Abstract
We have a fundamental responsibility as oncologists to deliver personalized care tailored to each individual. In addition to an unprecedented expansion of treatment options for patients, recent advances in molecular profiling and functional assessments have greatly improved our ability to predict risks, benefits, and outcomes for older patients with cancer.1,2 Molecular profiling identifies genomic abnormalities and allows oncologists to predict response to cancer therapy. Functional assessment such as a geriatric assessment allows oncologists to predict risks of treatment-related morbidity and mortality. Ongoing efforts aim to further refine our ability to predict outcomes for individuals by identifying relevant clinically meaningful thresholds (e.g., cut-off values for variant allele frequency, fitness criteria for a specific disease). Complex risk prediction models are now routinely used to integrate these data and produce personalized estimates of survival and response to cancer therapies, helping oncologists to provide personalized, high-quality care. Assessments of the disease and function of the patient, however, are insufficient to guide personalized treatment recommendations—we must understand patient preferences for treatment outcomes in order to tailor treatment.
Keywords: Geriatric Oncology, Patient Preference, Patient Outcome Assessment, Clinical Decision-Making, Patient-Centered Care
We have a fundamental responsibility as oncologists to deliver personalized care tailored to each individual. In addition to an unprecedented expansion of treatment options for patients, recent advances in molecular profiling and functional assessments have greatly improved our ability to predict risks, benefits, and outcomes for older patients with cancer.1,2 Molecular profiling identifies genomic abnormalities and allows oncologists to predict response to cancer therapy. Functional assessment such as a geriatric assessment allows oncologists to predict risks of treatment-related morbidity and mortality. Ongoing efforts aim to further refine our ability to predict outcomes for individuals by identifying relevant clinically meaningful thresholds (e.g., cut-off values for variant allele frequency, fitness criteria for a specific disease). Complex risk prediction models are now routinely used to integrate these data and produce personalized estimates of survival and response to cancer therapies, helping oncologists to provide personalized, high-quality care.
Assessments of the disease and function of the patient, however, are insufficient to guide personalized treatment recommendations—we must understand patient preferences for treatment outcomes in order to tailor treatment. Preferences, used in this context, refers to the patient’s value judgments of the trade-offs between the harms of potential therapies and the benefits. Preferences are the application of patient values, priorities, and goals to a specific treatment decision. Numerous stakeholders including patients, caregivers, advocacy organizations, and the United States Food and Drug Administration have consistently advocated for the increased incorporation of patient preferences into treatment decision-making.3–6 The reason for this is simple: different patients value different treatment outcomes.7–10 Some patients prefer to maximize the chance at long-term survival and others prefer to focus on maintaining quality of life. Older adults are more likely to prioritize outcomes such as maintaining functional independence and preserving cognitive abilities.11 Oncologists cannot deliver personalized care without knowing how patients value the outcomes that may be achieved or sacrificed with each treatment.
Personalized care delivery in oncology, therefore, requires assessment of three essential domains: disease, function, and patient preferences (Figure). The disease and functional assessments serve to establish which treatment options are reasonable for any given individual and the likely outcomes of those treatment options. For example, in acute myeloid leukemia (AML) the finding of a biallelic TP53 mutation nearly eliminates the future possibility of long-term disease-free survival for a patient even with highly aggressive therapy such as an allogeneic cell transplant, while the finding of an inversion of chromosome 16 opens the possibility of a cure with standard chemotherapy alone. Similarly, a poor functional status may increase the chance of side effects and significantly decrease the probability of achieving long-term benefits. Once the probability of benefits and harms with reasonable treatment options are identified, the assessment of patient preferences enable patients and oncologists make a final decision about personalized cancer treatment.
Figure. Essential elements for personalized treatment decision-making.
