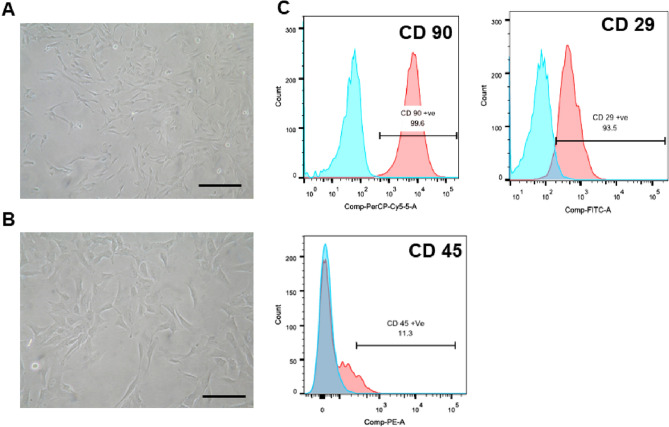
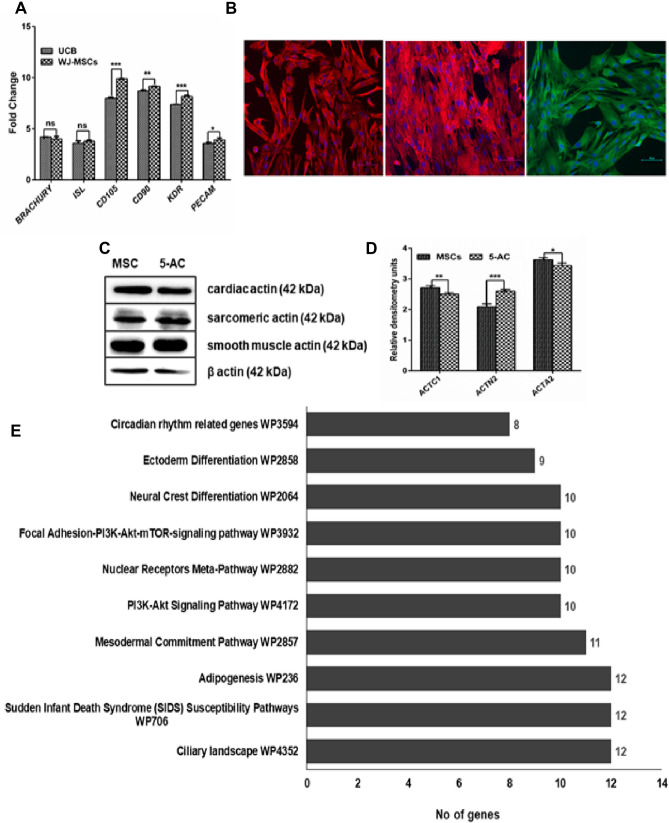
Passage 3 MSCs were used in the study. The isolated MSCs showed typical spindle fibroblastoid shaped morphology (Fig. 1 A, B) and FACS analysis showed positive expression for CD 29, and CD 90, markers, and negative for CD 45 (Fig. 1C). Tri-lineage differentiation induction studies confirmed the successful differentiation into adipocytes, osteocytes and chondrocytes, respectively (data not shown).
Fig. 1.
Isolation and characterization of MSCs. A, B Phase contrast microscopic analysis of a spindle fibroblastoid morphology of MSCs (Scale bar- A. 400 µm, B. 100 µm). C Immunophenotyping of WJ-MSCs showing positive for CD29, CD90, and negative for CD45
Microarray predictive gene expression analysis
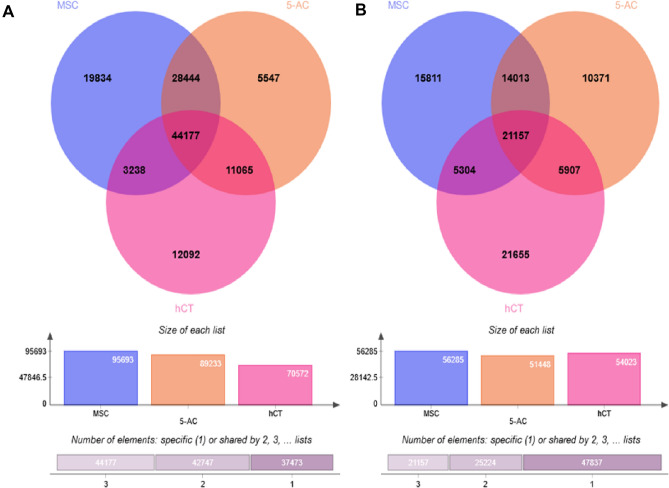
On a global scale, Venn diagram representation clearly depicted that native MSCs has higher number of hypomethylated CpGs, as represented in Fig. 2A, and in-depth sorted analysis of overlapped genes from other two-sample, we found higher number of open CpGs (n = 19,834) as similar to the previous pattern. Whereas the least unmethylated CpGs were found in 5-AC-treated MSCs, respectively. However, after segregating the overlapped genes from the samples, we found hCT have more hypermethylated CpGs (n = 21,655) may be due to its state of terminal differentiation (Fig. 2B). Whereas, the least methylated CpGs were found in 5-AC-treated MSCs that anticipated inhibitory effect due to the nonspecific methyl transferase inhibitory effect incurred due to 5-AC treatment. Thus, our microarray data evidently suggested that MSCs possess a more open promoter regions compared with 5-AC-treated and cardiac tissue (hCT), as shown in Fig. 2A. This clearly portrayed the stem cell property of MSCs having a more open and conducive environment for undergoing differentiation into various lineages.
