Abstract
Patients with type 2 diabetes mellitus (T2DM) are at increased risk of severe coronavirus disease 2019 (COVID-19) outcomes possibly because of dysregulated inflammatory responses. Glucose-regulating medications, such as glucagon-like peptide 1 receptor (GLP-1R) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and pioglitazone, are known to have anti-inflammatory effects that may improve outcomes in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In a multinational retrospective cohort study, we used the TriNetX COVID-19 Research Network of 56 large health care organizations to examine these medications in relation to the incidence of hospital admissions, respiratory complications, and mortality within 28 days after a COVID-19 diagnosis. After matching for age, sex, race, ethnicity, BMI, and significant comorbidities, use of GLP-1R agonists and/or pioglitazone was associated with significant reductions in hospital admissions (GLP-1R: 15.7% vs. 23.5%, risk ratio [RR] 0.67 [95% CI 0.57–0.79; P < 0.001]; pioglitazone: 20.0% vs. 28.2%; RR 0.71 [95% CI 0.54–0.93; P = 0.01]). Use of GLP-1R agonists was also associated with reductions in respiratory complications (15.3% vs. 24.9%, RR 0.62 [95% CI 0.52–0.73]; P < 0.001) and incidence of mortality (1.9% vs. 3.3%, RR 0.58 [95% CI 0.35–0.97]; P = 0.04). Use of DPP-4 inhibitors was associated with a reduction in respiratory complications (24.0% vs. 29.2%, RR 0.82 [95% CI 0.74–0.90]; P < 0.001), and continued use of DPP-4 inhibitors after hospitalization was associated with a decrease in mortality compared with those who discontinued use (9% vs. 19%, RR 0.45 [95% CI 0.28–0.72]; P < 0.001). In conclusion, use of glucose-regulating medications, such as GLP-1R agonists, DPP-4 inhibitors, or pioglitazone, may improve COVID-19 outcomes for patients with T2DM; randomized clinical trials are needed to further investigate this possibility.
Introduction
As of 14 April 2021, there were 137,811,552 confirmed cases and 2,964,835 (2.2%) deaths worldwide as a result of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These figures include 31,375,111 confirmed cases and 563,873 (1.8%) deaths in the U.S. alone (1). Given the time required to distribute a vaccine, an expedient solution to reduce morbidity and mortality as a result of COVID-19 is to rapidly identify therapeutics utilizing highly scalable agents that show promise. A randomized placebo-controlled trial found that remdesivir, a broad-spectrum antiviral with activity against SARS-CoV-2, reduced median recovery time from 15 to 11 days but did not reduce mortality (2,3). An open-label trial with the synthetic glucocorticoid dexamethasone suggested a reduction in mortality by one-third in patients requiring ventilator support and by one-fifth in patients requiring oxygen (4). Together, these interventions are proving helpful; however, the number of deaths as a result of COVID-19 continues to climb in the U.S. and around the world (1). Moreover, mortality for at-risk populations, such as those with type 2 diabetes mellitus (T2DM), is much higher (5–7). In China, risk of mortality was reported to be 7.2% for patients with T2DM (8), and a recent study in England found that patients with T2DM made up 31.4% of the COVID-19–related deaths (9). One hypothesis is that patients with T2DM are more susceptible to a dysregulated inflammatory response, or cytokine storm, which has been hypothesized to lead to severe outcomes in a subset of patients with COVID-19 (10,11). Additionally, recent data indicate dysregulation of glycometabolic control in patients with COVID-19 (12).
Because SARS-CoV-2 uses ACE2 as its receptor, we selected medications that are used to treat T2DM and that have been shown to increase ACE2 and have anti-inflammatory effects. The glucagon-like peptide 1 (GLP-1) agonist liraglutide has been shown to increase ACE2 expression in the lungs of rats and reduce acute lung injury caused by influenza virus in mice, which is particularly relevant to COVID-19–induced pulmonary disease (13–16). Dipeptidyl peptidase 4 (DPP-4) inhibitors may have similar effects as they increase endogenous GLP-1 levels by inhibiting the breakdown of GLP-1 by the DPP-4 enzyme. The DPP-4 inhibitor linagliptin has been shown to increase ACE2 activity and has the potential to exert anti-inflammatory effects (17). Finally, thiazolidinediones (TZDs) were selected because they have been shown to increase ACE2 levels in the aorta, liver, adipose tissue, and skeletal muscle (18,19). Because GLP-1 agonists, DPP-4 inhibitors, and TZDs increase ACE2, we hypothesized that these drugs would have protective effects in patients with COVID-19 and diabetes. Increased ACE2 leads to increased production of angiotensin-(1–7), which exhibits anti-inflammatory effects and prevents end-organ damage as a result of diabetes and may also potentially prevent end-organ damage caused by COVID-19.
Several medications used to regulate blood glucose have been hypothesized to improve COVID-19–related outcomes in patients with T2DM (20–28); however, no data currently support these hypotheses or provide rationale for a randomized trial. The objective of this multinational, retrospective cohort study was to investigate whether the use of T2DM medications is associated with improved outcomes in patients with COVID-19 and T2DM. One such class of medications includes GLP-1 receptor (GLP-1R) agonists. GLP-1 is a hormone produced by L cells in the small intestine that increases insulin release, decreases glucagon release, and decreases gastric emptying (29). GLP-1R agonists are an approved and effective treatment for obesity and T2DM (30–32) and may have a protective effect for severe outcomes of COVID-19 in patients with fatty liver disease (33). In patients with T2DM, GLP-1R agonists control blood glucose, reduce elevated glycated hemoglobin (HbA1c), and decrease both cardiovascular risk (34) and the frequency of major adverse cardiac events (35,36). GLP-1R agonists also have positive effects on body weight, BMI, blood pressure, and cholesterol (29,37). Of particular interest, GLP-1R agonists have been shown to decrease inflammatory cytokines in animals (38) and humans (37,39). Other medications for T2DM that have anti-inflammatory effects include DPP-4 inhibitors, which block the breakdown of GLP-1 and have been shown to regulate other coronaviruses (40), and pioglitazone, which is a TDZ (23,41). We hypothesized that the use of GLP-1R agonists, DPP-4 inhibitors, or pioglitazone within 6 months before the diagnosis of COVID-19 would reduce hospital admissions, respiratory complications, and mortality within 28 days after the diagnosis of COVID-19 in patients with T2DM.
