Abstract
In the present study, the efficiency of four different strains of Pseudomonas aeruginosa and their biosurfactants in the bioremediation process were investigated. The strains were found to be capable of metabolizing a wide range of hydrocarbons (HCs) with preference for high molecular weight aliphatic (ALP) over aromatic (ARO) compounds. After treating with individual bacteria and 11 different consortia, the residual crude oils were quantified and qualitatively analyzed. The bacterial strains degraded ALP, ARO, and nitrogen, sulphur, oxygen (NSO) containing fractions of the crude oil by 73–67.5, 31.8–12.3 and 14.7–7.3%, respectively. Additionally, the viscosity of the residual crude oil reduced from 48.7 to 34.6–39 mPa s. Further, consortium designated as 7 and 11 improved the degradation of ALP, ARO, and NSO HCs portions by 80.4–78.6, 42.7–42.4 and 21.6–19.2%, respectively. Moreover, addition of biosurfactant further increased the degradation performance of consortia by 81.6–80.7, 43.8–42.6 and 22.5–20.7%, respectively. Gas chromatographic analysis confirmed the ability of the individual strains and their consortium to degrade various fractions of crude oil. Experiments with biosurfactants revealed that polyaromatic hydrocarbons (PAHs) are more soluble in the presence of biosurfactants. Phenanthrene had the highest solubility among the tested PAHs, which further increased as biosurfactant doses raised above their respective critical micelle concentrations (CMC). Furthermore, biosurfactants were able to recover 73.5–63.4% of residual oil from the sludge within their respective CMCs. Hence, selected surfactant-producing bacteria and their consortium could be useful in developing a greener and eco-sustainable way for removing crude oil pollutants from soil.
Keywords: Pseudomonas aeruginosa, Hydrocarbon, Consortium, Biosurfactant, Critical micelle concentration (CMC)
Introduction
Spillage of crude oil on soil is one of the most common and a destructive types of pollution, posing a serious threat to a wide range of life forms (Alzahrani and Rajendran 2019). The accumulation of petroleum sludge, which is commonly emitted during production, refining, storage, and transportation, further exacerbates the degree of environmental contamination (Ren et al. 2020). Petroleum sludge poses a major threat to the surrounding ecological system and human health due to its deleterious chemical composition (Rudyk 2018). Furthermore, it has been classified as hazardous waste solids in a number of countries (Hui et al. 2020). Crude petroleum is a complex mixture of organic compounds that includes a wide variety of hydrocarbons (aliphatic and aromatic), non-hydrocarbons (containing sulfur, nitrogen, oxygen and various trace metals) and even some heavy metals (Saadoun 2015; Alzahrani and Rajendran 2019). These are mostly hazardous, particularly polyaromatic hydrocarbons (PAHs), because they are toxic, non-polar, poorly or non-degradable, and persistent in nature (Saadoun 2015). The most drastic changes in soil properties triggered by crude oil substances are clogged pore spaces, which minimize soil aeration and water penetration, thereby raising bulk density and restricting the adequate supply of nitrogen and phosphorus sources (Abosede 2013; Ahmed and Fakhruddin 2018). Such substantial changes negatively affect the germination and growth of plants in soils (Siddiqui and Adams 2002). Presence of such pollutants further weakens biological parameters of soil such as enzymatic activity and microbial counts (Zhan et al. 2010; Lipińska et al. 2013). Rhizospheric microbial diversity, which is linked to biogeochemical cycles, is impacted, resulting in the disruption of nutrient availability (Zhang et al. 2016; Yu et al. 2020). As a result, land use is limited, resulting in financial losses, environmental concerns, drop in soil quality for crop production and reduced long-term food security (Alzahrani and Rajendran 2019; Odukoya et al. 2019; Arjoon and Speight 2020). Besides, crops grown in a crude oil-polluted field are unsafe for human and animal consumption (Ordinioha and Brisibe 2013). These contaminants have a profound effect on soil fauna which includes micro-arthropods (Iloba and Jarret 2007), earthworm species (Brown et al. 2004; Contreras-Ramos et al. 2006), and terrestrial vertebrates (Alzahrani and Rajendran 2019). In humans, crude oil components have a negative impact on the sensory system (Riihimäki and Savolainen 1980; Wang et al. 2014), respiratory system (Peng et al. 2010; Altomare et al. 2013), reproductive system (Krivoshto et al. 2008; Ordinioha and Brisibe 2013), and renal system (Kum et al. 2007; Monteiro et al. 2017).
Thus, to safeguard the environment and public health, such hazardous wastes must be properly treated and handled. Effective remediation of soil contaminated with petroleum hydrocarbon has continued to be a matter of eminent concern. Chemical and physical methods of remediation are among the readily available solutions, but they are costly and do not ensure that contaminants are completely removed (Viamajala et al. 2007). Furthermore, these methods could cause secondary pollution and habitat destruction, negatively influencing humans and other species that are in close proximity to the polluted site (Koshlaf and Ball 2017). Bioremediation is a suitable alternative to physicochemical methods and regarded as one of the safer, cleaner, cost-effective, and sustainable approach for the removal of crude oil substances from contaminated soil (Guo et al. 2014). The United State Environmental Protection Agency (USEPA) has certified it as an environmentally benign waste management strategy that helps to restore contaminated areas and encourages sustainable development (Bharagava et al. 2020).
We previously explored four different strains of P. aeruginosa (PA) namely PA-OBP1, PA-OBP2, PA-OBP3, and PA-OBP4 and were capable of secreting rhamnolipid biosurfactant efficiently in the presence of aliphatic hydrocarbon such as n-hexadecane. The pattern of rhamnolipid congeners production differed between the strains, with di-rhamnolipid being comparatively prevalent over mono-rhamnolipid (Bharali and Konwar 2011; Bharali et al. 2018). In the present investigation, competence of the selected indigenous bacterial strains and their consortia in the degradation of crude oil was assessed. In addition, biosurfactant produced by these strains were also evaluated for their ability to solubilize PAHs and separate residual crude oil from sludge. The aim of this study was to concentrate on bioprospecting of possible indigenous bacteria in order to develop an efficient and environmentally friendly remediation technology for the management of crude oil polluted soil.
Material and methods
Bacterial strains and tested hydrocarbon
Four different strain of P. aeruginosa (PA), namely PA-OBP1, PA-OBP2, PA-OBP3, and PA-OBP4 with NCBI accession numbers JX843423, JX843422, JX843421, and JX843420, respectively, were used in the present investigation. For the cultivation of bacteria and associated biosurfactants, previously reported optimum culture conditions were applied (Bharali and Konwar 2011; Bharali et al. 2018). Different hydrocarbons, solvents, reagents, and other salts of analytical grade used in the present study were purchased from Merck Chemicals (India) and Hi-Media (Mumbai, India). The crude oil and sludge were kindly provided by the Oil and Natural Gas Corporation Limited (ONGC), Jorhat, Assam, India.
Screening of petroleum hydrocarbon utilizing ability
The pure cultures for each bacterial strain were prepared with Luria Bertani broth and cultivated in an orbital incubator shaker overnight at 37 °C and 180 rpm. A 100-μl aliquot of the aforesaid fresh culture broth containing 1 × 108 ml−1 microorganisms (as determined by the McFarland turbidity method) was inoculated into a 250-ml Erlenmeyer flask containing 100 ml of mineral salt medium (MSM) which consists of 1 g CH4N2O, 1 g (NH4)2SO4, 1.805 g Na2HPO4, 0.875 g KH2PO4, 0.1 MgSO4 7H2O, 25 mg CaCl2 2H2O, 0.5 mg FeSO4 7H2O, 25 μg CuSO4 7H2O, 5 μg MnSO4 5H2O, 5 μg H3BO3, 35 μg ZnSO4 7H2O, and 5 μg MnO3. The pH of the medium was tuned to 6.8 by using 6 N HCl. All of the cultures were given 1% (v/v) test hydrocarbon and incubated on an orbital incubator shaker at 37 °C and 180 rpm. Aliphatic hydrocarbons (pentane, n-hexane, heptane, iso-octane, dodecane, tridecane, n-hexadecane, octadecane, eicosane, triacontane, and liquid paraffin) and petroleum products (phenol, benzene, toluene, xylene, kerosene, diesel, lubricating oil, and crude oil), 1% (v/v) of each carbon source was added to the culture separately and in the case of polycyclic aromatic hydrocarbons (pyrene, anthracene, naphthalene, fluorine and phenanthrene), the culture medium was supplemented with 50 μg of each type of carbon source (Barkay et al. 1999). After 15 days of incubation, the bacterial cells were harvested from the culture by centrifuging at 8000 rpm followed by washing with hexane. The dry weight of bacterial biomass was determined through standard gravimetric method.
Growth characteristics of selected bacterial strains in crude oil
The initial cultivation steps were same as mentioned above. Individually, all of the cultures were supplemented with a range of crude oils (1–3% v/v) and incubated at 37 °C at 180 rpm in an orbital incubator shaker. The growth of the bacterial strain was monitored by determining the cell-forming unit (CFU ml−1) at a time interval of 3 days for 30 days. Biosurfactant was isolated from the acidified culture supernatant using acid precipitation followed by solvent extraction and expressed as g l−1 (Abdel-Mawgoud et al. 2009). The reduction in the surface tension of the culture supernatant was determined by Du Noüy ring method and expressed as mN m−1 (Abdel-Mawgoud et al. 2009; Bharali and Konwar 2011).
Designing of bacterial consortium
From four different bacterial strains, 11 different combinations were developed. Initially all the strains were grown in LB broth and incubated in an orbital shaker overnight at 37 °C with 180 rpm. For the preparation of consortium, aliquots of 100 μl of the aforementioned fresh culture broth of each bacterial strain containing 1 × 108 ml−1 microorganisms (as per McFarland constant) were inoculated into a 250-ml Erlenmeyer flask containing 100 ml of MSM as per the designed combination of strains. All of the cultures were supplemented with 2% crude oil (v/v) and incubated at 37 °C and 180 rpm in an orbital incubator shaker. All of the culture conditions, including the consortium composition, were maintained in the second phase of the experiment, with the exception of biosurfactant (45 mg l−1), which was added separately to the culture medium. The bacterial cells were extracted from the culture after 30 days of incubation by centrifuging at 8000 rpm and washing with hexane. Using a standard gravimetric approach, the dry weight of bacterial biomass was calculated. Degradation assay was carried out on the residual crude oil as described by Queiroga et al. (2003). The aliphatic hydrocarbons of the treated crude oil with selected consortia were analyzed using a gas chromatograph-mass spectrometer (Wongsa et al. 2004).
Reduction of viscosity
The culture broths of the bacterial strains after 4 weeks of incubation with crude oil were extracted thrice with the equal volume of dichloromethane, dried over anhydrous sodium sulphate, filtered, and concentrated in vacuum. Viscosity measurement was performed on Ostwald viscometers, which allows the determination of viscosity of the control/treated crude oil. All determinations were carried out at 25 °C using a concentration of 1 mg m1−1 of the extracted crude oil dissolved in hexane.