Fig. 2.
Global comparative methylation profiling in Venn diagram representation- Differentially methylated genes obtained in 180 K microarray analysis was represented in the form of Venn diagram depicting both overlapping genes and uniquely expressing in the samples compared, i.e., native MSCs, 5-AC-treated MSCs and human cardiac tissue. Comparative analysis obtained from native MSCs, 5-Aza-treated MSCs and cardiac tissue control. A Hypomethylated CpGs, B hypermethylated CpGs
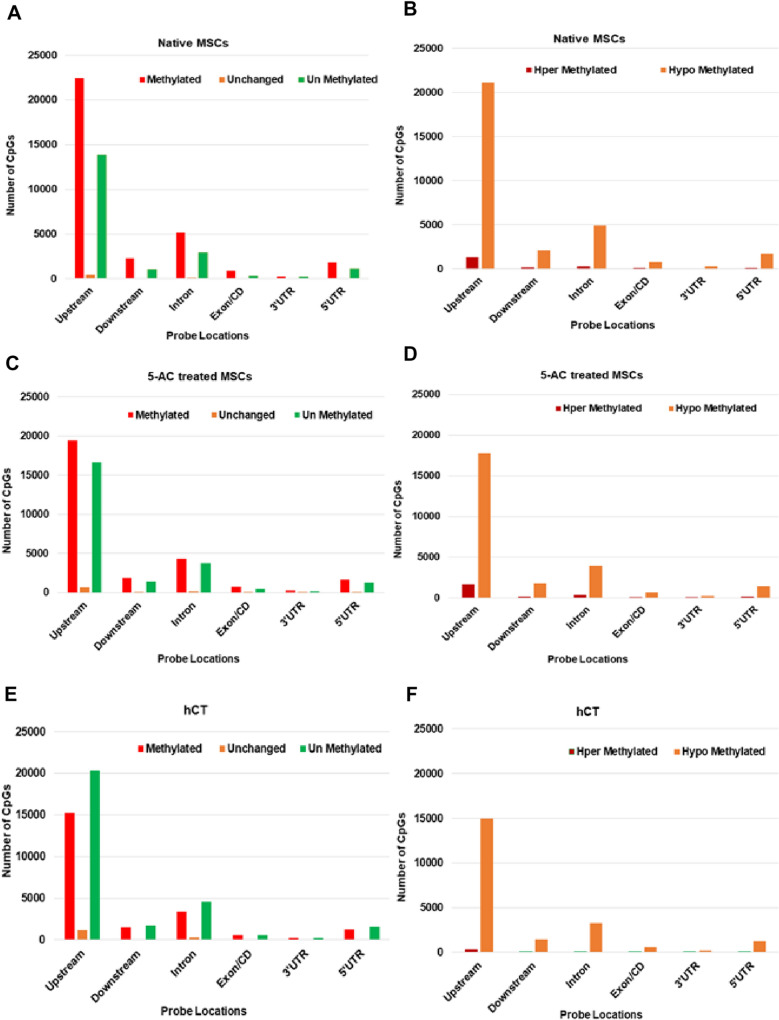
Furthermore, all the samples were subjected to the global functional genomic distribution CpGs location analysis. It showed a higher deposition of methylated and unmethylated CpG marks at the upstream location, which is known to constitute the gene promoter, enhancer, and silencer regions, respectively (Fig. 3 A, C, E). The least distributions of CpG marks were found in the region of 3'UTRs and Exon/CD domains of the gene (Fig. 3 A, C, E). Furthermore, we were interested in analyzing the distribution of Methyl-CpGs at the global scale across the genome for the occurrence of hyper- and hypomethylated state, i.e., densely methylated and sparsely methylated levels. Data analysis highlighted that native MSCs possess the most occurrence of least or sparsely methylated CpGs as compared with the other two samples. This demonstrates the higher presence of modifiable hypomethylated CpGs regions at the promoter region in native MSCs, which corresponds to the more plasticity of MSCs (Fig. 3 B, D, F). As expected, the least occurrence of hypomethylated CpGs in the cardiac tissue may be correlated with its terminally differentiated state (Fig. 3 B, D, F).
Fig. 3.