Research Design and Methods
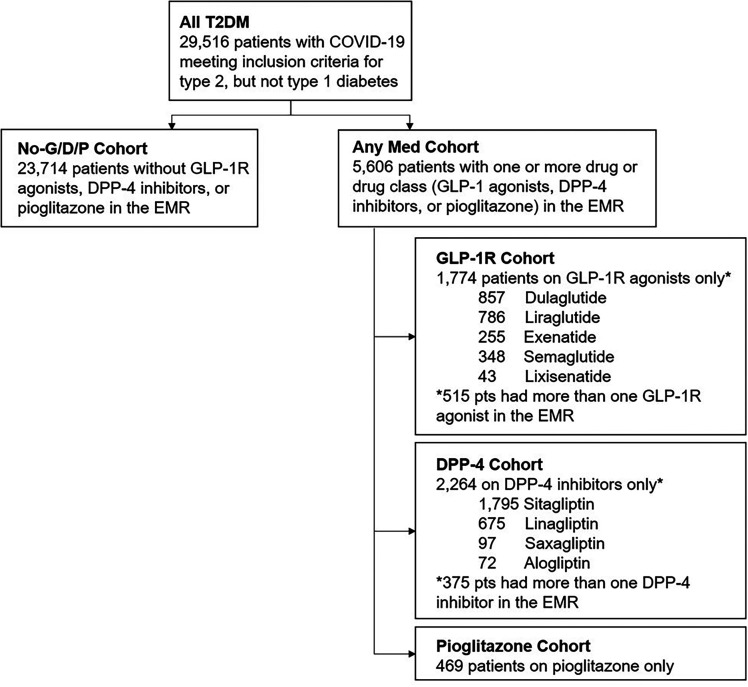
To determine whether treatment with GLP-1R agonists, DPP-4 inhibitors, or pioglitazone in patients with COVID-19 and T2DM was associated with better outcomes, we conducted a retrospective analysis of the TriNetX COVID-19 Research Network. This global federated research network provides access to statistics in electronic medical records (EMRs) across 56 large health care organizations predominately in the U.S. TriNetX provides aggregated counts and statistical summaries of deidentified information but no protected health information. Further details of the TriNetX networks have been previously described (42,43). All analyses were conducted using the browser-based real-time analytics feature of TriNetX.
We analyzed the EMRs of 64,936,797 patients with a diagnostic code for COVID-19 or positive laboratory test results for SARS-CoV-2 between 1 January 2020 and 1 September 2020. Analyses included patients with a diagnostic code for T2DM any time within 6 months preceding the first record of COVID-19 (Fig. 1). Because treatment for type 1 diabetes has some, but not total, overlap with T2DM treatment, patients with type 1 diabetes were excluded from analyses. There were 29,516 patients with T2DM included in the analyses. These patients were divided into cohorts based on medications listed in their EMR within 6 months preceding the first record of COVID-19 (see Supplementary Tables 1–3 for a comprehensive list of medications). These patient subsets included 1,774 patients treated with GLP-1R agonists only (dulaglutide, exenatide, liraglutide, semaglutide, or lixisenatide), 2,264 treated with DPP-4 inhibitors only (alogliptin, linagliptin, saxagliptin, or sitagliptin), and 469 patients treated with pioglitazone only. There were two control groups: The first consisted of 23,714 patients with T2DM who had none of the medications of interest (No-G/D/P cohort), and the second included 5,606 patients with T2DM who had more than one of the medications of interest (Any Med cohort) any time within 6 months preceding the first record of COVID-19.
Figure 1.
Study flowchart. pts, patients.
The primary outcome was mortality and defined as the presence of the term deceased in the EMR up to 28 days after the first record of COVID-19. Hospitalization and respiratory complications were secondary outcomes. Hospitalization was defined as a direct admission to inpatient care or observation in the EMR up to 28 days after the first record of COVID-19. Respiratory complications were defined as the presence of diagnostic codes for acute respiratory distress syndrome, acute and chronic respiratory failure, dependence on mechanical ventilation, acute respiratory distress, or respiratory arrest in the EMR within 28 days after the first record of COVID-19.
Risks for hospital admissions, respiratory complications, and mortality were first assessed for each patient cohort compared with the No-G/D/P cohort before propensity matching. Subsequent analyses were propensity matched for age, sex, race, ethnicity, BMI, and the presence of diagnostic codes for the following comorbidities: hypertension, ischemic heart disease, cerebrovascular disease, heart failure, chronic kidney disease, and overweight or obesity in the EMR within 6 months of the first record of COVID-19. Sex was used for propensity matching throughout these analyses and, thus, not considered a factor for comparison. Propensity scoring was calculated using logistic regression implemented by the function LogisticRegression of the scikit-learn package in Python version 3.7. The propensity score 1:1 matching used a greedy nearest neighbor matching approach, with a caliper distance of 0.1 pooled SDs of the logit of the propensity score. All laboratory values are the most recent value in the EMR within the designated time period unless otherwise stated. After propensity matching, the risks of hospital admissions, respiratory complications, and mortality were calculated for each patient cohort compared with the No-G/D/P cohort. Additionally, hospitalized patients who remained on their respective medications were compared with those whose medications were discontinued within each cohort.
Data and Resource Availability
The data that support the findings of this study are available from TriNetX, but restrictions apply to the availability of these data, which were used under license for the current study and, therefore, are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of TriNetX.