Solubilization of polyaromatic hydrocarbon (PHA)
The solubilization assay was carried out as described by Barkay et al. (1999). Three polyaromatic hydrocarbons (PHA) anthracene, phenanthrene, and naphthalene were selected. Biosurfactant solutions were used in the increasing concentrations between 100 and 800 μg l–1. All experiments were performed in triplicate.
Assay on residual petroleum
Verification experiment of hydrocarbon degradation was carried out on the residual petroleum products by using combined solvent extraction and column chromatographic techniques as described by Queiroga et al. (2003). All the extracts were dried at room temperature over anhydrous Na2SO4 and concentrated in vacuum. The residual hydrocarbon components were determined gravimetrically. All experiments were performed in triplicates.
Gas chromatographic (GC) analysis of degraded petroleum products
The separated aliphatic hydrocarbons of crude oil after treatment with selected bacteria and their consortium were further evaluated using a gas chromatograph-mass spectrometer, as described by Wongsa et al. (2004) (Clarus 600, Perkin Elmer, USA). The column temperature was kept at 50 °C for 5 min before being raised to 280 °C for crude oil analysis. All analyses were conducted with a 20:1 split ratio. The carrier gas was helium, with a flow rate of 0.8 ml min−1. The temperature of the injector was set at 250 °C.
Separation of residual crude oil from petroleum sludge
The petroleum sludge was mixed with acid-washed sterile sand to achieve the sludge concentration between 1 and 9% (w/w). The sludge samples weighing 20 g of the five concentrations were transferred to 250 ml Erlenmeyer flasks containing 100 ml of aqueous biosurfactant solution (0.001, 0.01 and 0.1% w/v) separately and kept at constant shaking (100–180 rpm) between 3 and 18 days at room temperature. After the treatment, the culture flasks were allowed to settle for few hours and the treated sludge samples were recovered. The total petroleum hydrocarbon (TPH) of the sludge sample after treatment was estimated and expressed as residual TPH (Joseph and Joseph 2009). Flask receiving sludge sample with only water was kept as control.
Results
Ability of the bacterial strains to utilize different petroleum hydrocarbons
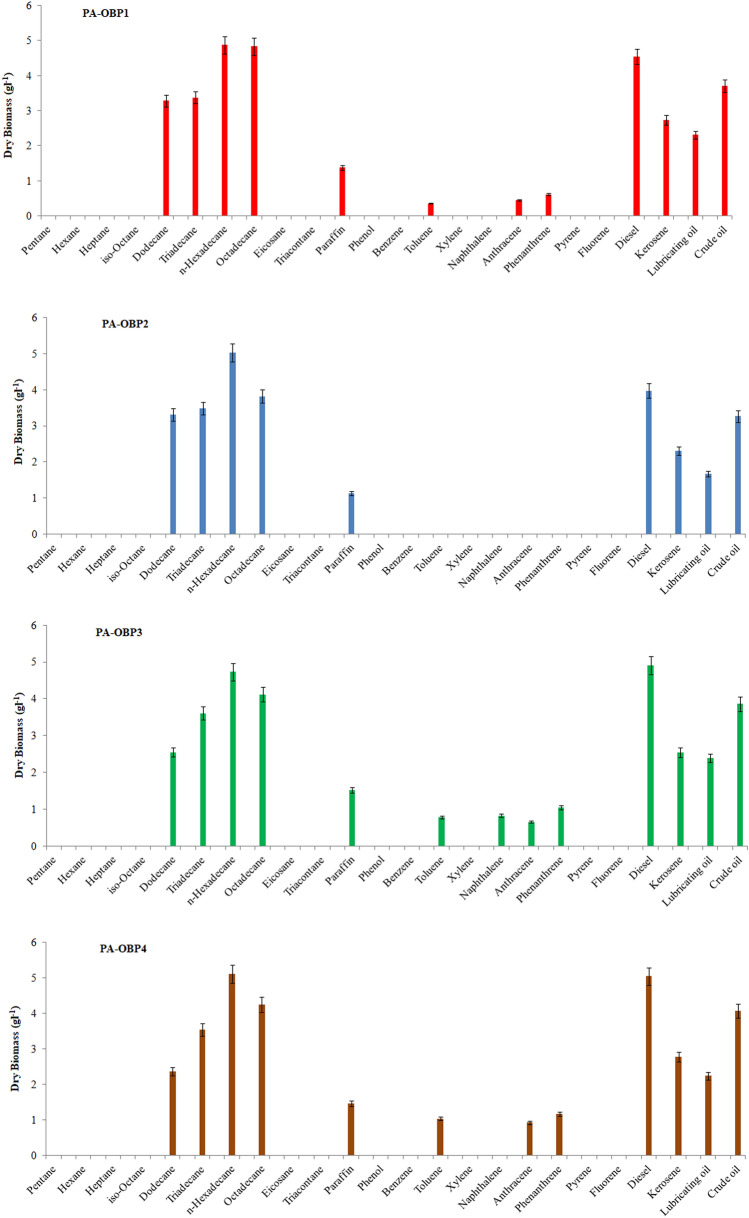
The growth performances of the bacterial strains on different hydrocarbon sources are presented in Fig. 1. The bacterial strain PA-OBP1 followed by PA-OBP3 and PA-OBP4 exhibited the highest growth in dodecane, tridecane, hexadecane- and octadecane-supplemented media as evident from the production of bacterial biomass. However, the bacterial isolate PA-OBP3 exhibited almost similar biomass production in hexadecane, and octadecane-supplemented media but lower in tridecane, and dodecane supplemented media. Among the tested aromatic hydrocarbons such as benzene, toluene, and xylene, the bacterial strains PA-OBP4, PA-OBP3 and PA-OBP1 exhibited growth on toluene but not by the PA-OBP2 strains. The bacterial strains PA-OBP4, PA-OBP3, and PA-OBP1 showed slow growth on the PAHs including phenanthrene and anthracene and exhibited no growth on pyrene and fluorene. Only PA-OBP3 grew on a naphthalene-supplemented medium, out of the four bacteria tested. The bacterial strains exhibited better growth in diesel-, crude oil-, and kerosene-supplemented media as evident from the bacterial biomass.
Fig. 1.
Ability of the bacterial strains to utilize different components of crude petroleum. Results represent mean ± SD of three individual experiments
Effect of crude oil on bacterial growth
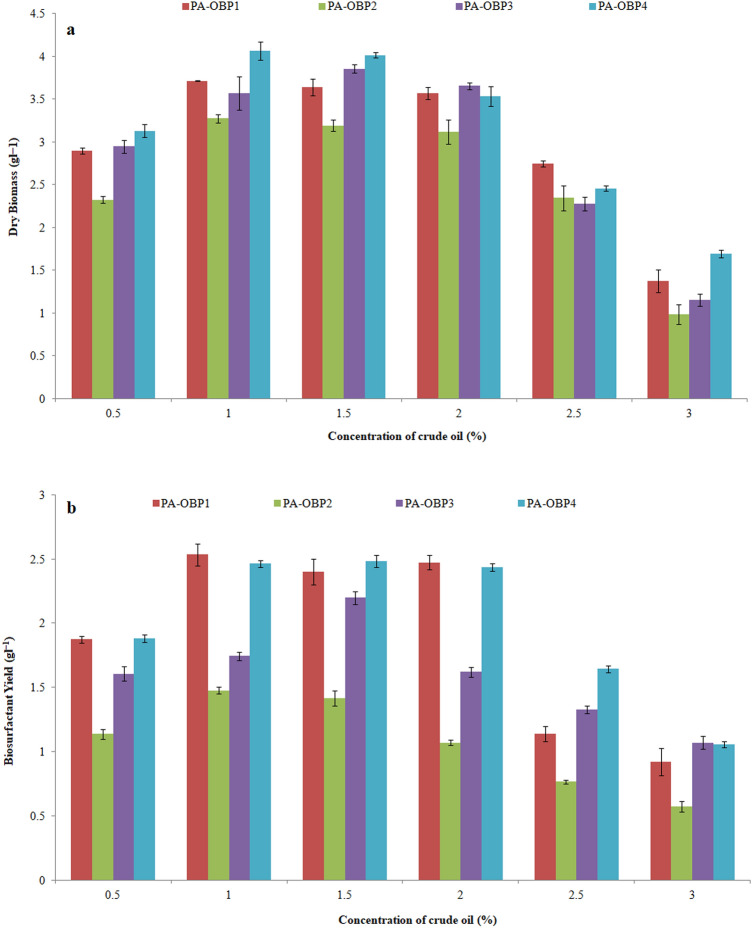
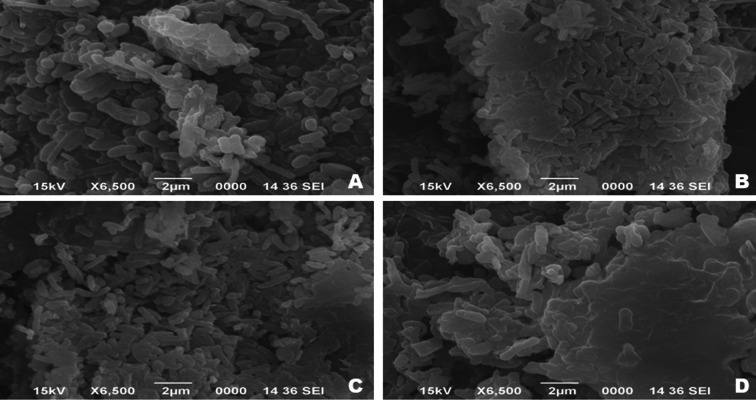
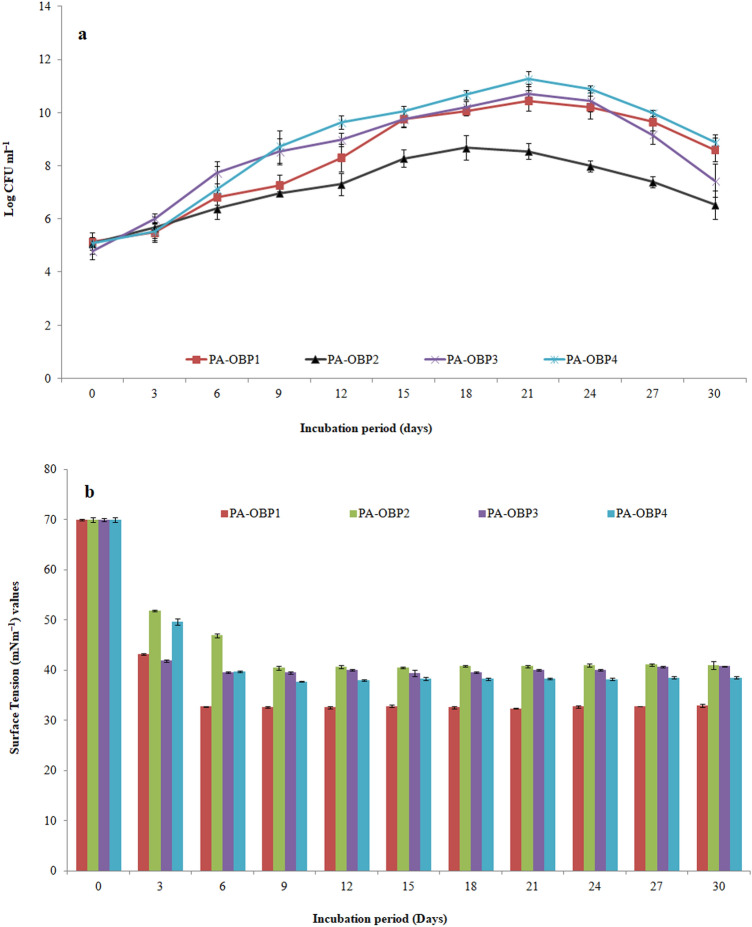
When the crude oil concentration exceeded 2.0%, the bacterial biomass production was significantly reduced as a result of the drastic reduction in growth. The same are presented in Fig. 2. Figure 3 shows scanning electron microscopic images of bacterial strains grown on MSM supplemented with crude oil. The bacterial cells appear in dense clumps, which could be due to the type of carbon source used. All four strains morphologically appeared as rods shaped with a short- to the mid-size range. The cell surfaces appeared to be smooth and devoid of any irregularities. Figure 4 represents the CFU ml−1 of the bacterial cultures, which were monitored over the course of 30 days.