Functional genomic distribution with probe locations of CpGs in samples analyzed—A–F) Bar charts representing overall genomic distribution of CpGs across the genome in all the three samples. Data portrayed the occurrence of more CpGs at the promoter region and the least at the 3’ prime UTR region, respectively
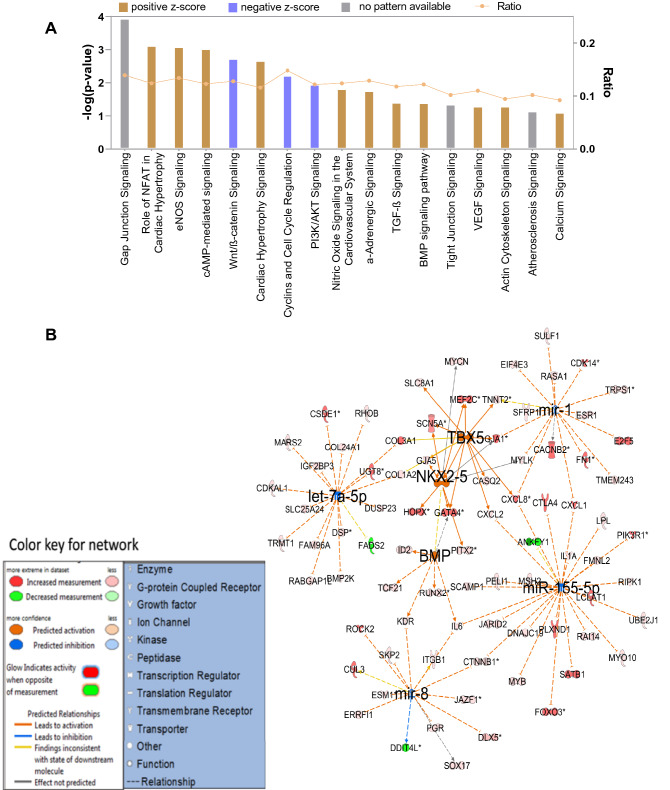
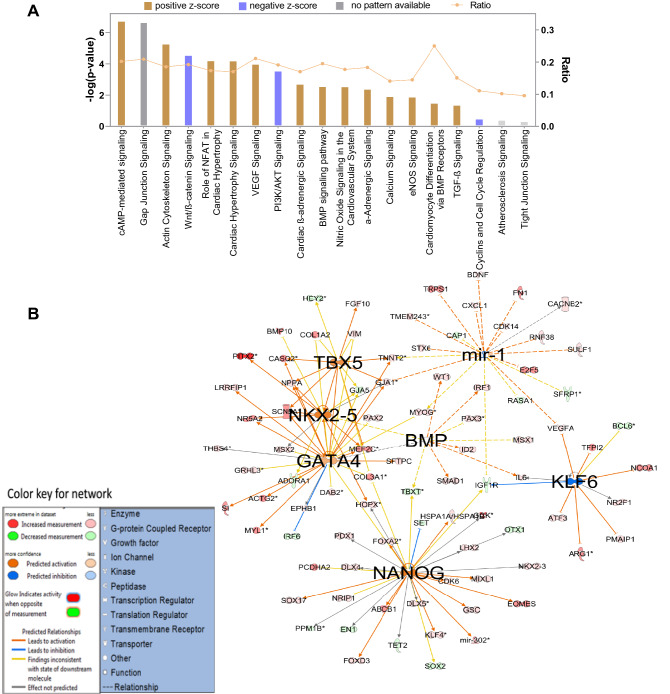
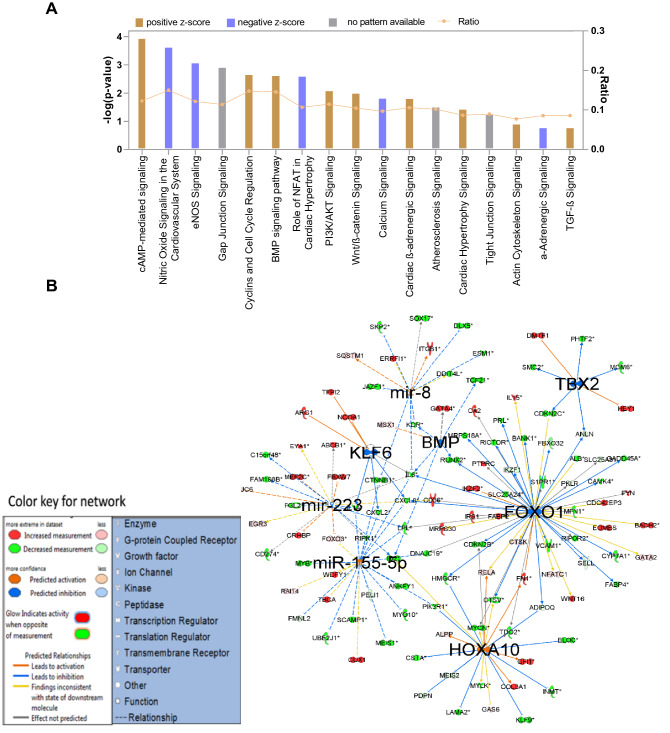
On subjecting the enriched genes into IPA pathway analysis software, we found that cAMP-mediated signaling, actin cytoskeleton signaling, NFAT signaling in cardiac hypertrophy, VEGF signaling, BMP pathway, and cardiomyocyte differentiation via BMP signaling pathways were found to be in the functional state in native MSCs (Fig. 4A). IPA gene networking analysis in Fig. 4B showed the activation nodes pertaining to Nanog, GATA4, NKX-2.5, and TBX5 again substantiated our previous study that WJ-MSCs showed inherent open methylation status with respect to cardiac transcription factors such as GATA4, NKX-2.5, and TBX5 had the unrestricted potential to become CM, provided with appropriate cues of induction (Govarthanan et al. 2020). In Fig. 5A, 5-AC-treated MSCs showed varied profiling of upregulated signaling pathway analysis compared with the native state. Here, signaling pathways linking to Nitric oxide signaling, calcium signaling, and α-adrenergic signaling have been shut down with negative z scores rendering a compromised state of differentiation has acquired; however, in the case of native MSCs, both pathways remain to be in an active state. This shows that treatment with a nonspecific de-methylating agent has triggered the process of mosaic differentiation patterns in treated MSCs. In addition, HOXA 10 gene network has known to interact with NKX 2.5 to induce cardiac mesoderm differentiation. Here, the expression of NKX-2.5 was not observed in an active state, thus showing a varied pattern in inducing differentiation into cardiac like (Fig. 5B). Interestingly, few miRs such as miR 223, miR 155-5p, and miR 8 were predicted to be dysregulated as compared between native hMSCs and hCT (Fig. 5B).