Results
Of the 229,809 patients with a diagnostic code for COVID-19 or positive laboratory result for SARS-CoV-2 present in the database, 2.2% were deceased within 28 days of the first record of COVID-19 (Table 1). Patients with a diagnosis of T2DM were more than four times as likely to be deceased (6.5%) within 28 days compared with those without a diagnosis of T2DM (1.6%). This increase in mortality was observed across all age-groups, with the greatest increase (22.8-fold) in patients <30 years of age. Group characteristics were first assessed for all patients with T2DM with COVID-19 who were alive at 28 days compared with those who were deceased at 28 days after the first record of COVID-19. Those who were deceased were older, more likely to be male, and had higher blood glucose, lower oxygen saturation, and lower blood pressure (Table 2). Additionally, patients with T2DM who were deceased within 28 days had a greater incidence of hypertension, ischemic heart disease, cerebrovascular disease, heart failure, chronic kidney disease, and overweight and obesity. Consequently, these factors were used for propensity matching in subsequent analyses (see Table 3 for baseline patient characteristics by drug cohort).
Table 1.
Cohort statistics
| Positive for SARS-CoV-2 | Deceased at 28 days* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients in database | Percent of database | n | % | n | % | |||||
| Without T2DM | 64,936,797 | 94.2 | 200,293 | 0.3 | 3,209 | 1.6 | ||||
| With T2DM | 4,022,267 | 5.8 | 29,516 | 0.7 | 1,921 | 6.5 | ||||
| Total | 68,959,064 | — | 229,809 | 0.3 | 5,130 | 2.2 | ||||
| Patients with COVID-19 without T2DM | Patients with COVID-19 with T2DM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | Deceased at 28 days, n (%) | Alive at 28 days, n | Total, n | Deceased at 28 days, n (%) | Alive at 28 days, n | Total, n | Fold increase in risk for T2DM | |||
| <30 | 44 (0.07) | 63,162 | 63,206 | 10 (1.59) | 619 | 629 | 22.8 | |||
| 30–40 | 63 (0.17) | 36,252 | 36,315 | 26 (1.49) | 1,715 | 1,741 | 8.6 | |||
| 40–50 | 128 (0.41) | 31,333 | 31,461 | 54 (1.36) | 3,931 | 3,985 | 3.3 | |||
| 50–60 | 272 (0.95) | 28,383 | 28,655 | 231 (3.38) | 6,611 | 6,842 | 3.6 | |||
| 60–70 | 525 (2.65) | 19,260 | 19,785 | 388 (5.28) | 6,954 | 7,342 | 2.0 | |||
| 70–80 | 793 (7.25) | 10,138 | 10,931 | 587 (10.77) | 4,863 | 5,450 | 1.5 | |||
| 80–90 | 959 (13.81) | 5,986 | 6,945 | 496 (17.45) | 2,346 | 2,842 | 1.3 | |||
| >90 | 425 (14.19) | 2,570 | 2,995 | 129 (18.83) | 556 | 685 | 1.3 | |||
| Total | 3,209 (1.60) | 197,084 | 200,293 | 1,921 (6.51) | 27,595 | 29,516 | 4.1 | |||
Patients with the term “deceased” in the medical record within 28 days of a diagnosis of COVID-19 or positive test results for SARS-CoV-2 were included in the deceased group, while all others were assigned to the alive group.
Table 2.
Baseline characteristics for all patients with T2DM with a diagnosis of COVID-19 or positive SARS-CoV-2 test results
| Characteristic | All T2DM (n = 29,516) | Alive at 28 days* (n = 27,595) | Deceased at 28 days (n = 1,921) | P | SMD |
|---|---|---|---|---|---|
| Age,† years, mean (SD) | 60.9 (15.0) | 60.0 (14.8) | 71.7 (12.8) | <0.001 | 0.78 |
| Sex, n (%) | |||||
| Female | 15,289 (51.8) | 14,518 (52.6) | 771 (40.1) | <0.001 | |
| Male | 14,227 (48.2) | 13,077 (47.4) | 1,149 (59.8) | <0.001 | |
| Race, n (%) | |||||
| White | 14,138 (47.9) | 13,356 (48.4) | 782 (40.7) | <0.001 | |
| Black or African American | 7,527 (25.5) | 6,014 (25.4) | 513 (26.7) | 0.14 | |
| Asian | 915 (3.1) | 840 (3.0) | 75 (3.9) | 0.16 | |
| Unknown | 5,903 (20.0) | 5,367 (19.4) | 536 (27.9) | <0.001 | |
| Ethnicity, n (%) | |||||
| Hispanic or Latino | 5,490 (18.6) | 5,338 (19.3) | 152 (7.9) | <0.001 | |
| Not Hispanic or Latino | 12,898 (43.7) | 12,198 (44.2) | 700 (36.4) | <0.001 | |
| Unknown | 11,128 (37.7) | 10,059 (36.5) | 1,069 (55.6) | <0.001 | |
| Vitals and key laboratory findings,‡ mean (SD) | |||||
| BMI, kg/m2 | 32.8 (8.9) | 32.7 (8.8) | 30.8 (8.7) | <0.001 | 0.21 |
| Glucose, mg/dL | 155.0 (78.6) | 155.0 (77.1) | 163.0 (85.1) | <0.001 | 0.10 |
| HbA1c | 0.65 | 0.05 | |||
| % | 7.7 (2.1) | 7.7 (2.1) | 7.8 (2.0) | ||
| mmol/mol | 61 (23) | 61 (23) | 62 (21.9) | ||
| Oxygen saturation, % | 81.9 (22.3) | 83.1 (20.9) | 79.4 (21.9) | <0.001 | 0.17 |
| Blood pressure, mmHg | |||||
| Systolic | 128.0 (21.5) | 130.0 (21.0) | 109.0 (31.4) | <0.001 | 0.98 |
| Diastolic | 73.3 (13.4) | 74.4 (12.8) | 57.7 (18.1) | <0.001 | 1.25 |
| Comorbidities,§ n (%) | |||||
| Essential (primary) hypertension | 14,079 (47.7) | 12,901 (46.8) | 1,178 (61.3) | <0.001 | |
| Overweight and obesity | 5,726 (19.4) | 5,190 (18.8) | 536 (27.9) | <0.001 | |
| Ischemic heart disease | 4,457 (15.1) | 3,708 (13.4) | 749 (39.0) | <0.001 | |
| Heart failure | 3,512 (11.9) | 2,868 (10.3) | 644 (33.5) | <0.001 | |
| Cerebrovascular disease | 1,800 (6.1) | 1,485 (5.4) | 315 (16.4) | <0.001 | |
| Chronic kidney disease | 4,575 (15.5) | 3,799 (13.8) | 776 (40.4) | <0.001 |
P values indicate significance between alive and deceased groups. SMD, standardized mean difference between alive and deceased groups.