Fig. 2.
Influence of different concentrations of crude oil on a growth and b biosurfactant production of P. aeruginosa strains within 30 days of incubation period. Results represent mean ± SD of three individual experiments
Fig. 3.
Scanning electron micrograph of bacterial strains showing growth crude oil A P. aeruginosa OBP1, B P. aeruginosa OBP2, C P. aeruginosa OBP3 and D P. aeruginosa OBP4
Fig. 4.
Time profile of a growth and b surface tension values of the culture broth by the P. aeruginosa strains in MSM supplemented with 2% crude oil. Results represent the mean of three independent experiments
Reduction in the viscosity of crude oil
The bacterial strains efficiently reduced the viscosity of the crude oil after 30 days of treatment under laboratory conditions. Results clearly indicated the ability of bacterial strains to degrade the different fractions of crude oil that in turn changed the physicochemical properties of the crude oil (Hao et al. 2004). When bacterial strains PA-OBP1, PA-OBP2, PA-OBP3, and PA-OBP4 were used, viscosity of crude oil was substantially reduced from 48.7 to 34.6, 38.9, 36.2, and 39.6 mPa s, respectively.
Biodegradation of crude oil by the bacteria strains
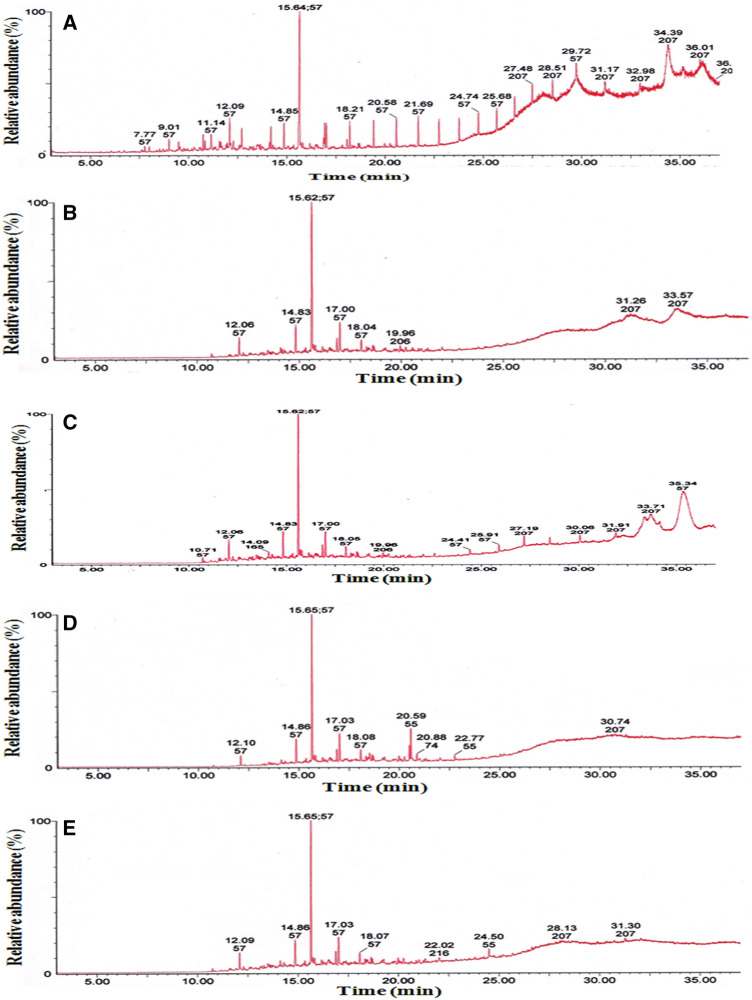
The bacterial strains were found to be efficient in hydrocarbon degradation. Table 1 shows that the strains PA-OBP4 and PA-OBP1 both showed high levels of aliphatic hydrocarbon degradation, with 73.0 and 72.8%, respectively. The strains PA-OBP3 and PA-OBP2, on the other hand, degraded aliphatic hydrocarbons at a rate of 71.8 and 67.5%, respectively. The bacterial strain PA-OBP3, which was accompanied by PA-OBP4, PA-OBP1, and PA-OBP2, demonstrated greater degradation of the aromatic fraction, with 31.8, 30.3, 25.2, and 12.3% efficiency, respectively. The strain PA-OBP4 degraded NSO compounds the most (14.7%), followed by PA-OBP3, PA-OBP1, and PA-OBP2, which degraded 13.5, 11.5, and 7.3%, respectively. Figure 5 depicts the GC profiles of aliphatic fractions of crude oil after 30 days of treatment with the bacterial strains and a control.
Table 1.
Degradation of aliphatic, aromatic and NSO fractions of crude oil by P. aeruginosa strains after 30 days of treatment in liquid culture
| Bacterial strain | Aliphatic fraction degraded (%) | Aromatic fraction degraded (%) | NSO compounds degraded (%) |
|---|---|---|---|
| Control | 12.6 ± 0.8 | 10.3 ± 0.6 | 04.7 ± 0.2 |
| P. aeruginosa OBP1 | 72.8 ± 0.2 | 25.2 ± 0.6 | 11.5 ± 0.5 |
| P. aeruginosa OBP2 | 67.5 ± 0.7 | 12.3 ± 0.9 | 07.3 ± 0.1 |
| P. aeruginosa OBP3 | 71.8 ± 0.3 | 31.8 ± 0.8 | 13.5 ± 0.4 |
| P. aeruginosa OBP4 | 73.0 ± 0.3 | 30.3 ± 0.3 | 14.7 ± 0.7 |
Determination based on crude oil 2.0 ml (1.857 g). Results represented mean ± SD of three individual experiments
Fig. 5.
Gas chromatographic analysis of the saturate fraction of crude oil after treatment with bacterial strains. A Control without treatment, B P. aeruginosa OBP1, C P. aeruginosa OBP2, D P. aeruginosa OBP3, and E P. aeruginosa OBP4
Biodegradation of crude oil by bacterial consortium
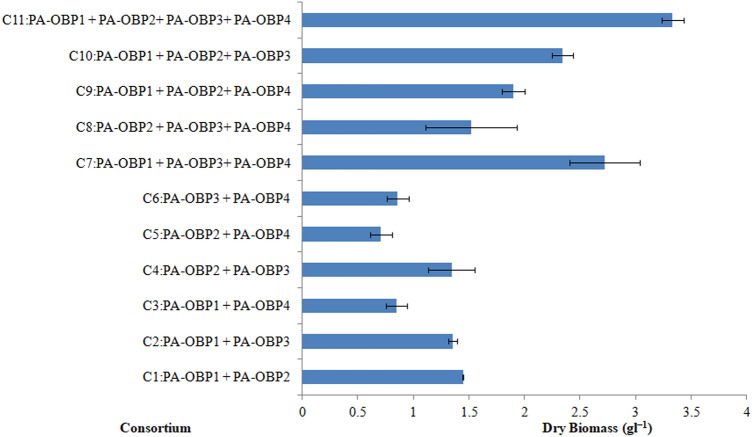
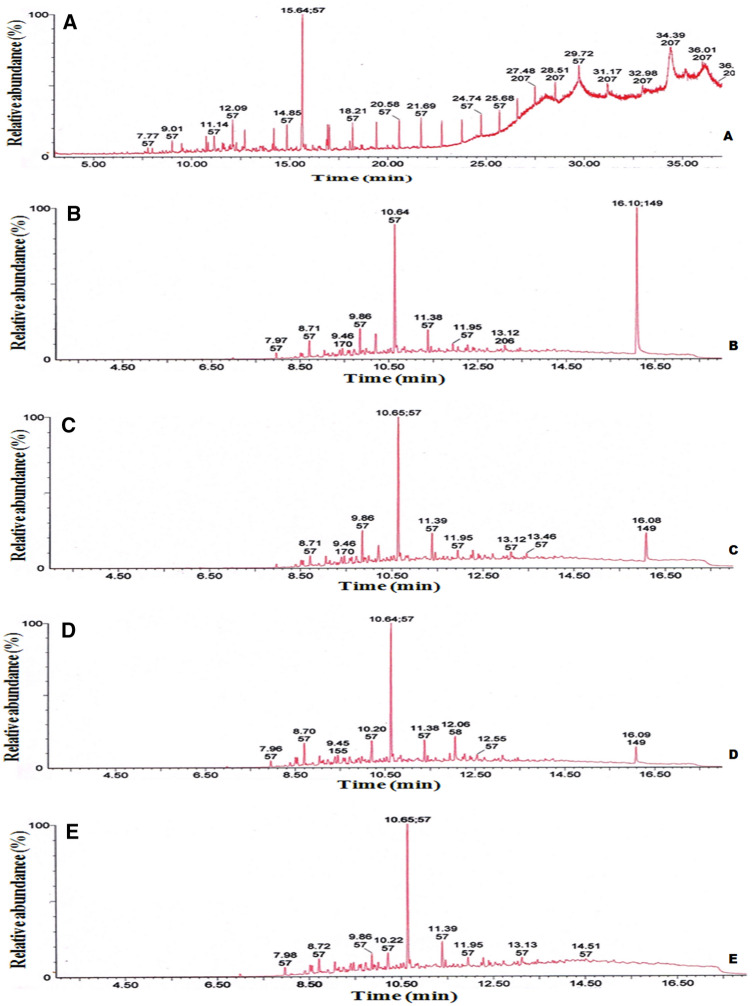
The dry biomass yield from 11 different consortia are presented in Fig. 6. Combination number 7 and 11, named as consortium I and II exhibited the highest dry biomass production of 2.72 ± 0.3 and 3.33 ± 0.1 g l−1, respectively, in the MSM supplemented with crude oil within 30 days. Consortium I and II were efficient in degrading 78.6–80.4% of aliphatic, 42.4–42.7% of aromatic, and 21.6–19.2% of NSO compounds in 30 days of culture (Table 2). The addition of biosurfactant (45 mg l−1) produced by the bacterial strain PA-OBP1 to both consortia did not result in a substantial increase in crude oil degradation, implying that externally added biosurfactant has a minimal undeviating positive effect on the biodegradation process (Table 2). Gas chromatographic analyses of the aliphatic fractions of treated crude oil with individual bacterial consortia I and II and in combination with biosurfactant are presented in Fig. 7.