Fig. 4.
IPA pathway analysis of genes enriched in native MSCs. A Bar graph representing the active status of signaling pathway obtained in the enriched set of genes in MSCs, B gene interactive networks active in the MSCs and its domains of interaction
Fig. 5.
IPA pathway analysis of genes enriched in 5-AC-treated MSCs. A Bar graph representing the active status of signaling pathway obtained in the enriched set of genes in 5-AC-treated MSCs, B Gene interactive networks active in the 5-AC-treated MSCs and its domains of interaction
In Fig. 6A, our signaling pathway analysis showed a positive score in the following pathways, such as eNOS signaling, α-adrenergic signaling, calcium signaling, VEGF signaling, α-actin cytoskeleton signaling, etc. in hCT samples. The aforesaid signaling status was matched with already known cardiac signaling pathways, which in turn substantiated the prediction analysis accuracy. On the other hand, the gene interaction nodes portrayed similar expected activation domains of cardiac-specific transcription factors, such as BMP, NKX 2.5, and TBX5, where except BMP, the remaining others were found to be active in the native state itself (Fig. 6B). Paradoxically, negative expression of neuron specific-miRNA let-7a-5p and hematopoietic specific miR 155-5p and its putative role in human heart development and differentiation is yet to be underscored.
We also performed the global dysregulated pathways analysis by comparing the open CpGs or active promoter regions of native MSCs and human cardiac tissue samples. The rationale behind the analysis is to understand the pathways, which are commonly upregulated in MSCs and its relevance in undergoing the other lineage commitment and differentiation. Here, we found that a plethora of pathways attributes to mesodermal commitment, ectoderm, and neural crest differentiation were upregulated in native MSCs (Fig. 7D). In addition, the focal adhesion PI3K–Akt–mTOR signaling pathways were found to be dysregulated in MSCs after treatment with 5-AC (Fig. 7D). This predictive data is very crucial in selecting or identifying the triggering or induction agent to regulate the pathway and to overcome the concerns leading to a successive differentiation event. The molecular functional pathway analysis also suggested that genes binding to DNA, RNA, RNA polymerase II specific regulatory region, and other genes related to transcriptional machinery were upregulated in native MSCs. Hence, predicting that MSCs are highly transcriptionally active cell types having greater proliferative property and higher plasticity (Fig. S1).
Fig. 7.
A–C Validation of cardiovascular lineage specific markers in WJ-MSCs. A Gene expression analysis of native MSCs showing upregulated expression of cardiovascular lineage specific markers, such as Brachury, ISL, KDR, PECAM, CD105, CD90, B ICC analysis showing innate MSCs profoundly expression Vimentin, SERCA and alpha smooth muscle actin (Scale bar-100 microns), C Immunoblot showing expression of actins, such as cardiac, smooth muscle specific, and sarcomeric actin in MSCs and 5-AC-treated MSCs, D Densitometry analysis of Immunoblot C panel. E Gene Ontology Wiki pathway analysis of dysregulated genes—Comparative analysis of pathways that are specifically active in MSCs with hCT
Gene expression analysis
We further validated our microarray findings for the expression of genes specific for cardiovascular lineages in MSCs using real time gene expression analysis. We used mononuclear fraction obtained from umbilical cord blood (UCB) to normalize and study the cardiovascular markers expression profiles in MSCs derived from Wharton’s jelly. Since UCB is rich in common progenitor populations harboring cardiovascular subset populations. We found that the mesodermal markers (Brachury, ISL1), endothelial markers (KDR, PECAM (CD31), endoglin (CD105), and fibroblast marker (CD90) were consistently upregulated in native MSCs obtained from umbilical cord Wharton’s jelly (Fig. 7A). The MSCs default markers are not uniquely specific for MSCs; however, it shares common markers for mesoderm such as CD105 (endoglin), CD31 (PECAM), KDR, Brachury inferring that the MSCs from WJ region has multifaceted lineage differentiation potential with respect to cardiovascular lineage. The gene expression analysis showed a significant fold change in KDR (0.8-fold, ***p < 0.001), PECAM (0.3-fold *p < 0.05), CD90 (0.45-fold, **p < 0.01), and CD105 (1.91-folds, ***p < 0.001); whereas no significant change was obtained in Brachury (0.19-fold), and ISL1 (0.19-fold), respectively.