Patients with the term “deceased” in the medical record within 28 days of a diagnosis of COVID-19 or positive test results for SARS-CoV-2 were included in the deceased group, while all others were assigned to the alive group.
Age is defined as the age of the patient at the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
Vitals and key laboratory findings are the most recent value recorded in the EMR within 6 months up to the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
Comorbidities are assessed as presence of a diagnostic code for the six major comorbidities within the EMR within 6 months up to the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
Table 3.
Baseline characteristics for patients with T2DM with a COVID-19 diagnosis or positive SARS-CoV-2 test results by medication cohort
| Characteristic | No-G/D/P* (n = 23,714) |
GLP-1 (n = 1,774) |
P | SMD | DPP-4 (n = 2,264) |
P | SMD | Pioglitazone (n = 469) |
P | SMD | Any Med† (n = 5,606) |
P | SMD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age,‡ years, mean (SD) | 60.9 (15.3) | 55.0 (12.7) | <0.001 | 0.39 | 64.6 (13.5) | <0.001 | 0.25 | 63.1 (13.5) | 0.003 | 0.15 | 60.2 (13.5) | 0.006 | 0.05 |
| Sex, n (%) | |||||||||||||
| Female | 12,142 (51.2) | 1,079 (60.8) | < 0.001 | 1,152 (50.9) | 0.80 | 219 (46.7) | 0.19 | 3,066 (54.7) | <0.001 | ||||
| Male | 11,572 (48.8) | 695 (39.2) | <0.001 | 1,112 (49.1) | 0.79 | 250 (53.3) | 0.19 | 2,540 (45.3) | <0.001 | ||||
| Race, n (%) | |||||||||||||
| White | 11,792 (49.7) | 609 (52.3) | 0.46 | 1,114 (49.2) | 0.64 | 178 (52.4) | 0.32 | 2,790 (50.5) | 0.18 | ||||
| Black or African American | 5,874 (24.8) | 334 (28.7) | <0.001 | 603 (26.6) | 0.05 | 79 (23.2) | 0.06 | 1,669 (30.2) | <0.001 | ||||
| Asian | 783 (3.3) | 10 (0.9) | <0.001 | 116 (5.1) | < 0.001 | 12 (3.5) | 0.85 | 170 (3.1) | 0.36 | ||||
| Unknown | 4,808 (20.3) | 200 (17.2) | <0.001 | 409 (18.1) | 0.01 | 63 (18.5) | 0.005 | 830 (15.0) | <0.001 | ||||
| Ethnicity, n (%) | |||||||||||||
| Hispanic or Latino | 4,755 (20.1) | 239 (20.5) | 0.17 | 354 (15.6) | < 0.001 | 85 (25.0) | 0.72 | 929 (16.8) | <0.001 | ||||
| Not Hispanic or Latino | 10,407 (43.9) | 659 (56.6) | <0.001 | 1,076 (47.5) | < 0.001 | 172 (50.6) | <0.001 | 2,679 (48.5) | <0.001 | ||||
| Unknown | 8,552 (36.1) | 266 (22.9) | <0.001 | 834 (36.8) | 0.46 | 83 (24.4) | <0.001 | 1,912 (34.6) | 0.01 | ||||
| Vitals and key laboratory findings,§ mean (SD) | |||||||||||||
| BMI, kg/m2 | 32.3 (8.7) | 37.5 (9.3) | <0.001 | 0.58 | 31.4 (8.1) | 0.02 | 0.10 | 31.9 (8.0) | 0.82 | 0.04 | 34.3 (9.0) | <0.001 | 0.22 |
| Glucose, mg/dL | 152.0 (77.5) | 168.0 (81.4) | <0.001 | 0.20 | 167.0 (80.2) | <0.001 | 0.19 | 159.0 (79.9) | 0.40 | 0.09 | 168.0 (81.2) | <0.001 | 0.20 |
| HbA1c | <0.001 | 0.43 | <0.001 | 0.24 | 0.16 | 0.14 | <0.001 | 0.33 | |||||
| % | 7.5 (2.1) | 8.4 (2.2) | 8.0 (2.0) | 7.8 (2.0) | 8.2 (2.0) | ||||||||
| mmol/mol | 58 (23) | 68 (24) | 64 (21.9) | 62 (21.9) | 66 (21.9) | ||||||||
| Blood pressure, mmHg | |||||||||||||
| Systolic | 129.0 (22.0) | 129.0 (19.7) | 0.03 | 0.00 | 130.0 (21.3) | 0.06 | 0.05 | 128.0 (23.0) | 0.72 | 0.05 | 130.0 (20.4) | 0.03 | 0.05 |
| Diastolic | 73.3 (13.4) | 75.8 (12.8) | <0.001 | 0.19 | 71.7 (13.5) | <0.001 | 0.12 | 71.8 (14.6) | 0.19 | 0.11 | 74.1 (13.1) | 0.009 | 0.06 |
| Comorbidities,¶ n (%) | |||||||||||||
| Essential (primary) hypertension | 10,648 (44.9) | 651 (55.9) | <0.001 | 1,266 (55.9) | <0.001 | 177 (52.1) | 0.003 | 3,166 (57.4) | <0.001 | ||||
| Overweight and obesity | 4,161 (17.5) | 416 (35.7) | <0.001 | 416 (18.4) | 0.32 | 71 (20.9) | 0.13 | 1,444 (26.2) | <0.001 | ||||
| Ischemic heart disease | 3,521 (14.9) | 161 (13.8) | 0.17 | 451 (19.9) | <0.001 | 51 (15.0) | 0.99 | 881 (16.0) | 0.04 | ||||
| Heart failure | 2,786 (11.7) | 99 (8.5) | < 0.001 | 344 (15.2) | <0.001 | 32 (9.4) | 0.18 | 613 (11.1) | 0.18 | ||||
| Cerebrovascular disease | 1,467 (6.2) | 37 (3.2) | < 0.001 | 177 (7.8) | 0.002 | 22 (6.5) | 0.83 | 307 (5.6) | 0.11 | ||||
| Chronic kidney disease | 3,528 (14.9) | 150 (12.9) | 0.06 | 508 (22.4) | <0.001 | 59 (17.4) | 0.22 | 941 (17.0) | <0.001 |
P values indicate significance compared with the No-G/D/P cohort. SMD, standardized mean difference between drug cohorts and the No-G/D/P cohort.