Fig. 6.
Biomass of bacterial consortia in MSM supplemented with 2% crude oil. Results represented mean ± SD of three individual experiments
Table 2.
Degradation of aliphatic, aromatic and NSO fractions of crude oil by bacterial consortia and in presence of biosurfactant after 30 days
| Bacterial strain | Aliphatic fraction degraded (%) | Aromatic fraction degraded (%) | NSO compound degraded (%) |
|---|---|---|---|
| Control | 12.2 ± 0.5 | 08.9 ± 0.5 | 05.2 ± 0.7 |
| Consortium I | 78.6 ± 0.5 | 42.7 ± 0.7 | 21.6 ± 0.3 |
| Consortium II | 80.4 ± 0.8 | 42.4 ± 0.4 | 19.2 ± 0.4 |
| Consortium I + biosurfactant | 80.7 ± 0.3 | 43.8 ± 0.5 | 22.5 ± 0.7 |
| Consortium II + biosurfactant | 81.6 ± 0.7 | 42.6 ± 0.2 | 20.7 ± 0.5 |
Determination on the basis of crude oil 2.0 ml (1.849 g). Results represented mean ± SD of three individual experiments
Fig. 7.
Gas chromatographic analysis of the saturate fraction of crude oil after treatment with A control, B Consortium I, C Consortium II, D Consortium I + biosurfactant, and E Consortium II + biosurfactant
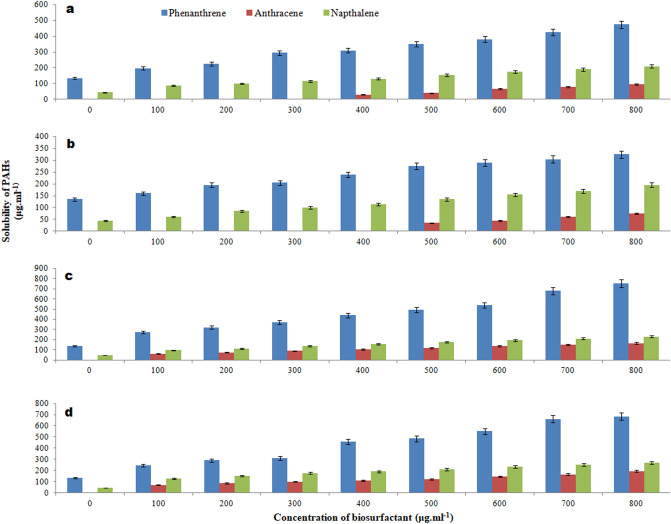
Biosurfactant-mediated solubilization of polyaromatic hydrocarbons (PAH)
In the presence of biosurfactants, the impact of biosurfactants on the solubility of PAHs including phenanthrene, anthracene, and naphthalene was studied. Figure 8 shows that biosurfactants had a significant impact on the solubilization of the three PAHs studied. Biosurfactant from strain PA-OBP3 showed the highest phenanthrene solubilization, followed by PA-OBP4. When it came to anthracene, the biosurfactant from the bacterial strain PA-OBP4 displayed a higher degree of solubilization than the biosurfactants from the PA-OBP3 and PA-OBP1 strains. Only the biosurfactant from PA-OBP4 stain was found to substantially solubilize naphthalene.
Fig. 8.
PAH solubilization assay showing the decrease in the available phenanthrene, anthracene and naphthalene concentration with increasing concentration of biosurfactants produced by a P. aeruginosa OBP1, b P. aeruginosa OBP2, c P. aeruginosa OBP3, and d P. aeruginosa OBP4. Values are the mean of three independent experiments ± standard deviation
Separation of residual crude oil from the petroleum sludge by the biosurfactant
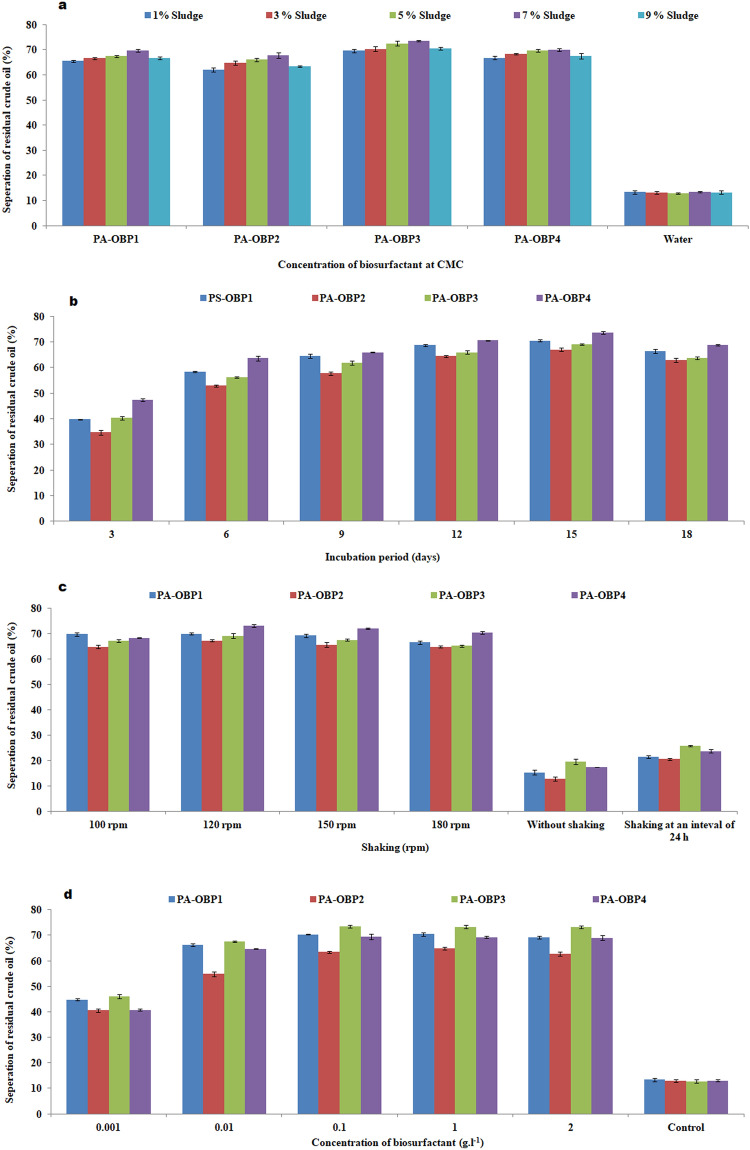
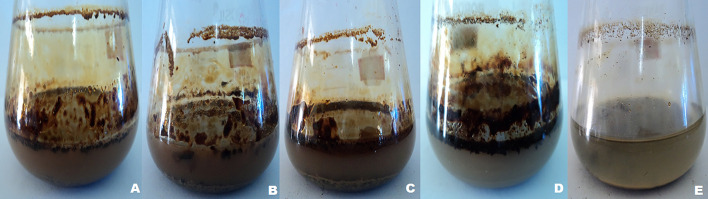
Results demonstrated that biosurfactants are more effective at removing total petroleum hydrocarbons (TPH) from petroleum sludge than water alone. The overall petroleum hydrocarbon content of the sludge was estimated to be around 785 ± 130 g kg−1. Oil separation was possible up to 7% (w/w) sludge concentrations, but there was no further increase in the release of residual crude oil from the sludge above this concentration because they were unable to form homogeneous slurry, as shown in Fig. 9a. The amount of residual crude oil released increased over time, but there was no further after 15 days of treatment in the presence of constant shaking, as seen in Fig. 9b, release in the volume of residual oil escalated when the flasks were shaken constantly as compared to when they were shaken only occasionally or not at all. The release of oil increased as the rpm value increased, but there was a substantial decrease in residual oil separation above 120 rpm as shown in Fig. 9c. As shown in Fig. 9d, PA-OBP4 biosurfactant separated the most (73.5%), followed by PA-OBP1, PA-OBP3, and PA-OBP2, which separated 70.3, 69.5, and 63.4% of residual crude oil, respectively, from petroleum sludge after 15 days of treatment. Water was used as a control, and it separated between 13% of the residual crude oil. The dense deposition of crude oil layer near the surface of the washing solution in an Erlenmeyer flask containing sludge and aqueous biosurfactant solution indicates the effective separation process (Fig. 10).
Fig. 9.
Effect of different parameters on the separation of residual crude oil from the petroleum sludge a sludge concentration, b treatment period, c shaking, and d biosurfactant concentration. Values are the mean of three independent experiments ± standard deviation
Fig. 10.
Separation of residual crude oil from the petroleum sludge after washing with biosurfactant solution of A P. aeruginosa OBP1, B P. aeruginosa OBP2, C P. aeruginosa OBP3 and D P. aeruginosa OBP4
It could be seen that the separated crude oil from the sludge in the presence of aqueous biosurfactant solution gets adhered over the surface of the Erlenmeyer flask..
Discussion
All the four strains of P. aeruginosa were initially cultured in MSM supplemented with 23 different hydrocarbons along with crude oil individually to distinguish whether or not they can utilize petroleum as its sole carbon and energy source. Among the tested hydrocarbons, bacterial strains performed well in MSM supplemented with n-hexadecane, as we have previously mentioned in Bharali et al. (2018). The four strains have quite different preferences for aliphatic hydrocarbons. As evidenced by the generation of biomass, dodecane, tridecane, n-hexadecane, and octadecane were favoured above the others. None of the strains was capable to utilize eicosane and triacontane. As indicated by the bacterial strains’ utilization of crude oil, which is detailed later in the section, the biosurfactant produced by the bacterial strains undoubtedly aids in the assimilation of hydrophobic compounds. On the other hand, the inability to use specific types of aliphatic hydrocarbons is most likely owing to the inherent capacities of selected bacterial stains. The n-alkane hydroxylase system, for example, is necessary for the degradation of long-chain alkanes, and its efficiency differs across bacteria that degrade hydrocarbons (Ji et al. 2013; Pi et al. 2016). Moreover, with the increase in the chain length of alkanes, the hydrophobicity of the molecule increases which in turn makes the molecules less soluble in water and reduces the bioavailability (Zhang et al. 2005). Several studies reported the capability to use aliphatic chains in the range of C5–C13, C8–C16, C12–C24, C12–C34, C6–C28, C12–C32 and C12–C28 by various of Pseudomonas spp. (Smits et al. 2002; Chaerun et al. 2005; Naik and Sakthivel 2006; Mehdi and Giti 2008). Such a wide range of aliphatic alkane preferences among the genus Pseudomonas spp. strongly suggests strain specificity.