Immunocytochemistry (ICC) analysis
Furthermore, the expression of mesenchymal specific proteins expression was validated by ICC and immunoblot analysis. Our ICC analysis revealed that inherent profound expression of cardiovascular lineage specific markers, such as Vimentin, α smooth muscle cell actin, and SERCA in native MSCs, respectively (Fig. 7B). The above said markers were also expressed in mesodermal derived lineage cells, such as CM, Smooth muscle cells and endothelial cells, respectively.
Immunoblotting findings
Our immunoblot analysis also shown the default expression of distinct actins specific for cardiac, smooth muscle cell and sarcomeric actin in native MSCs and as well as in the 5-AC-treated MSCs (Fig. 7C). Our findings showed the common cardiovascular lineage markers such as α-smooth muscle actin (ACTA2), cardiac actins (ACTC1) and sarcomeric actin (ACTN2) were expressed in significant levels in native MSCs as suggested mesodermal origin MSCs has inherent expression of actins specific to cardiovascular lineages at protein levels.
Discussion
Cell-based regenerative therapy ideally contributes to the regeneration of damaged myocardium and favors the effective neovascularization that eventually improves the clinical treatment lacking severe side effects. Although numerous clinical trials were conducted employing autologous bone marrow MSCs, still the results revealed poor prognosis due to its restricted proliferation, homing, and differentiation potential in aged CVD patients. Therefore, a manifestation of multipotent cardiovascular progenitors from an allogenic source such as the umbilical cord offers huge anticipations of the unrestrained supply of such populations for therapeutic purposes pertaining to CVDs. Perhaps, a detailed investigation of MSC's propensity to undergo cardiovascular lineage differentiation would refine our knowledge regarding the putative cardiogenic potential of MSCs. Here, in our current study, we investigated the prospective developmental pathways enriched with differentially methylated regions in Wharton's jelly MSCs and its predictive gene integration network for its potential to undergo efficient downstream lineage differentiation programs.
Previously, our group has reported that MSCs from WJ source has a permissive cardiomyogenic fate with respect to cardiac-specific genes using 180 K DNA methylation microarray analysis. Furthermore, we also demonstrated WJ-MSCs were inherently known to express cardiac markers in its native state and could be considered as an apparent candidate for cardiac regenerative therapy. (Govarthanan et al. 2020). It is widely reported that MSCs by default, have endowed with the restricted lineage differentiation potential, and restriction is mainly correlated with the age and source of the isolation (Hill et al. 2019). Furthermore, in our study, we engrossed to perform an in-depth global microarray analysis in MSCs, 5-AC-treated MSCs, and through which we handpicked the enriched gene promoter region for further pathway prediction analysis. In the current study, we probed for the overall lineage interaction networks through the enriched gene prediction tools and studied its global impact on MSCs in inducing differentiation. Our analysis predicted that the pathways related to lineage commitment, cardiac hypertrophy, and upregulation of myomiRs related to myoblast proliferation and differentiation were picked up in the initial sorting process. Interestingly we observed that WJ-MSCs showed enriched differentially hypomethylated regions at the aforesaid pathways. It is understood that MSCs from WJ origin showed activation profiles having permissive methylome for cardiovascular lineage and could be an ideal candidate cell type for cardiovascular regenerative therapies. An earlier study by Amable et al., 2014 demonstrated the pro-angiogenic factor secretion by WJ-MSCs, was corroborated with our prediction data; WJ-MSCs exhibited a hypomethylated open promoter region for angiogenesis and eNOS signaling pathway and thereby gained an increased propensity to undergo endothelial cell lineage (Amable et al. 2014). Similarly, in another study, Rammal et al. 2017 demonstrated the differentiation of WJ-MSCs was more efficient under synergistic culture conditions in polyallylamine hydrochloride/polystyrene sulfonate coated surface and angiogenic factors into endothelial-like cells. Differentiated WJ-MSCs exhibited upregulated expression of endothelial markers, such as KDR, VEGF-R2, CD31, and CDH5 gene as compared with bone marrow MSCs (Rammal et al. 2017). Our promoter enriched pathway analysis further strengthens the previously demonstrated in vitro data, showing that WJ-MSCs versatile differentiation potential into the cardiovascular lineage.