*The No-G/D/P cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who did not have any GLP-1R agonists, DPP-4 inhibitors, or pioglitazone in the EMR.
†The Any Med cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who had GLP-1R agonists, DPP-4 inhibitors, and/or pioglitazone in the EMR.
‡Age is defined as the age of the patient at the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
§Vitals and key laboratory findings are the most recent values recorded in the EMR within 6 months up to the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
¶Comorbidities are the presence of a diagnostic code for the six major comorbidities within the EMR within 6 months up to the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
Hospital Admissions
Collectively, 27.3% of all patients with T2DM with COVID-19 were admitted to the hospital within 28 days of the first record of COVID-19 (Table 4). Among patients with T2DM in the No-G/D/P cohort, 28.4% were admitted, reflecting a 4.3% relative increase in incidence of hospital admissions compared with all patients with T2DM (risk ratio [RR] 1.04 [95% CI 1.01–1.07]; P = 0.004). Compared with the No-G/D/P cohort, there was a 47.1% relative reduction in hospitalizations for the GLP-1R cohort (15.1% vs. 28.4%, RR 0.53 [95% CI 0.47–0.59]; P < 0.001), 19.0% relative reduction for the pioglitazone cohort (23.0% vs. 28.4%, RR 0.81 [95% CI 0.69–0.96]; P = 0.01), and 21.0% relative reduction for the Any Med cohort (22.5% vs. 28.4%, RR 0.79 [95% CI 0.75–0.83]; P < 0.001). These cohorts maintained significant reductions in hospitalizations after propensity matching, with the GLP-1R cohort showing a 33.0% relative decrease (15.7% vs. 23.5%, RR 0.67 [95% CI 0.57–0.79]; P < 0.001) followed by the pioglitazone cohort, which had a 29.2% relative decrease (20.0% vs. 28.2%, RR 0.71 [95% CI 0.54–0.93]; P = 0.01), and the Any Med cohort, which had a 16.2% relative decrease in hospitalizations (22.3% vs. 26.6%, RR 0.84 [95% CI 0.78–0.90]; P < 0.001). Patients in the DPP-4 cohort did not differ from the No-G/D/P cohort before propensity matching (29.5% vs. 28.4%, RR 1.04 [95% CI 0.97–1.11]; P = 0.30) or after propensity matching (29.5% vs. 30.2%, RR 0.98 [95% CI 0.89–1.07]; P = 0.63).
Table 4.
Risk of adverse outcomes within 28 days after COVID-19 diagnosis or positive SARS-CoV-2 test results
| Before propensity matching | After propensity matching* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | RR (95% CI) | P | Relative change, % | n after matching | n (%) | RR (95% CI) | P | Relative change, % | |
| Hospital admissions | |||||||||
| No-G/D/P† (n = 23,714) | 6,741 (28.4) | 1.04 (1.01–1.07) | 0.004 | 4.3 | |||||
| All T2DM (n = 29,516) | 8,046 (27.3) | ||||||||
| GLP-1 (n = 1,774) | 267 (15.1) | 0.53 (0.47–0.59) | <0.001 | −47.1 | 1,163 | 183 (15.7) | 0.67 (0.57–0.79) | <0.001 | −33.0 |
| No-G/D/P† (n = 23,714) | 6,741 (28.4) | 1,163 | 273 (23.5) | ||||||
| DPP-4 (n = 2,264) | 667 (29.5) | 1.04 (0.97–1.11) | 0.30 | 3.6 | 2,264 | 667 (29.5) | 0.98 (0.89–1.07) | 0.63 | −2.5 |
| No-G/D/P† (n = 23,714) | 6,741 (28.4) | 2,264 | 684 (30.2) | ||||||
| Pioglitazone (n = 469) | 108 (23.0) | 0.81 (0.69–0.96) | 0.01 | −19.0 | 340 | 68 (20.0) | 0.71 (0.54–0.93) | 0.01 | −29.2 |
| No-G/D/P† (n = 23,714) | 6,741 (28.4) | 340 | 96 (28.2) | ||||||
| Any Med‡ (n = 5,606) | 1,259 (22.5) | 0.79 (0.75–0.83) | <0.001 | −21.0 | 5,520 | 1,229 (22.3) | 0.84 (0.78–0.90) | <0.001 | −16.2 |
| No-G/D/P† (n = 23,714) | 6,741 (28.4) | 5,520 | 1,467 (26.6) | ||||||
| Respiratory complications | |||||||||
| No-G/D/P† (n = 23,714) | 6,432 (27.1) | 1.06 (1.03–1.09) | <0.001 | 5.7 | |||||
| All T2DM (n = 29,516) | 7,577 (25.7) | ||||||||
| GLP-1 (n = 1,774) | 269 (15.2) | 0.56 (0.50–0.63) | <0.001 | −44.1 | 1,163 | 178 (15.3) | 0.62 (0.52–0.73) | <0.001 | −38.4 |
| No-G/D/P† (n = 23,714) | 6,432 (27.1) | 1,163 | 289 (24.9) | ||||||
| DPP-4 (n = 2,264) | 543 (24.0) | 0.88 (0.82–0.95) | 0.001 | −11.6 | 2,264 | 543 (24.0) | 0.82 (0.74–0.90) | <0.001 | −18.0 |
| No-G/D/P† (n = 23,714) | 6,432 (27.1) | 2,264 | 662 (29.2) | ||||||
| Pioglitazone (n = 469) | 112 (23.0) | 0.88 (0.75–1.04) | 0.12 | −12.0 | 340 | 85 (25.0) | 0.89 (0.70–1.14) | 0.34 | −11.5 |
| No-G/D/P† (n = 23,714) | 6,432 (27.1) | 340 | 96 (28.2) | ||||||
| Any Med‡ (n = 5,606) | 1,101 (19.6) | 0.72 (0.68–0.77) | <0.001 | −27.6 | 5,520 | 1,083 (19.6) | 0.73 (0.68–0.78) | <0.001 | −27.2 |
| No-G/D/P† (n = 23,714) | 6,432 (27.1) | 5,520 | 1,488 (27.0) | ||||||