The growth of bacterial strains on aromatic hydrocarbons was insignificant as compared to that on aliphatic hydrocarbons. Among the aromatic compounds, only toluene was found to be utilized by the strains, but in limited quantity. The results are consistent with the observation of Jones and Edington (1968) who reported that only 0.5% of a large group of soil organisms could utilize aromatic hydrocarbons. The preference for PAHs as carbon source was found to be quite diverse among the strains. Cerniglia (1993) reported that only a small number of bacteria were capable of completely mineralizing large molecular weight PAHs. Preference for toluene, anthracene and phenanthrene as the carbon source could be attributed to their site of isolation from those environments, which are frequently contaminated by aromatic and polyaromatic fractions of crude oil. As evidenced by the amount of bacterial biomass, significant growth was seen in diesel-, crude oil-, and kerosene-supplemented media. A greater portion of diesel, crude oil and kerosene contains aliphatic hydrocarbons and are reported to be more prone to microbial degradation (Wongsa et al. 2004; Adebusoye et al. 2007; Mehdi and Giti 2008). The preference for carbon substrates among the studied bacterial strains of P. aeruginosa is quite different, and the aliphatic rich hydrocarbons were found to be better than complex hydrocarbons. Such behaviour, clearly confirms the efficient alkane utilizing ability. The overall preference of hydrocarbons was found to be in the order of n-hexadecane > octadecane > diesel > crude oil > tridecane > dodecane > kerosene > lubricating oil as evident from the increase in the bacterial biomass production. Such anomalous behavior of the bacterial strains clearly suggests that the preference towards carbon sources for their sustenance entirely depends on the type of bacterial strains. Moreover, the effect of the nutrient medium and particularly the carbon source on the synthesis of biosurfactant is still under study (Perfumo et al. 2006). According to Primeia et al. (2020), microorganisms that produce biosurfactants build their own micro-environment, which promotes emulsification by releasing certain compounds such as biosurfactants through various mechanisms such as quorum sensing.
The growth of all four strains accelerated with rising crude oil concentrations until they reached their maximum value, after which they levelled off. Within 2% of crude oil, all four strains grew faster, but after that, growth slowed dramatically, as seen by a decrease in CFU. The exact reason for such behavior is not well understood, but it is thought to be related to one or more of the following primary factors: crude oil nature and quantity (Ma et al. 2015), enzyme inhibition (Leahy and Colwell 1990) and oxygen limitation (Wongsa et al. 2004), all of which are considered to have a negative impact on degradation. Furthermore, the ability to tolerate different crude oil concentrations is determined by the type of bacterium, which differs between species and even strains. Strict aerobic P. aeruginosa uses oxygen for hydrocarbon degradation and as an acceptor in cell metabolic processes (Koshlaf and Ball 2017). Since oxygen molecules are bound to the C terminal cluster and sub-terminal chain of n-alkanes by monooxygenase or dioxygenase enzymes during the early stages of hydrocarbon biodegradation, an adequate supply of oxygen is essential (Sierra-Garcia and de Oliveira 2013). Wongsa et al. (2004) stated that the inadequate availability of oxygen in shake-flask cultures might be accountable for the reduced growth of P. aeruginosa WatG on petroleum refined products like kerosene and diesel, and reported it to be an oxygen-intensive metabolic process. Leahy and Colwell (1990) reported that the effects of toxicity, enzyme inhibition, and oxygen limitation were minimized by using relatively low concentrations of hydrocarbons. According to Mehdi and Giti (2008) the high concentration of crude oil reduced the growth rate of the different crude oil degrading bacterial strains of Pseudomonas, Rhodococcus and Bacillus.
In the crude oil-supplemented media, the bacterial strains grew in a nearly identical fashion. Initially, bacterial growth accelerated within the 6–9 days of the cultivation process. Rise in the bacterial growth may be due to the accumulation of alcoholic compounds, which are produced as result of terminal and sub-terminal oxidation pathways of alkanes. Apparent exponential phases were seen between 09 and 24 days of incubation, which was believed to be caused by the accumulation of fatty acid derivatives. Fatty acids are formed when aldehydes or methyl ketones are oxidized (Sierra-Garcia and de Oliveira 2013). Further, towards 27 days of cultivation, CFUs of the bacterial cultures declined indicating that they had reached the stationary phase. This phase is generally characterized by an equipoise state of cell multiplication and death. The most possible reason for the reduction in bacterial growth may be due to nutrient scarcity or the adaptation of bacteria to use compounds that are more complex in nature (Shao and Wang 2013). After 06 days of inoculation, the surface tension in the cultures was reduced to around 32.7–46.8 mN m−1, indicating that the bacteria were towards the end of lag phase of their growth. The possible cause for the initiation of biosurfactant production in the early exponential phase could be due to availability of hydrocarbons as the source of carbon through pseudo-solubilization. After, 09–12 days of bacterial growth, the surface tension of the cultures were further reduced about 32.5–40.6 mN m−1, which was mainly towards the stationary phase of growth. Such behaviour might be due to the release of cell bound biosurfactant into the culture broth (Déziel et al. 1999). Biosurfactants can help with hydrocarbon degradation efficiently by two key mechanisms. first, by increasing the microorganisms' access to substrate. Second, by modifying the bacterial cell surface to increase its surface hydrophobicity and hydrophobic substrates can be combined more easily (Mulligan and Gibbs 2004). As a result, hydrophobic substrates can more readily interact with bacterial cells. The steady surface activities between 15 and 24 days of culture indicated the optimum level of biosurfactant production by the bacterial strains near to their stationary phase. Biosurfactant production increased as growth progressed from the late exponential to the early stationary period, indicating that it was produced as a secondary metabolite. Various strains of P. aeruginosa were reported to show an over-production of rhamnolipid when cultures reached their stationary phase of growth (Lotfabad et al. 2009; Makkar et al. 2011; Abbasi et al. 2012; Müller et al. 2012). Despite maintaining the same culture conditions, the four P. aeruginosa strains showed a significant difference in growth behaviour and biosurfactant development patterns. Discrepancies in growth dynamics may be linked to the constitutive aspect of the organism’s hydrocarbon assimilation capacity or to the strains’ adaptation to previous exogenous hydrocarbon exposure. This could be followed by simultaneous development of the capability to use the oil and/or its catabolic products as carbon and energy sources (Adebusoye et al. 2007).
In comparison to the control, the leftover crude oil after treatment with the bacterial strain had lower viscosity. The use of microbes in degrading long-chain alkanes might have several benefits such as minimizing paraffin precipitation or deposition problem along the production flow line, reduction of the viscosity of crude oil, increase in API gravity value and finally reduction of both pour point and paraffin content of crude oil (Etoumi et al. 2008). Moreover, the biosurfactants produced during the bacterial growth on crude oil additionally reduces its viscosity by altering the surface and interfacial energy of the system, thereby increasing the mobility of crude oil in the pipelines. She et al. (2011) employed indigenous Bacillus strains that produce biosurfactants to degrade the higher fractions of crude oil. They also reported the improvement in the flow characteristics of crude oil after the treatment in a petroleum reservoir of Daqing Oilfield. According to Gudiña et al. (2012) Bacillus strains were able to degrade long alkyl chains and thereby lowered the viscosity of hydrocarbon mixtures. These reports supported our view of using the indigenous biosurfactant producing bacterial strains in reducing the viscosity of crude oil.
The bacterial strains were found to be effective at degrading hydrocarbons. The degree of degradation of aliphatic, aromatic, and NSO-containing hydrocarbons varied greatly across the bacterial strains. This is generally linked to the enzyme efficiency and the biosurfactant released by the bacterium. It has been reported that P. aeruginosa possesses two homologous AlkB hydroxylases that are activated in different ways depending on the length of the hydrocarbon chain present, allowing it to degrade a wide range of alkanes (Liu et al. 2014; Muriel-Millán et al. 2019). Results clearly established that the strains of P. aeruginosa are efficient in degrading aliphatic fractions as compared to other existing components of crude oil. The evidence of petroleum hydrocarbons being used by bacterial strains supports these results. Further, the degradation of crude oil by the bacterial strains was verified through GC. GC analyses of the aliphatic fractions of treated crude oil with the bacterial strains showed a similar response of degradation, but different in details. Bioconversion of crude oil components leads to the enrichment of the lighter fractions of hydrocarbons having shorter retention time. The bacterial strains were competent in degrading crude oil and efficiently degraded n-alkanes in the range of C9–C18. Previous studies showed that alkane with C14–C20 carbon atoms permits abundant growth for most of the bacteria (Mishra et al. 2004; Balachandran et al. 2012). In the present investigation, bacterial strains were not much efficient in utilizing aromatic and polyaromatic compounds. The predominance of mineralization of aliphatic over aromatic hydrocarbons with greater rate of degradation by the bacterial community was reported by several authors (Lal and Khanna 1996; Vinas et al. 2002; Adebusoye et al. 2007). The batch culture of each bacterial strain has a specific selection of preferred substrates, rendering it less effective at using the complex hydrocarbon mixtures found in crude oil (Adams and Jackson 1996). According to Lal and Khanna (1996), bacteria usually degrade crude oil by using alkanes or light aromatic fractions, while heavier molecular weight aromatics, resins, and asphaltenes are recalcitrant. Adebusoye et al. (2007) reported that the individual organisms such as Corynebacterium spp., Acinetobacter lwoffii and P. aeruginosa could only metabolize a restricted spectrum of hydrocarbon substrates. Biodegradation studies conducted by Sharma and Pant (2000) showed that 50% of the aliphatic fractions of the crude oil of Assam (India) were degraded by the isolates of Rhodococcus. Several studies have found that the kind of oil and its molecular makeup are closely related to the extent of oil and total petroleum hydrocarbon biodegradation (Balachandran et al. 2012). The present study showed that the presence of crude oil in the culture medium had no detrimental effect on the bacterial strain’s ability to synthesize biosurfactant and might assist in the biodegradation of high molecular weight n-alkanes (C12–C18). Lee et al. (2018) reported that when indigenous bacteria degrade crude oil, they produce biosurfactants that enhance emulsification and make crude oil degradation more favorable.