Bone Morphogenetic Proteins (BMP) were predicted to be negatively dysregulated correlated with the successive activation of Notch, Wnt, and Bmp signaling, essential during the specification and differentiation of cardiac progenitor cells (Klaus et al. 2012). Many studies have also demonstrated that bone morphogenetic protein (BMP) signaling plays a central role in the induction of cardiac myogenesis. Under the influence of BMPs, the conversion of pre-cardiac cells to cardiac-like cells was observed via upregulation of cardiac-specific transcription factors NKX-2.5 and GATA4 (Schultheiss et al. 1997). In addition, KLF6 was predicted to be dysregulated and often reported to trigger the myoblast proliferation and its survival with enhanced myoblast differentiation (Behrens et al. 2013). Lupu et al. 2011 demonstrated the intrinsic integration potential of WJ MSCs into the in vitro ischemic embryonic ventricular slices model study. The research group has identified the existence of a novel MSCs population having a shared characteristic feature of endothelial-colony-forming cells at the functional level. Hence, the studies showing the MSCs cardioprotective response to home towards the cardiac injury site in turn, naturally due to the prevalence of enriched cardiovascular lineage progenitors in the specific source (Lupu et al. 2011). Concisely, it is well evident with our predicted and previously demonstrated data (Sturzu and Wu 2011) that WJ-MSCs have enhanced potential to differentiate into cardiovascular lineage sub-types; and could be considered as a clinically ideal source for cardiovascular tissue engineering approaches.
The IPA gene interaction analysis showed active HOXA 10 gene nodes predicting the differentiation is slightly inclined to osteogenic lineage, as it is already known that HOXA 10 expression is often co-related with osteogenesis (Hassan et al. 2007). However, another master regulator for osteogenesis FOXO1 seems to be negatively regulated in treated conditions showing that the treatment caused spontaneous mosaic differentiation into various nonspecific lineages, such as osteogenic and cardiomyocytes. Noticeably, 5-AC treatment was observed with a negative score in cyclin and cell cycle regulation network, Wnt/Beta-catenin pathway, and PI3/AKT pathway clearly demonstrated that the treated MSCs population were no longer proliferative in state and depicts the loss of stemness has incurred after treatment. In 5-AC-treated MSCs the predicted activation node in HOXA 10 gene network suggested that the treatment might have induced expression; however, the known interactive network partner for HOXA 10 gene network was NKX 2.5. The downstream absence of expression of NKX-2.5 suggested a varied pattern in inducing differentiation into cardiac such as in the differentiation induced 5-AC-treated MSCs (Fig. 5B).
In addition, it was essential for the specification and differentiation of early cardiac progenitors by modulating NKX 2.5 and GATA4, respectively (Behrens et al. 2013). FOXO1 was also reported to impair cardiac hypertrophy by interacting with the autophagy pathway in cardiac cells (Sengupta et al. 2009). Remarkably, a small number of miRs such as miR 223, miR 155-5p, and miR 8 were anticipated to be dysregulated as compared between native hMSCs and hCT (Fig. 5B). Thus, our data predicted new targets, which may potentially find to have a putative function in converting MSCs into fully functional cardiac-like cells. Hence, we are suggesting via our differential methylation microarray data that MSCs have enormous potential to differentiate into cardiac-like cells with respect to its DNA methylation status. However, the effective and efficient differentiation will be achieved by careful screening of small molecules that fine-tunes the cardiac enriched histone expressive marks.
Further validation of cardiovascular lineage markers, such as Brachury, Isl, KDR, CD105, CD90 and PECAM in MSCs at native population, suggesting MSCs inherent cardiovascular lineage supporting potential. Expression of Brachury and Isl in the MSCs from UC were in corroboration with the previous findings of Kattman et al. 2006; Moretti et al. 2006; Wu et al. 2006. We believe that MSCs from extraembryonic source derived would be of rich in progenitor population having multipotent nature. Further our hypothesis was strengthened by the report of existence of bi-potent meso-endothelial progenitors in human placenta exhibiting the expression profile of CD45−/CD34+/CD144+ /CD31low (Shafiee et al. 2018). In addition, these CD73high MSCs conferred heightened anti-inflammatory property via attenuating the microphage infiltration and augmenting the upregulated anti-inflammatory gene expression in the infarcted site. Absence of such events in CD73low MSCs subpopulation showing its higher propensity to regenerate cardiovascular damage with augmented anti-inflammatory property (Liu et al. 2019). Another study demonstrated that murine pre-cardiac adipose tissue derived MSCs profiled with high CD73 expression shown to ameliorate the structural and functional repair via angiogenesis in mouse myocardial infarction model (Li et al. 2021). In our study also MSCs expressing CD73high (data not shown), exhibiting the inherent expression of markers pertaining to mesodermal specific cardiovascular lineage revealing WJ-MSCs could be considered as a versatile stem cell hoping to resolve the complications and management of CVDs in future. Our study scope is to evaluate the expression of cardiovascular lineage specific markers in native and 5-AC-treated MSCs as predicted by 180 K methylation specific microarray analysis. We have limited our study by screening specific markers at its native state, however, further inducing corresponding differentiation and followed by its validation would give us the clear picture of our predicted analysis.