| Mortality | |||||||||
| No-G/D/P† (n = 23,714) | 1,443 (6.1) | 1.04 (0.98–1.12) | 0.21 | −6.5 | |||||
| All T2DM (n = 29,516) | 1,921 (6.5) | ||||||||
| GLP-1 (n = 1,774) | 44 (2.5) | 0.41 (0.30–0.55) | <0.001 | −59.2 | 1,163 | 22 (1.9) | 0.58 (0.35–0.97) | 0.04 | −42.1 |
| No-G/D/P† (n = 23,714) | 1,443 (6.1) | 1,163 | 38 (3.3) | ||||||
| DPP-4 (n = 2,264) | 169 (7.5) | 1.23 (1.05–1.43) | 0.009 | 22.7 | 2,264 | 169 (7.5) | 1.03 (0.84–1.26) | 0.78 | 3.1 |
| No-G/D/P† (n = 23,714) | 1,443 (6.1) | 2,264 | 164 (7.2) | ||||||
| Pioglitazone (n = 469) | 21 (4.5) | 0.74 (0.48–1.12) | 0.15 | −26.4 | 340 | 17 (5.0) | 1.06 (0.55–2.07) | 0.86 | 6.3 |
| No-G/D/P† (n = 23,714) | 1,443 (6.1) | 340 | 16 (4.7) | ||||||
| Any Med‡ (n = 5,606) | 266 (4.7) | 0.78 (0.69–0.89) | <0.001 | −22.0 | 5,520 | 254 (4.6) | 0.85 (0.72–1.00) | 0.05 | −15.1 |
| No-G/D/P† (n = 23,714) | 1,443 (6.1) | 5,520 | 299 (5.4) | ||||||
P values indicate significance compared with the No-G/D/P cohort.
Propensity matching balanced cohorts according to age, sex, race, ethnicity, BMI, and the presence of essential (primary) hypertension, ischemic heart disease, cerebrovascular disease, heart failure, chronic kidney disease, or overweight and obesity in the EMR within 6 months before COVID-19 diagnosis.
The No-G/D/P cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who did not have any GLP-1R agonists, DPP-4 inhibitors, or pioglitazone in the EMR.
The Any Med cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who had GLP-1R agonists, DPP-4 inhibitors, and/or pioglitazone in the EMR.
Incidence of Respiratory Complications
Of all patients with T2DM, 25.7% experienced respiratory complications within 28 days of the first record of COVID-19 (Table 4). Patients in the No-G/D/P cohort had a 5.7% relative increase in incidence compared with all patients with T2DM (27.1% vs. 25.7%, RR 1.06 [95% CI 1.03–1.09]; P < 0.001). Compared with the No-G/D/P cohort, there was a 44.1% relative decrease in respiratory complications for the GLP-1R cohort (15.2% vs. 27.1%, RR 0.56 [95% CI 0.50–0.63]; P < 0.001), 11.6% relative decrease for the DPP-4 cohort (24.0% vs. 27.1%, RR 0.88 [95% CI 0.82–0.95]; P = 0.001), and 27.6% relative decrease for the Any Med cohort (19.6% vs. 27.1%, RR 0.72 [95% CI 0.68–0.77]; P < 0.001). These cohorts maintained significant reductions in respiratory complications after propensity matching. The GLP-1R cohort had a 38.4% relative decrease (15.3% vs. 24.9%, RR 0.62 [95% CI 0.52–0.73]; P < 0.001), the DPP-4 cohort had a 18.0% relative decrease (24.0% vs. 29.2%, RR 0.82 [95% CI 0.74–0.90]; P < 0.001), and the Any Med cohort had a 27.2% relative decrease (19.6% vs. 27.0%, RR 0.73 [95% CI 0.68–0.78]; P < 0.001) in respiratory complications. Patients in the pioglitazone cohort did not differ from the No-G/D/P cohort before propensity matching (23.0% vs. 27.1%, RR 0.88 [95% CI 0.75–1.04]; P = 0.12) or after propensity matching (25.0% vs. 28.2%, RR 0.89 [95% CI 0.70–1.14]; P = 0.34).
Mortality
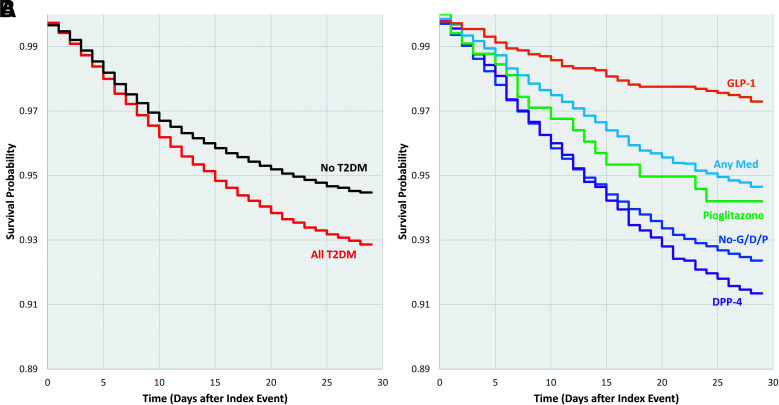
Among all patients with T2DM and COVID-19, 6.5% died within 28 days of the first record of COVID-19 (Fig. 2 and Table 4). Patients with T2DM in the No-G/D/P cohort did not differ significantly from all patients with T2DM (6.1% vs. 6.5%) in risk for mortality. Compared with the No-G/D/P cohort, patients with T2DM on GLP-1R agonists had a 59.2% relative decrease in risk of mortality (2.5% vs. 6.1%, RR 0.41 [95% CI 0.30–0.55]; P < 0.001), and those in the Any Med cohort had a 22.0% relative decrease in risk for mortality (4.7% vs. 6.1%, RR 0.78 [95% CI 0.69–0.89]; P = 0.001). After propensity matching, both cohorts had a significant relative reduction in risk for mortality, with statistically significant reductions of 42.1% for the GLP-1R (1.9% vs. 3.3%, RR 0.58 [95% CI 0.35–0.97]; P = 0.04) and 15.1% for the Any Med (4.6% vs. 5.4%, RR 0.85 [95% CI 0.72–1.00]; P = 0.05) cohorts. The DPP-4 cohort had a significant 22.7% relative increase in mortality risk (7.5% vs. 6.1%, RR 1.23 [95% CI 1.05–1.43]; P = 0.009); however, this was not significant after propensity matching (7.5% vs. 7.2%, RR 1.03 [95% CI 0.84–1.26]; P = 0.78). Patients in the pioglitazone cohort did not differ from those in the No-G/D/P cohort before propensity matching (4.5% vs. 6.1%, RR 0.74 [95% CI 0.48–1.12]; P = 0.15) or after propensity matching (5.0% vs. 4.7%, RR 1.06 [95% CI 0.55–2.07]; P = 0.86).