The properties and rate of breakdown of complex mixtures of petroleum hydrocarbon vary greatly which determines the degree of bioremediation (Yasin et al. 2013). Formulation of efficacious microbial consortium could be a troubleshooter to the issue of limited remediation process. Individual bacterial strains can only metabolize a restricted spectrum of hydrocarbon substrates; thus, heterogeneous populations with high enzymatic capabilities are needed to speed up and expand petroleum biodegradation (Ghazali et al. 2004). In 30 days of culture, combination number 7 and 11, labelled consortium I and II, were found to be effective in degrading 78.6–80.4% of aliphatic, 42.4–42.7% of aromatic, and 21.6–19.2% of NSO containing compounds. Both consortia, which included all four distinct strains of P. aeruginosa except PA-OBP2 in consortium I, were found to digest crude oil efficiently. The consortia were able to degrade up to 42.7% of the aromatic fraction of crude oil, which no single bacteria could do (Table 2). This is most likely due to the fact that the consortium members’ enzyme and biosurfactant work in tandem. Mixed cultures have the ability to secrete a wider range of enzymes that are needed to catalyze various reactions involved in degradation process compared to a single bacterial culture (Ghazali et al. 2004). Degradation of aromatic fraction supports the co-metabolism behaviour and synergistic interactions among members of the consortium. The metabolic intermediates produced by one bacterial strain could be utilized by the other members of the consortium as the substrate for their growth and biosurfactant production (Mulligan 2005). According to Ghazali et al. (2004), biodegradation of complex hydrocarbons frequently necessitated the cooperation of multiple species. This is especially true for pollutants like crude oil, which are made up of a variety of chemical chemicals. In designing a consortium, solubility and accessibility of the hydrophobic compounds available in the crude oil are the two key aspects. The rate of solubility of the crude oil is a significant limiting factor in its biodegradation. Since only 0.02% of crude oil is soluble in water, hence there is a need for emulsification of the crude oil in the medium (Chhatre et al. 1996). To analyze the influence of biosurfactant (45 mg l−1) produced by the bacterial strain PA-OBP1 on the degradation process, it was added to both consortia. Since the biosurfactant from the PA-OBP1 strain has higher surface activity than biosurfactants from other strains, it was selected (Bharali et al. 2018). Both consortia II and I appeared to degrade aliphatic and aromatic components of crude oil at identical rates in the presence and absence of additional biosurfactant (Table 2). Our previous experiments showed that in the presence of crude oil, all members of the consortium could produce biosurfactant. Therefore, it is conceivable that the total biosurfactant, which includes both externally added and internally produced biosurfactants, accumulates in the culture medium. Further, through the mobilization process, such accumulated biosurfactants may aid in the bioavailability of crude oil to bacterial cells. Biosurfactants support the rate of biodegradation by emulsifying and increasing the solubility of hydrophobic compounds. By emulsifying the hydrocarbons, biosurfactants aid in microbial uptake of crude oil and degradation. Biosurfactants can raise the pseudo-solubilization of hydrophobic substrates because of their specificity and degradability (Karlapudi et al. 2018). Further, the GC analysis of the aliphatic fractions of crude oil confirmed the enhancement in the degradation process (Fig. 7). Gas chromatographic profile of the saturated fraction of the crude oil inoculated with Consortia I and II exhibited much reduced noise level as compared to the non-inoculated medium (Fig. 7). Presence of certain distinct unreduced peaks indicates the accumulation of bacterial by-products which are not degraded further by the members of the consortia. The results clearly established that both consortia along with the biosurfactant were capable of degrading the various components of crude oil.
Water solubility of some of the hydrophobic organic compounds (HOCs) could be increased with the addition of synthetic surfactant or biosurfactant (Eddouaouda et al. 2012). The solubilization assays of selected PAHs clearly showed that with the increase in the concentration of biosurfactants upto a range of 45–120 mg l−1 could reduce the amount of available undissolved PAHs in the aqueous mixture. PAHs were effectively solubilized by biosurfactants at levels below or above their CMC. However, when the concentration of biosurfactants produced by the bacterial strains was increased above their respective CMC values, solubilization was far more pronounced. Such behaviour of the biosurfactants is in view of the fact that the concentration of biosurfactants above the CMC enhances the formation of micelle causing the undissolved organic components to dissolve within the micelle structure facilitating microbial uptake and bioremediation (Yin et al. 2009). Phenanthrene was solubilized to a noticeable extent by biosurfactants from all four bacterial strains. Yin et al. (2009) reported the solubilization of phenanthrene at 50 mg l−1 of rhamnolipid produced by P. aeruginosa strain S6. Das et al. (2008) showed that the solubilization of anthracene increases with the increase in rhamnolipid concentration beyond 100 mg l−1. Further, differences in the degree of solubilization by the biosurfactants might be due to differences in the physicochemical characters of the tested PAHs and the types of rhamnolipid congeners present in the biosurfactants (Abdel-Mawgoud et al. 2009; Salihu et al. 2009).
Petroleum sludge not only contains large amounts of harmful environmental contaminants, but also carries a substantial portion of recyclable resources, such as extractable and refractory petroleum hydrocarbons with high potential value (Hu et al. 2020). Recycling of petroleum sludge can be used to reduce not only the quantity and level of contaminants, but also the consumption of non-renewable resources (Hui et al. 2020). Currently, there is a growing understanding and popularity of cleaner development, and green operating strategies that promote sustainability and other eco-friendly techniques. Among these technologies, recycling has emerged as the most environmentally friendly alternative for petroleum sludge disposal and treatment. As a result, in recent years, more emphasis has been placed on developing technologies for the recycling and safe treatment of petroleum sludge (Hu et al. 2020). In this regard, use of bacterial-derived biosurfactant for petroleum sludge recycling is desirable because it meets both environmental and ecological sustainability goals. In fact, it was reported that the preparation of homogeneous slurry could be a critical factor in the treatment of sludge, which could limit the process (Déziel et al. 2000). The disturbance caused by the continuous shaking further separated the loosely bound oil droplets from the soil particles due to the reduction of interfacial tension. With the increase in the biosurfactant concentration, there was increase in the removal of residual crude oil from the sludge. The decrease in surface and interfacial tension caused a gradual decrease in the capillary force that holds soil and oil together. This further enhanced the contact angle between soil and oil, resulting in a change in the wettability of the system. The interfacial tension of the system decreased gradually until it reached its CMC after which it remained constant. The effect is most likely caused by the physicochemical features of biosurfactants and the coupled behaviour of surfactant/crude oil/soil systems (Urum and Pekdemir 2004). The CMC of the biosurfactant developed by PA-OBP1, PA-OBP2, PA-OBP3, and PA-OBP4 has previously been determined and reported to be 45, 105, 90, and 65 mg 1−1, respectively (Bharali and Konwar 2011; Bharali et al. 2018). A successful attempt in using biosurfactant to recover oil from the sludge was also reported by Yan et al. (2012), Chirwa et al. (2017) and Liu et al. (2018, 2020). Joseph and Joseph (2009) separated the residual oil from the petroleum sludge generated from the crude oil refinery by directly inoculating the strains of Bacillus spp. and with the addition of the cell-free culture supernatant of the bacteria. Helmy et al. (2010) used a biosurfactant generated by Azotobacter vinelandii AV01 to improve oil recovery from oil sludge and recovered up to 15% of the oil. According to Saikia and Deka (2013), P. aeruginosa RS29, a biosurfactant-producing strain, could extract up to 55.5% of hydrocarbon from refinery sludge using CFCS. Synthetic surfactant such as sodium dodecyl sulphate (SDS) is effective for the separation of residual oil from the sludge but is severely hazardous to the environmental components (del Mar Sánchez-Peinado et al. 2010; Wyrwas et al. 2011). Therefore, the application of biosurfactant appears to be more beneficial over the chemical surfactants because of their biodegradability, lower toxicity and effectiveness at severe temperatures, pH and salinity (Abdel-Mawgoud et al. 2009; Lotfabad et al. 2009; Makkar et al. 2011; Abbasi et al. 2012; Bharali et al. 2018).
Conclusion
In the present investigation, the indigenous four biosurfactant-producing P. aeruginosa strains were found to be effectively utilizing a wide spectrum of petroleum as supported by increase in biomass and degradation profiles. Two effective bacterial consortia, i.e. consortium I, which includes all the strains except for P. aeruginosa PA-OBP2, and consortium II, with all the four strains, revealed to biodegrade different components of crude oil more efficiently than the individual strains. Such behaviour supports the idea that the most efficient combination of microbes in a mixed culture will ensure the effectiveness of the petroleum degradation process. Furthermore, incorporating biosurfactant externally to the consortium improved the biodegradation process. The biosurfactants produced by the strains were effective at solubilizing PAHs within their CMCs and were able to recover a significant amount of residual crude oil from the petroleum sludge. Thus, bacterial strains and consortiums that can synthesize biosurfactant and degrade crude oil could be used as an efficient bioremediation tool. Application of such approaches reduces toxic contaminants and ensures environmental protection, and thereby contributes to achieving ecological sustainability.
Author contributions
PB, YB, AR, ND, PM designed the experiments, analyzed the data. PB, YB, AR, ND, PM, conducted most of the experiments. VS, AT, VV, PDN, BKK provided the experimental materials and performed few experiments. PB, BKK, YB, AT, VS, AR wrote the manuscript. All authors read and approved the manuscript.
Funding
PB acknowledges ONGC, India for the fellowship and funding the research (Sanction number: ONGCA/CHAIRS/PU/2010-Dated 31-03-2010 for the year 2009–2014).
Availability of data and materials
This manuscript and its additional information files contain all of the data generated or analyzed during the study.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors agreed and gave their permission for the manuscript to be published.