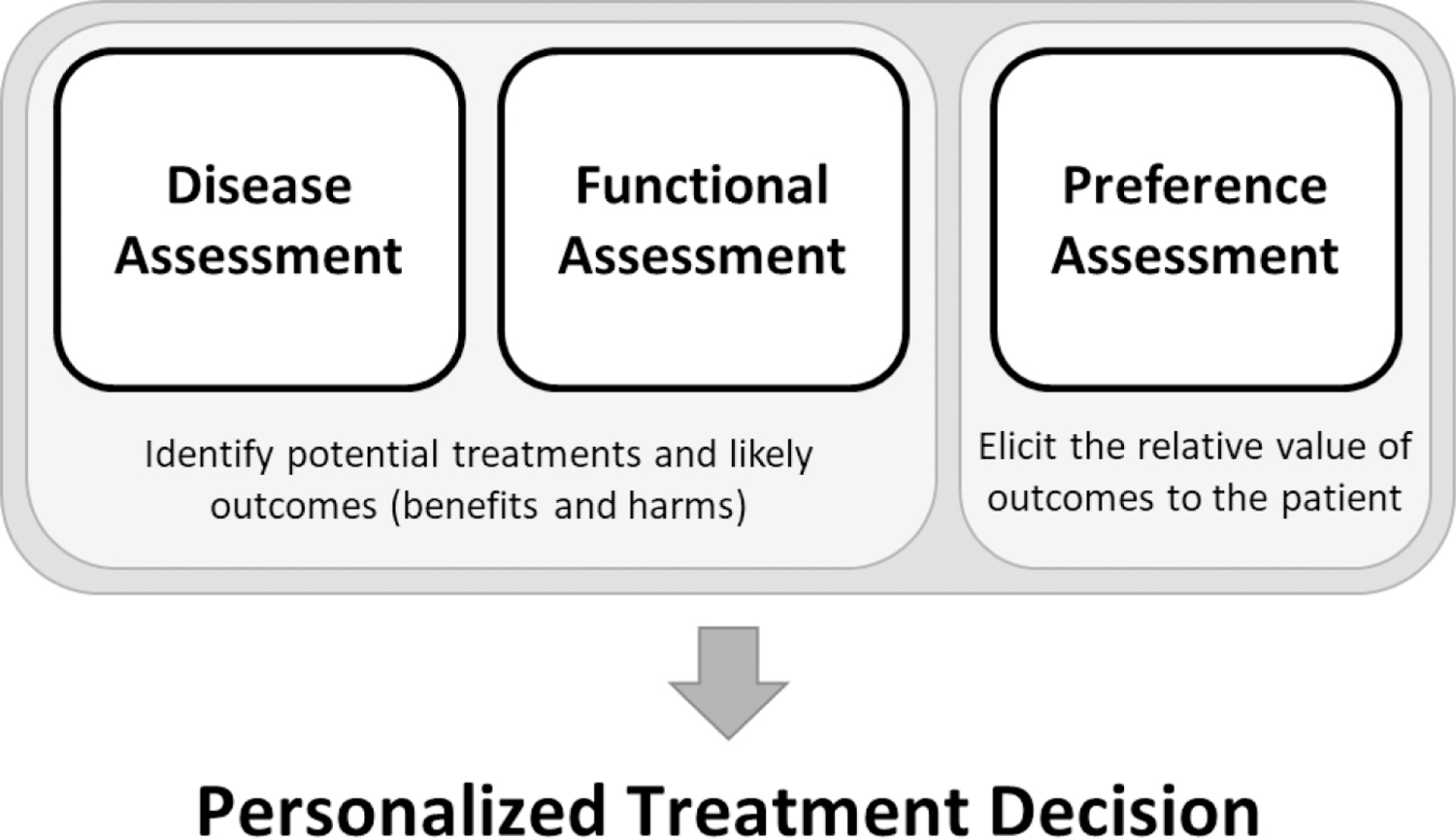
Disease and functional assessments are necessary to establish the potential treatment options available for the patient and to determine likely outcomes with each treatment option. This includes a personalized evaluation of the probability of experiencing harms and achieving benefits. A preference assessment is necessary to establish the relative value of each of these outcomes. Specifically, this should include how the patient would prefer to trade-off the potential harms of treatments in order to achieve potential benefits. Personalized treatment decisions arrived at through the process of shared decision-making require all three elements.
Routine shared decision-making (SDM), as it is currently practiced, is inadequate to reliably capture patient preferences.12–16 Multiple systematic reviews have demonstrated consistent discordance after routine SDM between physician perceptions of what matters most to patients and their actual prefrences.16,17 The reasons for this are yet to be fully explored, but several are apparent: enormous patient distress at the time of treatment decision-making, the complexity of cancer care, and substantial time constraints in the clinical encounter.18–20 Survey methods to elicit and quantify patient preferences are increasingly being used to inform regulatory medical-decision making as part of a larger vision to incorporate the patient voice into drug development.21–23 Applying these quantitative preference elicitation methods to individual SDM may overcome known barriers to personalized treatment decision-making, though substantial work remains to develop strategies to implement these methods into routine clinical care and to demonstrate their effectiveness.