Figure 2.
Survival probability by cohort up to 28 days after the first record of COVID-19 (diagnosis code for COVID-19 or positive test results for SARS-CoV-2). A: All patients without T2DM (n = 200,293) and all patients with T2DM (n = 29,516). B: Patients in the drug comparison cohorts (No-G/D/P, n = 23,714; GLP-1R agonists, n = 1,774; DPP-4 inhibitors, n = 2,264; pioglitazone, n = 469; Any Med, n = 5,606).
Continuation of Medication After Hospital Admission
In a follow-up analysis, hospitalized patients within each cohort who remained on their respective medications were compared with those whose medications were discontinued (Table 5). Of those who were hospitalized, 35.2% (94 of 267) in the GLP-1 cohort, 39.6% (264 of 667) in the DPP-4 cohort, 39.8% (43 of 108) in the pioglitazone cohort, and 38.6% (486 of 1,259) of the Any Med cohort remained on their respective medications (see Supplementary Table 4 for baseline patient characteristics and Supplementary Tables 4 and 5 for laboratory values at admission and during hospitalization). There were no differences in the incidence of respiratory complications for patients who continued compared with those who discontinued GLP-1R agonists or pioglitazone both before (GLP-1R: 39% vs. 44%, RR 0.87 [95% CI 0.65–1.18; P = 0.37]; pioglitazone: 36% vs. 51%, RR 0.70 [95% CI 0.44–1.11; P = 0.11]) and after (GLP-1R: 46% vs. 54%, RR 0.86 [95% CI 0.61–0.1.21; P = 0.39]; pioglitazone: 43% vs. 47%, RR 0.93 [95% CI 0.53–1.63; P = 0.80]) propensity matching (Table 6). Hospitalized patients in the DPP-4 cohort and Any Med cohort who remained on their medications had a 59% and 52% relative decrease in the incidence of mortality compared with patients who discontinued their medication (DPP-4: 9% vs. 21%, RR 0.42 [95% CI 0.27–0.64; P < 0.001]; Any Med: 8% vs. 16%, RR 0.48 [95% CI 0.34–0.69; P < 0.001]). This maintained significance after propensity matching, with the DPP-4 cohort having a 55% relative reduction (9% vs. 19%, RR 0.45 [95% CI 0.28–0.72]; P < 0.001) and the Any Med cohort having a 49% reduction in mortality (8% vs. 15%, RR 0.51 [95% CI 0.35–0.73]; P < 0.001). Differences in mortality as a function of the continuation of medication could not be compared within the GLP-1R or pioglitazone cohorts following propensity matching because of small group size.
Table 5.
Baseline characteristics of hospitalized patients who continued or discontinued specified medications
| Before propensity matching | After propensity matching* | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic and cohort | Continued† | Discontinued | P | SMD | Continued† | Discontinued | P | SMD |
| Age,‡ years, mean (SD) | ||||||||
| No-G/D/P§ | 64.9 (14.8) | — | — | — | — | — | ||
| GLP-1 | 58.3 (13.1) | 57.5 (13.5) | 0.70 | 0.05 | 59.2 (13.6) | 57.6 (12.8) | 0.49 | 0.11 |
| DPP-4 | 66.4 (13.0) | 68.5 (12.7) | 0.03 | 0.14 | 67.1 (12.7) | 66.4 (12.4) | 0.50 | 0.05 |
| Pioglitazone | 65.3 (12.7) | 69.5 (11.6) | 0.08 | 0.28 | 67.2 (12.4) | 66.4 (12.7) | 0.81 | 0.05 |
| Any Med‖‖ | 64.1 (13.3) | 65.3 (13.6) | 0.15 | 0.08 | 64.3 (13.1) | 64.7 (13.4) | 0.62 | 0.03 |
| Male sex, n/N (%) | ||||||||
| No-G/D/P§ | 3,661/6,742 (54) | — | — | — | — | — | ||
| GLP-1 | 40/94 (43) | 76/173 (44) | 0.95 | 31/67 (46) | 29/67 (43) | 0.73 | ||
| DPP-4 | 143/264 (54) | 212/403 (53) | 0.65 | 133/253 (53) | 138/253 (55) | 0.66 | ||
| Pioglitazone | 24/43 (56) | 32/65 (49) | 0.45 | 13/30 (43) | 16/30 (53) | 0.44 | ||
| Any Med‖‖ | 254/486 (52) | 378/773 (49) | 0.25 | 249/482 (52) | 237/482 (49) | 0.41 | ||
| BMI, kg/m2, mean (SD) | ||||||||
| No-G/D/P§ | 30.8 (8.5) | — | — | — | — | — | ||
| GLP-1 | 30.9 (6.2) | 40.2 (11.3) | 0.009 | 1.04 | 31.4 (6.6) | 39.4 (13.6) | 0.11 | 0.90 |
| DPP-4 | 29.1 (5.9) | 30.6 (8.1) | 0.14 | 0.17 | 29.1 (5.9) | 29.2 (7.9) | 0.92 | 0.01 |
| Pioglitazone | 33.1 (11.1) | 30.0 (6.9) | 0.29 | 0.35 | 32.3 (12.8) | 32.4 (9.2) | 0.97 | 0.01 |
| Any Med‖‖ | 31.5 (7.4) | 32.8 (9.1) | 0.12 | 0.15 | 31.5 (7.4) | 31.7 (9.7) | 0.85 | 0.02 |
| Comorbidities,¶ n/N (%) | ||||||||
| Essential (primary) hypertension | ||||||||