References
- Abbasi H, Hamedi MM, Lotfabad TB, Zahiri HS, Sharafi H, Masoomi F, Moosavi-Movahedi AA, Ortiz A, Amanlou M, Noghabi KA. Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: physicochemical and structural characteristics of isolated biosurfactant. J Biosci Bioeng. 2012;113(2):211–219. doi: 10.1016/j.jbiosc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA. Characterization of rhamnolipid produced by Pseudomonas aeruginosa isolate Bs20. Appl Biochem Biotechnol. 2009;157(2):329–345. doi: 10.1007/s12010-008-8285-1. [DOI] [PubMed] [Google Scholar]
- Abosede EE. Effect of crude oil pollution on some soil physical properties. J Agric Vet Sci. 2013;6(3):14–17. [Google Scholar]
- Adams PZ, Jackson PP (1996) Bioremediation of oil spill. Theory and Practice. In: Nigeria National Petroleum Corporation Publication, Department of Petroleum Resources, pp 3−10
- Adebusoye SA, Ilori MO, Amund OO, Teniola OD, Olatope SO. Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J Microbiol Biotechnol. 2007;23(8):1149–1159. [Google Scholar]
- Ahmed F, Fakhruddin AN. A review on environmental contamination of petroleum hydrocarbons and its biodegradation. J Environ Sci Nat Resour. 2018;11(3):1–7. doi: 10.19080/IJESNR.2018.11.555811. [DOI] [Google Scholar]
- Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M, Dragonieri S, Memeo V, De Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg. 2013;100(1):144–150. doi: 10.1002/bjs.8942. [DOI] [PubMed] [Google Scholar]
- Alzahrani AM, Rajendran P (2019) Petroleum hydrocarbon and living organisms. In: Ince M, Ince OK (eds) Hydrocarbon pollution and its effect on the environment. IntechOpen. 10.5772/intechopen.86948
- Arjoon K, Speight JG. Chemical and physical analysis of a petroleum hydrocarbon contamination on a soil sample to determine its natural degradation feasibility. Inventions. 2020;5(3):43. doi: 10.3390/inventions5030043. [DOI] [Google Scholar]
- Balachandran C, Duraipandiyan V, Balakrishna K, Ignacimuthu S. Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour Technol. 2012;112:83–90. doi: 10.1016/j.biortech.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Barkay T, Navon-Venezia S, Ron EZ, Rosenberg E. Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl Environ Microbiol. 1999;65(6):2697–2702. doi: 10.1128/aem.65.6.2697-2702.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharagava RN, Saxena G, Mulla SI. Introduction to industrial wastes containing organic and inorganic pollutants and bioremediation approaches for environmental management. In: Saxena G, Bharagava R, editors. Bioremediation of industrial waste for environmental safety. Singapore: Springer; 2020. pp. 1–18. [Google Scholar]
- Bharali P, Konwar BK. Production and physico-chemical characterization of a biosurfactant produced by Pseudomonas aeruginosa OBP1 isolated from petroleum sludge. Appl Biochem Biotechnol. 2011;164(8):1444–1460. doi: 10.1007/s12010-011-9225-z. [DOI] [PubMed] [Google Scholar]
- Bharali P, Singh SP, Yasir B, Nipu D, Konwar BK, Singh CB. Characterization and assessment of biosurfactant producing indigenous hydrocarbonoclastic bacteria: potential application in bioremediation. Nova Biotechnol Chim. 2018;17(2):103–114. [Google Scholar]
- Brown PJ, Long SM, Spurgeon DJ, Svendsen C, Hankard PK. Toxicological and biochemical responses of the earthworm Lumbricus rubellus to pyrene, a non-carcinogenic polycyclic aromatic hydrocarbon. Chemosphere. 2004;57(11):1675–1681. doi: 10.1016/j.chemosphere.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4(3):331–338. [Google Scholar]
- Chaerun SK, Tazaki K, Asada R, Kogure K. Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: implications for bioremediation. Clay Miner. 2005;40(1):105–114. doi: 10.1180/0009855054010159. [DOI] [Google Scholar]
- Chhatre S, Purohit H, Shanker R, Khanna P. Bacterial consortia for crude oil spill remediation. Water Sci Technol. 1996;34(10):187–193. [Google Scholar]
- Chirwa EM, Mampholo CT, Fayemiwo OM, Bezza FA. Biosurfactant assisted recovery of the C5–C11 hydrocarbon fraction from oily sludge using biosurfactant producing consortium culture of bacteria. J Environ Manag. 2017;196:261–269. doi: 10.1016/j.jenvman.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Contreras-Ramos SM, Alvarez-Bernal D, Dendooven L. Eisenia fetida increased removal of polycyclic aromatic hydrocarbons from soil. Environ Pollut. 2006;141(3):396–401. doi: 10.1016/j.envpol.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Das P, Mukherjee S, Sen R. Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere. 2008;72(9):1229–1234. doi: 10.1016/j.chemosphere.2008.05.015. [DOI] [PubMed] [Google Scholar]
- del Mar Sánchez-Peinado M, González-López J, Martínez-Toledo MV, Pozo C, Rodelas B. Influence of linear alkylbenzene sulfonate (LAS) on the structure of Alphaproteobacteria, Actinobacteria, and Acidobacteria communities in a soil microcosm. Environ Sci Pollut Res. 2010;17(3):779–790. doi: 10.1007/s11356-009-0180-y. [DOI] [PubMed] [Google Scholar]
- Déziel E, Lépine F, Dennie D, Boismenu D, Mamer OA, Villemur R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim Biophys Acta. 1999;1440(2–3):244–252. doi: 10.1016/s1388-1981(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Déziel E, Lépine F, Milot S, Villemur R. Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa strain 57RP. Biochem Biophys Acta. 2000;1485(2–3):145–152. doi: 10.1016/s1388-1981(00)00039-1. [DOI] [PubMed] [Google Scholar]
- Eddouaouda K, Mnif S, Badis A, Younes SB, Cherif S, Ferhat S, Mhiri N, Chamkha M, Sayadi S. Characterization of a novel biosurfactant produced by Staphylococcus sp. strain 1E with potential application on hydrocarbon bioremediation. J Basic Microbiol. 2012;52(4):408–418. doi: 10.1002/jobm.201100268. [DOI] [PubMed] [Google Scholar]
- Etoumi A, El Musrati I, El Gammoudi B, El Behlil M. The reduction of wax precipitation in waxy crude oils by Pseudomonas species. J Ind Microbiol Biotechnol. 2008;35(11):1241–1245. doi: 10.1007/s10295-008-0420-z. [DOI] [PubMed] [Google Scholar]
- Ghazali FM, Rahman RN, Salleh AB, Basri M. Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeterior Biodegrad. 2004;54(1):61–67. doi: 10.1016/j.ibiod.2004.02.002. [DOI] [Google Scholar]
- Gudiña EJ, Pereira JF, Rodrigues LR, Coutinho JA, Teixeira JA. Isolation and study of microorganisms from oil samples for application in microbial enhanced oil recovery. Int Biodeterior Biodegrad. 2012;68:56–64. [Google Scholar]
- Guo J, Feng R, Ding Y, Wang R. Applying carbon dioxide, plant growth-promoting rhizobacterium and EDTA can enhance the phytoremediation efficiency of ryegrass in a soil polluted with zinc, arsenic, cadmium and lead. J Environ Manag. 2014;141:1–8. doi: 10.1016/j.jenvman.2013.12.039. [DOI] [PubMed] [Google Scholar]
- Hao R, Lu A, Zeng Y. Effect on crude oil by thermophilic bacterium. Pet Sci Eng. 2004;43(3–4):247–258. [Google Scholar]
- Helmy Q, Kardena E, Nurachman Z. Application of biosurfactant produced by Azotobacter vinelandii AV01 for enhanced oil recovery and biodegradation of oil sludge. Int J Civ Environ Eng. 2010;10:7–14. [Google Scholar]
- Hu G, Feng H, He P, Li J, Hewage K, Sadiq R. Comparative life-cycle assessment of traditional and emerging oily sludge treatment approaches. J Clean Prod. 2020;251:119594. doi: 10.1016/j.jclepro.2019.119594. [DOI] [Google Scholar]
- Hui K, Tang J, Lu H, Xi B, Qu C, Li J. Status and prospect of oil recovery from oily sludge: a review. Arab J Chem. 2020;13(8):6523–6543. doi: 10.1016/j.arabjc.2020.06.009. [DOI] [Google Scholar]
- Iloba BN, Jarret IE. Effect of crude oil spills and on the abundance and distribution of soil microartropods at different depths. Int J Zool Res. 2007;3(1):24–32. [Google Scholar]
- Ji Y, Mao G, Wang Y, Bartlam M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front Microbiol. 2013;4:58. doi: 10.3389/fmicb.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JG, Edington MA. An ecological survey of hydrocarbon-oxidizing micro-organisms. Microbiology. 1968;52(3):381–390. [Google Scholar]
- Joseph PJ, Joseph A. Microbial enhanced separation of oil from a petroleum refinery sludge. J Hazard Mater. 2009;161(1):522–525. doi: 10.1016/j.jhazmat.2008.03.131. [DOI] [PubMed] [Google Scholar]
- Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, ramu Dirisala V, Kodali VP. Role of biosurfactants in bioremediation of oil pollution—a review. Petroleum. 2018;4(3):241–249. [Google Scholar]
- Koshlaf E, Ball A. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017;3(1):25–49. doi: 10.3934/microbiol.2017.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoshto IN, Richards JR, Albertson TE, Derlet RW. The toxicity of diesel exhaust: implications for primary care. J Am Board Fam Med. 2008;21(1):55–62. doi: 10.3122/jabfm.2008.01.070139. [DOI] [PubMed] [Google Scholar]
- Kum C, Sekkin S, Kiral F, Akar F. Effects of xylene and formaldehyde inhalations on renal oxidative stress and some serum biochemical parameters in rats. Toxicol Ind Health. 2007;23(2):115–120. doi: 10.1177/0748233707078218. [DOI] [PubMed] [Google Scholar]
- Lal B, Khanna S. Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenes odorans. J Appl Bacteriol. 1996;81(4):355–362. doi: 10.1111/j.1365-2672.1996.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Leahy JG, Colwell RR. Microbial degradation of hydrocarbons in the environment. Microbiol Mol Biol Rev. 1990;54(3):305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Lee H, Kwon BO, Khim JS, Yim UH, Kim BS, Kim JJ. Biosurfactant-assisted bioremediation of crude oil by indigenous bacteria isolated from Taean beach sediment. Environ Pollut. 2018;241:254–264. doi: 10.1016/j.envpol.2018.05.070. [DOI] [PubMed] [Google Scholar]
- Lipińska A, Kucharski J, Wyszkowska J. Urease activity in soil contaminated with polycyclic aromatic hydrocarbons. Pol J Environ Stud. 2013;22(5):1393–1400. [Google Scholar]
- Liu H, Xu J, Liang R, Liu J. Characterization of the medium-and long-chain n-alkanes degrading Pseudomonas aeruginosa strain SJTD-1 and its alkane hydroxylase genes. PLoS One. 2014;9(8):e105506. doi: 10.1371/journal.pone.0105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang Y, Sun S, Huang L, Yu L, Liu X, Lai R, Luo Y, Zhang Z, Zhang Z. Oil recovery from tank bottom sludge using rhamnolipids. J Petrol Sci Eng. 2018;170:14–20. [Google Scholar]