We are currently developing methods to better understand and elicit patient preferences, priorities, and values to improve treatment decision-making for older adults with AML.7,24,25 One central challenge we have encountered is defining a primary endpoint. If our goal in developing these interventions is to align care to achieve outcomes that are most important to each patient, our primary endpoint, i.e., our goal in delivering therapy, will vary by each patient. This fact makes developing and evaluating interventions particularly challenging. We not only anticipate that outcomes will vary for patients but also recognize that if we are effective at improving personalized treatment decisions, treatment teams will be actively attempting to achieve different outcomes for different patients. For some, they will attempt to maximize survival, others reduction in symptoms, others they will be working to help them enjoy their time at the beach. Successful interventions that improve the delivery of personalized care, therefore, may or may not improve survival, improve symptoms, or even quality of life.
To overcome this methodological challenge, we are implementing several strategies in our studies that evaluate both the process of personalized treatment decision-making and its outcomes. First, in addition to standard disease and functional assessments, we are assessing and documenting patient preferences for treatment outcomes prior to a treatment decision. This enables an accurate assessment of the effectiveness of treatments to achieve outcomes most valued by patients. Second, we are evaluating the process of how patient preferences are integrated into care delivery. There is no standardized way to do this, but we suggest recording conversations between oncologists and patients or, at minimum, documenting how patients perceived that the chosen treatment is aimed at achieving those outcomes most important to them. In addition, following input from patients and oncologists, we are considering using a modified version of the Overall Treatment Utility (OTU) outcome in our studies.26 The modified OTU combines clinical efficacy, tolerability, and the patient’s assessment of treatment value and acceptability. Although susceptible to confirmation bias, the OTU could provide an approximation as to how valuable the patient perceived the treatment to be.
Future work in this area should aim to address key remaining questions. First, how can we reliably assess patient preferences to inform treatment decisions? Effective interventions, whether surveys, choice tasks, mobile applications, or communication tools, must be adaptable to individual treatment contexts as patient preferences are highly context dependent (e.g., patients may be more likely to value survival when their disease is in remission and they have minimal side effects in contrast to when they are enduring significant treatment-related morbidity). In addition, strategies must be responsive to changes over time as patient priorities, values, and preferences change throughout the course of disease. Interventions must also be both accessible and understandable to older adults and be able to produce clinically meaningful results for the oncology team.
Second, how can we better engage patients in treatment decision-making when they are in emotional distress? A cancer diagnosis is associated with significant emotional distress and fear about treatment and dying, and this distress interferes with complex cognitive processes.27,28 We do not yet know to what extent this undermines patients’ ability to meaningfully reflect on their own priorities and values and to engage with their oncology teams. Interventions to facilitate treatment decision-making need to account for and ideally reduce emotional distress.
Lastly, fundamental questions remain about how to systematically improve the actual practice of treatment decision-making across oncology. Substantial improvements in personalized care will not be accomplished by the development of novel decisional interventions alone; the very practice of engaging patients in decision-making must be improved. Widespread and lasting improvements will only be achieved with recognition of the problem and the development of a systematic process to implement effective strategies. This process of culture change must include all relevant stakeholders including patients, clinicians, and healthcare systems to be effective.
The recent expansion of therapeutic options across oncology together with the development of more accurate outcome prediction models has enabled an unprecedented opportunity for oncologists to tailor treatment to individuals. Only by developing a strategy to assess patient preferences for treatment outcomes will we be able to reliably tailor these treatments to achieve what matters most to each patient.
Acknowledgements:
We would like to thank the Cancer and Aging Research Group (R33 AG059206) that helps foster collaboration between DRR and KPL and acknowledge the Stakeholders for Care in Oncology and Research for our Elders Board (SCOREBoard) for their input on our projects.