Conclusion
The current study predicts that pathways pertaining to the cardiovascular lineage found to be active in the native MSCs, suggesting its higher propensity to undergo cardiac, smooth muscle cell, and endothelial lineages in vitro. Our study lacks the functional validation via inducing cardiovascular linear differentiation; however, our preliminary data validation analysis laid the strong foundation for future aforementioned studies. Human umbilical cord derived MSCs inherently expressed cardiovascular lineage markers might be pertaining to its common mesodermal origin. Henceforth, our study underpinned that WJ-MSCs may be the ideal candidates for regenerative studies relevant to cardiovascular lineages as prophesied by our putative gene ontology analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wished to acknowledge Dr. Ravi, Dr. Nand Kishore Kapadia, and Ms. Anita from Malar hospital, Chennai, Tamil Nadu, India, for obtaining the consented cardiac biopsy samples for the study. KG, VS, BD, RY, DR, BZE, are thankful to the University Grant Commission (UGC) and Ministry of Human Resource and Development (MHRD), Govt. of India for providing the fellowship. Dr. Piyush Kumar Gupta is thankful to the Sharda University for the infrastructure and research facilities. Prof. Rama Shanker Verma is grateful to the Indian Institute of Technology Madras for providing the infrastructure and research facilities.
Author contributions
KG designed the whole study, performed all the experiments with data analysis, and wrote the manuscript. PKG, BD and RY aided in data interpretation and manuscript preparation. DR, BZE, and VS assisted in manuscript preparation and figure compilation. PK and DS contributed their role in data analysis. RSV conceived the whole study and was in charge of designed work. All authors finally provided their critical feedbacks and contributed to manuscript preparation.
Declarations
Competing interests
The authors declare no competing interests.
Ethical disclosure
The proposed study was performed with human medical wastes such as umbilical cord, umbilical cord blood, human auricular cardiac biopsy sample which were approved by the Institute Stem Cells Ethical Committee (IEC) of Indian Institute of Technology Madras, India (IEC/2016/01/RSV/-4/20). All procedures performed during the study involving human participants were in accordance with the ethical standards of the institutional and/ or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. 2014;5(2):53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol. 2016;17(10):643–658. doi: 10.1038/nrm.2016.76. [DOI] [PubMed] [Google Scholar]
- Behrens AN, Iacovino M, Lohr JL, Ren Y, Zierold C, Harvey RP, Kyba M, Garry DJ, Martin CM. Nkx2-5 mediates differential cardiac differentiation through interaction with Hoxa10. Stem Cells Dev. 2013;22(15):2211–2220. doi: 10.1089/scd.2012.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. DNA methylation in stem cell renewal and multipotency. Stem Cell Res Ther. 2011;2(5):42. doi: 10.1186/scrt83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakouros D, Gronthos S (2019) Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis 10(1):174–189. 10.14336/AD.2017.1213 [DOI] [PMC free article] [PubMed]
- Cimmino L. Methylation maintains HSC division fate. Proc Natl Acad Sci U S A. 2017;114(2):192–194. doi: 10.1073/pnas.1619390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler SA, Deutsch MA, Lange R, Krane M. Cardiac regeneration: current therapies-future concepts. J Thorac Dis. 2013;5(5):683–697. doi: 10.3978/j.issn.2072-1439.2013.08.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen J, Zirkel A, Blake J, Rath B, Benes V, Papantonis A, Wagner W. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell. 2017;16(1):183–191. doi: 10.1111/acel.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K. Use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplant. 2003;32(Suppl 1):S25–S27. doi: 10.1038/sj.bmt.1703940. [DOI] [PubMed] [Google Scholar]
- Govarthanan K, Gupta PK, Ramasamy D, Kumar P, Mahadevan S, Verma RS. DNA methylation microarray uncovers a permissive methylome for cardiomyocyte differentiation in human mesenchymal stem cells. Genomics. 2020;112(2):1384–1395. doi: 10.1016/j.ygeno.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Guo X, Bai Y, Zhang L, Zhang B, Zagidullin N, Carvalho K, Du Z, Cai B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications. Stem Cell Res Ther. 2018;9(1):44. doi: 10.1186/s13287-018-0773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Tare R, Lee SH, Mandeville M, Weiner B, Montecino M, van Wijnen AJ, Stein JL, Stein GS, Lian JB. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol Cell Biol. 2007;27(9):3337–3352. doi: 10.1128/MCB.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill ABT, Bressan FF, Murphy BD, Garcia JM. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res Ther. 2019;10(1):44. doi: 10.1186/s13287-019-1145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Klaus A, Müller M, Schulz H, Saga Y, Martin JF, Birchmeier W. Wnt/β-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc Natl Acad Sci U S A. 2012;109(27):10921–10926. doi: 10.1073/pnas.1121236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna KA, Krishna KS, Berrocal R, Rao KS, Sambasiva Rao KR. Myocardial infarction and stem cells. J Pharm Bioallied Sci. 2011;3(2):182–188. doi: 10.4103/0975-7406.80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14(3):286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Le HQ, Ghatak S, Yeung CY, Tellkamp F, Günschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, Wickström SA. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18(8):864–875. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hore TA, Reik W. Reprogramming the methylome: erasing memory and creating diversity. Cell Stem Cell. 2014;14(6):710–719. doi: 10.1016/j.stem.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, Yu H, Jin S, Wei Z, Chen F, Jin Y. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009;18(22):4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- Li Q, Hou H, Li M, Yu X, Zuo H, Gao J, Zhang M, Li Z, Guo Z. CD73+ mesenchymal stem cells ameliorate myocardial infarction by promoting angiogenesis. Front Cell Dev Biol. 2021;9:637239. doi: 10.3389/fcell.2021.637239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rui T, Zhang S, Ding Z. Heterogeneity of MSC: origin, molecular identities, and functionality. Stem Cells International. 2019 doi: 10.1155/2019/8717694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu M, Khalil M, Andrei E, Iordache F, Pfannkuche K, Neef K, Georgescu A, Buzila C, Brockmeier K, Maniu H, Hescheler J. Integration properties of Wharton's jelly-derived novel mesenchymal stem cells into ventricular slices of murine hearts. Cell Physiol Biochem. 2011;28(1):63–76. doi: 10.1159/000331714. [DOI] [PubMed] [Google Scholar]
- Maalouf WE, Liu Z, Brochard V, Renard JP, Debey P, Beaujean N, Zink D. Trichostatin A treatment of cloned mouse embryos improves constitutive heterochromatin remodeling as well as developmental potential to term. BMC Dev Biol. 2009;9(1):1. doi: 10.1186/1471-213X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M, Atoui R. Therapeutic use of stem cells for myocardial infarction. Bioengineering (basel) 2018;5(2):28. doi: 10.3390/bioengineering5020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova YA, Nava MM, Wickström SA. Emerging roles of mechanical forces in chromatin regulation. J Cell Sci. 2017;130(14):2243–2250. doi: 10.1242/jcs.202192. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Moscoso I, Centeno A, López E, Rodriguez-Barbosa JI, Santamarina I, Filgueira P, Sánchez MJ, Domínguez-Perles R, Peñuelas-Rivas G, Domenech N. Differentiation, "in vitro" of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transplant Proc. 2005;37(1):481–482. doi: 10.1016/j.transproceed.2004.12.247. [DOI] [PubMed] [Google Scholar]
- Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379(9819):933–942. doi: 10.1016/S0140-6736(12)60075-0. [DOI] [PubMed] [Google Scholar]
- Qian Q, Qian H, Zhang X, Zhu W, Yan Y, Ye S, Peng X, Li W, Xu Z, Sun L, Xu W. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev. 2012;21(1):67–75. doi: 10.1089/scd.2010.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh S, Govarthanan K, Ostrovidov S, et al. Cardiac differentiation of mesenchymal stem cells: impact of biological and chemical inducers. Stem Cell Rev and Rep. 2021 doi: 10.1007/s12015-021-10165-3. [DOI] [PubMed] [Google Scholar]
- Rammal H, Harmouch C, Maerten C, Gaucher C, Boulmedais F, Schaaf P, Voegel JC, Laurent-Maquin D, Menu P, Kerdjoudj H. Upregulation of endothelial gene markers in Wharton's jelly mesenchymal stem cells cultured on polyelectrolyte multilayers. J Biomed Mater Res A. 2017;105(1):292–300. doi: 10.1002/jbm.a.35868. [DOI] [PubMed] [Google Scholar]
- Razin A, Shemer R. DNA methylation in early development. Hum Mol Genet. 1995 doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Russo VE, Martienssen RA, Riggs AD (1996) Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11(4):451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton's Jelly of umbilical cord. Methods Cell Biol. 2008;86:101–119. doi: 10.1016/S0091-679X(08)00006-X. [DOI] [PubMed] [Google Scholar]
- Shafiee A, Patel J, Hutmacher DW, Fisk NM, Khosrotehrani K. Meso-Endothelial Bipotent Progenitors from Human Placenta Display Distinct Molecular and Cellular Identity. Stem Cell Reports. 2018;10(3):890–904. doi: 10.1016/j.stemcr.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits AM, Vliet PV, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human Cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4(2):232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- Sørensen AL, Jacobsen BM, Reiner AH, Andersen IS, Collas P. Promoter DNA methylation patterns of differentiated cells are largely programmed at the progenitor stage. Mol Biol Cell. 2010;21(12):2066–2077. doi: 10.1091/mbc.e10-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzu AC, Wu SM. Developmental and regenerative biology of multipotent cardiovascular progenitor cells. Circ Res. 2011;108(3):353–364. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, Verma S, Ramirez V, Qin G, Losordo DW, Kishore R. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res. 2012;111(2):180–190. doi: 10.1161/CIRCRESAHA.112.270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the redefinition of myocardial infarction universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Yang J, Song T, Wu P, Chen Y, Fan X, Chen H, Zhang J, Huang C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol Med Rep. 2012;5(1):108–113. doi: 10.3892/mmr.2011.611. [DOI] [PubMed] [Google Scholar]
- Yang G, Xiao Z, Ren X, Long H, Ma K, Qian H, Guo Y. Obtaining spontaneously beating cardiomyocyte-like cells from adipose-derived stromal vascular fractions cultured on enzyme-crosslinked gelatin hydrogels. Sci Rep. 2017;7(1):1–1. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.