- Liu C, Xu Q, Hu X, Zhang S, Zhang P, You Y. Optimization of process parameters of rhamnolipid treatment of oily sludge based on response surface methodology. ACS Omega. 2020;5(45):29333–29341. doi: 10.1021/acsomega.0c04108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf B Biointerface. 2009;69(2):183–193. doi: 10.1016/j.colsurfb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Ma YL, Lu W, Wan LL, Luo N. Elucidation of fluoranthene degradative characteristics in a newly isolated Achromobacter xylosoxidans DN002. Appl Biochem Biotechnol. 2015;175:1294–1305. doi: 10.1007/s12010-014-1347-7. [DOI] [PubMed] [Google Scholar]
- Makkar RS, Cameotra SS, Banat IM. Advances in utilization of renewable substrates for biosurfactant production. AMB Express. 2011;1(1):1–9. doi: 10.1186/2191-0855-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi H, Giti E. Investigation of alkane biodegradation using the microtiter plate method and correlation between biofilm formation, biosurfactant production and crude oil biodegradation. Int Biodeterior Biodegrad. 2008;62(2):170–178. [Google Scholar]
- Mishra S, Sarma PM, Lal B. Crude oil degradation efficiency of a recombinant Acinetobacter baumannii strain and its survival in crude oil-contaminated soil microcosm. FEMS Microbiol Lett. 2004;235(2):323–331. doi: 10.1016/j.femsle.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Monteiro M, Moreira N, Pinto J, Pires-Luís AS, Henrique R, Jerónimo C, Bastos MD, Gil AM, Carvalho M, Guedes de Pinho P. GC-MS metabolomics-based approach for the identification of a potential VOC-biomarker panel in the urine of renal cell carcinoma patients. J Cell Mol Med. 2017;21(9):2092–2105. doi: 10.1111/jcmm.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Kügler JH, Henkel M, Gerlitzki M, Hörmann B, Pöhnlein M, Syldatk C, Hausmann R. Rhamnolipids—next generation surfactants? J Biotechnol. 2012;162(4):366–380. doi: 10.1016/j.jbiotec.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Mulligan CN. Environmental applications for biosurfactants. Environ Pollut. 2005;133(2):183–198. doi: 10.1016/j.envpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mulligan CN, Gibbs BF. Types, production and applications of biosurfactants. Proc Indian Acad Sci Part B. 2004;70(1):31–56. [Google Scholar]
- Muriel-Millán LF, Rodríguez-Mejía JL, Godoy-Lozano EE, Rivera-Gómez N, Gutierrez-Rios RM, Morales-Guzmán D, Trejo-Hernández MR, Estradas-Romero A, Pardo-López L. Functional and genomic characterization of a Pseudomonas aeruginosa strain isolated from the southwestern Gulf of Mexico reveals an enhanced adaptation for long-chain alkane degradation. Front Mar Sci. 2019;6:572. doi: 10.3389/fmars.2019.00572. [DOI] [Google Scholar]
- Naik PR, Sakthivel N. Functional characterization of a novel hydrocarbonoclastic Pseudomonas sp. strain PUP6 with plant-growth-promoting traits and antifungal potential. Res Microbiol. 2006;157(6):538–546. doi: 10.1016/j.resmic.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Odukoya J, Lambert R, Sakrabani R. Understanding the impacts of crude oil and its induced abiotic stresses on agrifood production: a review. Horticulturae. 2019;5(2):47. doi: 10.3390/horticulturae5020047. [DOI] [Google Scholar]
- Ordinioha B, Brisibe S. The human health implications of crude oil spills in the Niger delta, Nigeria: an interpretation of published studies. Niger Med J J Niger Med Assoc. 2013;54(1):10–16. doi: 10.4103/0300-1652.108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Tisch U, Haick H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer. 2010;103(4):542–551. doi: 10.1038/sj.bjc.6605810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfumo A, Banat IM, Canganella F, Marchant R. Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. Appl Microbiol Biotechnol. 2006;72(1):132–138. doi: 10.1007/s00253-005-0234-0. [DOI] [PubMed] [Google Scholar]
- Pi Y, Meng L, Bao M, Sun P, Lu J. Degradation of crude oil and relationship with bacteria and enzymatic activities in laboratory testing. Int Biodeterior Biodegrad. 2016;106:106–116. [Google Scholar]
- Primeia S, Inoue C, Chien MF. potential of biosurfactants’ production on degrading heavy oil by bacterial consortia obtained from tsunami-induced oil-spilled beach areas in Miyagi, Japan. J Mar Sci Eng. 2020;8(8):577. doi: 10.3390/jmse8080577. [DOI] [Google Scholar]
- Queiroga CL, Nascimento LR, Serra GE. Evaluation of paraffins biodegradation and biosurfactant production by Bacillus subtilis in the presence of crude oil. Brazil J Microbiol. 2003;34(4):321–324. [Google Scholar]
- Ren H, Zhou S, Wang B, Peng L, Li X. Treatment mechanism of sludge containing highly viscous heavy oil using biosurfactant. Colloids Surf A Physicochem Eng Asp. 2020;585:124117. doi: 10.1016/j.colsurfa.2019.124117. [DOI] [Google Scholar]
- Riihimäki V, Savolainen K. Human exposure to m-xylene. Kinetics and acute effects on the central nervous system. Ann Occup Hyg. 1980;23(4):411–422. doi: 10.1093/annhyg/23.4.411. [DOI] [PubMed] [Google Scholar]
- Rudyk S. Relationships between SARA fractions of conventional oil, heavy oil, natural bitumen and residues. Fuel. 2018;216:330–340. doi: 10.1016/j.fuel.2017.12.001. [DOI] [Google Scholar]
- Saadoun IM. Impact of oil spills on marine life. In: Larramendy M, Soloneski S, editors. Emerging pollutants in the environment-current and further implications. Croatia: Intech; 2015. pp. 75–104. [Google Scholar]
- Saikia RR, Deka S. Removal of hydrocarbon from refinery tank bottom sludge employing microbial culture. Environ Sci Pollut Res. 2013;20(12):9026–9033. doi: 10.1007/s11356-013-1888-2. [DOI] [PubMed] [Google Scholar]
- Salihu A, Abdulkadir I, Almustapha MN. An investigation for potential development on biosurfactants. Biotechnol Mol Biol Rev. 2009;4(5):111–117. [Google Scholar]
- Shao Z, Wang W. Enzymes and genes involved in aerobic alkane degradation. Front Microbiol. 2013;4:116. doi: 10.3389/fmicb.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SL, Pant A. Biodegradation and conversion of alkanes and crude oil by a marine Rhodococcus. Biodegradation. 2000;11(5):289–294. doi: 10.1023/a:1011185806974. [DOI] [PubMed] [Google Scholar]
- She YH, Zhang F, Xia JJ, Kong SQ, Wang ZL, Shu FC, Hu JM. Investigation of biosurfactant-producing indigenous microorganisms that enhance residue oil recovery in an oil reservoir after polymer flooding. Appl Biochem Biotechnol. 2011;163(2):223–234. doi: 10.1007/s12010-010-9032-y. [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Adams WA. The fate of diesel hydrocarbons in soils and their effect on the germination of perennial ryegrass. Environ Toxicol. 2002;17(1):49–62. doi: 10.1002/tox.10032. [DOI] [PubMed] [Google Scholar]
- Sierra-Garcia IN, de Oliveira VM. Microbial hydrocarbon degradation: efforts to understand biodegradation in petroleum reservoirs. In: Chamy R, Rosenkranz F, editors. Biodegradation−engineering and technology, vol 10, p 55920. London: IntechOpen; 2013. [Google Scholar]
- Smits TH, Balada SB, Witholt B, Van Beilen JB. Functional analysis of alkane hydroxylases from gram-negative and gram-positive bacteria. J Bacteriol. 2002;184(6):1733–1742. doi: 10.1128/JB.184.6.1733-1742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urum K, Pekdemir T. Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere. 2004;57(9):1139–1150. doi: 10.1016/j.chemosphere.2004.07.048. [DOI] [PubMed] [Google Scholar]
- Viamajala S, Peyton BM, Richards LA, Petersen JN. Solubilization, solution equilibria, and biodegradation of PAH’s under thermophilic conditions. Chemosphere. 2007;66(6):1094–1106. doi: 10.1016/j.chemosphere.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Vinas M, Grifoll M, Sabaté J, Solanas AM. Biodegradation of a crude oil by three microbial consortia of different origins and metabolic capabilities. J Ind Microbiol Biotechnol. 2002;28(5):252–260. doi: 10.1038/sj/jim/7000236. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen S, Wang Z. Electrophysiological follow-up of patients with chronic peripheral neuropathy induced by occupational intoxication with n-hexane. Cell Biochem Biophys. 2014;70(1):579–585. doi: 10.1007/s12013-014-9959-7. [DOI] [PubMed] [Google Scholar]
- Wongsa P, Tanaka M, Ueno A, Hasanuzzaman M, Yumoto I, Okuyama H. Isolation and characterization of novel strains of Pseudomonas aeruginosa and Serratia marcescens possessing high efficiency to degrade gasoline, kerosene, diesel oil, and lubricating oil. Curr Microbiol. 2004;49(6):415–422. doi: 10.1007/s00284-004-4347-y. [DOI] [PubMed] [Google Scholar]
- Wyrwas B, Chrzanowski Ł, Ławniczak Ł, Szulc A, Cyplik P, Białas W, Szymański A, Hołderna-Odachowska A. Utilization of Triton X-100 and polyethylene glycols during surfactant-mediated biodegradation of diesel fuel. J Hazard Mater. 2011;197:97–103. doi: 10.1016/j.jhazmat.2011.09.060. [DOI] [PubMed] [Google Scholar]
- Yan P, Lu M, Yang Q, Zhang HL, Zhang ZZ, Chen R. Oil recovery from refinery oily sludge using a rhamnolipid biosurfactant-producing Pseudomonas. Bioresour Technol. 2012;116:24–28. doi: 10.1016/j.biortech.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Yasin G, Bhanger MI, Ansari TM, Naqvi SM, Ashraf M, Ahmad K, Talpur FN. Quality and chemistry of crude oils. J Pet Technol Altern Fuels. 2013;4(3):53–63. doi: 10.5897/JPTAF12.025. [DOI] [Google Scholar]
- Yin H, Qiang J, Jia Y, Ye J, Peng H, Qin H, Zhang N, He B. Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Process Biochem. 2009;44(3):302–308. [Google Scholar]
- Yu Y, Zhang Y, Zhao N, Guo J, Xu W, Ma M, Li X. Remediation of crude oil-polluted soil by the bacterial rhizosphere community of Suaeda Salsa revealed by 16S rRNA genes. Int J Environ Res Public Health. 2020;17(5):1471. doi: 10.3390/ijerph17051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Wu W, Zhou L, Liang J, Jiang T. Interactive effect of dissolved organic matter and phenanthrene on soil enzymatic activities. J Environ Sci. 2010;22(4):607–614. doi: 10.1016/s1001-0742(09)60139-x. [DOI] [PubMed] [Google Scholar]
- Zhang GL, Wu YT, Qian XP, Meng Q. Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ Sci Biol. 2005;6(8):725–730. doi: 10.1631/jzus.2005.B0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu Z, Luc NT, Yu Q, Liu X, Liang X. Impacts of soil petroleum contamination on nutrient release during litter decomposition of Hippophae rhamnoides. Environ Sci Proc Impact. 2016;18(3):398–405. doi: 10.1039/c5em00602c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript and its additional information files contain all of the data generated or analyzed during the study.