Financial Support:
DRR is supported by the Conquer Cancer - Harry F. Bisel, MD, Endowed Young Investigator Award from The ASCO Foundation. KPL is supported by the National Cancer Institute at the National Institute of Health (K99CA237744) and Wilmot Research Fellowship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Richardson reports no conflicts of interest. Dr. Loh has served as a consultant to Pfizer and Seattle Genetics, and has received honoraria from Pfizer.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Saad M, Loh KP, Tooze JA, et al. Geriatric assessment and survival among older adults receiving postremission therapy for acute myeloid leukemia. Blood 2020;136(23):2715–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loh KP, Tooze JA, Nicklas BJ, et al. Inflammatory biomarkers, geriatric assessment, and treatment outcomes in acute myeloid leukemia. J Geriatr Oncol 2020;11(3):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter NL, O’Callaghan KM, Califf RM. Engaging Patients Across the Spectrum of Medical Product Development: View From the US Food and Drug Administration. JAMA 2015;314(23):2499. [DOI] [PubMed] [Google Scholar]
- 4.Rocque G, Miller-Sonnet E, Balch A, et al. Engaging Multidisciplinary Stakeholders to Drive Shared Decision-Making in Oncology. J Palliat Care 2019;34(1):29–31. [DOI] [PubMed] [Google Scholar]
- 5.Bonamici S. H.R.34 – 114th Congress (2015–2016): 21st Century Cures Act 2016;
- 6.Wicker RF. S.1597 – 114th Congress (2015–2016): Patient-Focused Impact Assessment Act of 2016 2016;
- 7.Richardson DR, Crossnohere NL, Seo J, et al. Age at Diagnosis and Patient Preferences for Treatment Outcomes in AML: A Discrete Choice Experiment to Explore Meaningful Benefits. Cancer Epidemiol. Biomarkers Prev 2020;29(5):942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges JFP, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: A conjoint analysis. Lung Cancer 2012;77(1):224–231. [DOI] [PubMed] [Google Scholar]
- 9.Bien DR, Danner M, Vennedey V, et al. Patients’ Preferences for Outcome, Process and Cost Attributes in Cancer Treatment: A Systematic Review of Discrete Choice Experiments. Patient 2017;10(5):553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability ofadvanced cancer in older adults. Cancer 2019;125(14):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously illpatients. N Engl J Med 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 12.Aiello Bowles EJ, Tuzzio L, Wiese CJ, et al. Understanding high-quality cancer care: a summary of expertperspectives. Cancer 2008;112(4):934–942. [DOI] [PubMed] [Google Scholar]
- 13.Wagner EH, Aiello Bowles EJ, Greene SM, et al. The quality of cancer patient experience: perspectives ofpatients, family members, providers and experts. Qual Saf Health Care 2010;19(6):484–489. [DOI] [PubMed] [Google Scholar]
- 14.Beaussant Y, Mathieu-Nicot F, Pazart L, et al. Is shared decision-making vanishing at the end-of-life? Adescriptive and qualitative study of advanced cancer patients’ involvement in specific therapies decision-making. BMC Palliat Care 2015;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brom L, De Snoo-Trimp JC, Onwuteaka-Philipsen BD, et al. Challenges in shared decision making inadvanced cancer care: a qualitative longitudinal observational and interview study. Health Expect 2017;20(1):69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open 2017;7(5):e014719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy 2013;11(3):163–180. [DOI] [PubMed] [Google Scholar]
- 18.Covvey JR, Kamal KM, Gorse EE, et al. Barriers and facilitators to shared decision-making in oncology: asystematic review of the literature. Support Care Cancer 2019;27(5):1613–1637. [DOI] [PubMed] [Google Scholar]
- 19.Pel-Littel RE, Snaterse M, Teppich NM, et al. Barriers and facilitators for shared decision making in olderpatients with multiple chronic conditions: a systematic review. BMC Geriatrics 2021;21(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne S, Oliffe JL, Stajduhar KI. Communicating shared decision-making: cancer patient perspectives. Patient Educ Couns 2013;90(3):291–296. [DOI] [PubMed] [Google Scholar]
- 21.Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical Methods for the Analysis ofDiscrete Choice Experiments: A Report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value in Health 2016;19(4):300–315. [DOI] [PubMed] [Google Scholar]
- 22.Bridges JFP, Hauber AB, Marshall D, et al. Conjoint Analysis Applications in Health—a Checklist: A Report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health 2011;14(4):403–413. [DOI] [PubMed] [Google Scholar]
- 23.Janssen EM, Marshall DA, Hauber AB, Bridges JFP. Improving the quality of discrete-choice experimentsin health: how can we assess validity and reliability? Expert Review of Pharmacoeconomics & Outcomes Research 2017;17(6):531–542. [DOI] [PubMed] [Google Scholar]
- 24.Richardson DR, Oakes AH, Crossnohere NL, et al. Prioritizing the Worries of AML Patients: QuantifyingPatient Experience using Best-Worst Scaling. Psycho-Oncology 2021; [DOI] [PMC free article] [PubMed]
- 25.Loh KP, Abdallah M, Kadambi S, et al. Treatment decision-making in acute myeloid leukemia: aqualitative study of older adults and community oncologists. Leukemia & Lymphoma 2020;0(0):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients withmetastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. The Lancet 2011;377(9779):1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medicine I of. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs 2007.
- 28.Kahneman D. Thinking, fast and slow New York: Farrar, Straus and Giroux; 2013. [Google Scholar]