| No-G/D/P§ | 3,486/6,741 (52) | — | — | — | — | — | ||
| GLP-1 | 55/94 (59) | 109/173 (63) | 0.58 | 38/67 (57) | 44/67 (66) | 0.29 | ||
| DPP-4 | 163/264 (62) | 250/403 (62) | 0.88 | 156/253 (62) | 163/253 (64) | 0.52 | ||
| Pioglitazone | 27/43 (63) | 39/65 (60) | 0.77 | 19/30 (63) | 18/30 (60) | 0.79 | ||
| Any Med‖‖ | 303/486 (62) | 478/773 (62) | 0.89 | 300/482 (62) | 304/482 (63) | 0.79 | ||
| Overweight and obesity | ||||||||
| No-G/D/P§ | 1,793/6,741 (27) | — | — | — | — | — | ||
| GLP-1 | 32/94 (34) | 86/173 (50) | 0.02 | 26/67 (39) | 25/67 (37) | 0.86 | ||
| DPP-4 | 73/264 (28) | 101/403 (25) | 0.46 | 65/253 (26) | 60/253 (24) | 0.61 | ||
| Pioglitazone | 18/43 (42) | 24/65 (37) | 0.59 | 12/30 (40) | 13/30 (43) | 0.79 | ||
| Any Med‖‖ | 152/486 (31) | 264/773 (34) | 0.28 | 151/482 (31) | 152/482 (32) | 0.95 | ||
| Ischemic heart disease | ||||||||
| No-G/D/P§ | 1,598/6,741 (24) | — | — | — | — | — | ||
| GLP-1 | 26/94 (28) | 38/173 (22) | 0.29 | 16/67 (24) | 14/67 (21) | 0.68 | ||
| DPP-4 | 75/264 (28) | 129/403 (32) | 0.31 | 71/253 (28) | 71/253 (28) | >0.99 | ||
| Pioglitazone | <10/43 (—) | 22/65 (34) | — | <10/30 (—) | <10/30 (—) | — | ||
| Any Med‖‖ | 127/486 (26) | 226/773 (29) | 0.22 | 127/482 (26) | 120/482 (25) | 0.56 | ||
| Heart failure | ||||||||
| No-G/D/P§ | 1,598/6,741 (19) | — | — | — | — | — | ||
| GLP-1 | 17/94 (18) | 31/173 (18) | 0.94 | 12/67 (18) | 12/67 (18) | >0.99 | ||
| DPP-4 | 60/264 (23) | 111/403 (28) | 0.13 | 59/253 (23) | 56/253 (22) | 0.75 | ||
| Pioglitazone | <10/43 (-) | <10/65 (—) | — | <10/30 (—) | <10/30 (—) | — | ||
| Any Med‖‖ | 93/486 (19) | 179/773 (23) | 0.09 | 93/482 (19) | 94/482 (20) | 0.94 | ||
| Cerebrovascular disease | ||||||||
| No-G/D/P§ | 593/6,741 (9) | — | — | — | — | — | ||
| GLP-1 | <10/94 (—) | <10/173 (—) | — | <10/67 (-) | <10/67 (—) | — | ||
| DPP-4 | 26/264 (10) | 51/403 (13) | 0.25 | 25/253 (10) | 30/253 (12) | 0.56 | ||
| Pioglitazone | <10/43 (—) | <10/65 (—) | — | <10/30 (-) | <10/30 (—) | — | ||
| Any Med‖‖ | 45/486 (9) | 82/773 (11) | 0.48 | 44/482 (9) | 48/482 (10) | 0.67 | ||
| Chronic kidney disease | ||||||||
| No-G/D/P§ | 1,598/6,741 (25) | — | — | — | — | — | ||
| GLP-1 | 18/94 (19) | 41/173 (24) | 0.45 | 14/67 (21) | 18/67 (27) | 0.42 | ||
| DPP-4 | 75/264 (28) | 154/403 (38) | 0.008 | 78/253 (31) | 85/253 (34) | 0.51 | ||
| Pioglitazone | <10/43 (—) | 16/65 (25) | — | <10/30 (—) | <10/30 (—) | — | ||
| Any Med‖‖ | 118/486 (24) | 244/773 (32) | 0.005 | 119/482 (25) | 114/482 (24) | 0.72 | ||
P values indicate significance between continued and discontinued groups. SMD, standardized mean difference between continued and discontinued groups.
Propensity matching balanced cohorts according to age, sex, race, ethnicity, BMI, and the presence of essential (primary) hypertension, ischemic heart disease, cerebrovascular disease, heart failure, chronic kidney disease, or overweight and obesity in the EMR within 6 months before COVID-19 diagnosis.
Patients with the specified medication listed in the EMR after hospitalization within 28 days of a diagnosis of COVID-19 or positive test results for SARS-CoV-2 were included in the continued group, while those without the specified medications were assigned to the discontinued group.
Age is defined as the age of the patient at the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
The No-G/D/P cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who did not have any GLP-1R agonists, DPP-4 inhibitors, or pioglitazone in the EMR. Values for the No-G/D/P group represent all hospitalized patients in this cohort.
The Any Med cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who had GLP-1R agonists, DPP-4 inhibitors, and/or pioglitazone in the EMR.
Comorbidities are assessed as presence of a diagnostic code for the six major comorbidities within the EMR within 6 months up to the time of diagnosis of COVID-19 or positive test results for SARS-CoV-2.
Table 6.
Risk of adverse outcomes within 28 days after COVID-19 diagnosis or positive SARS-CoV-2 test results among hospitalized patients who continued or discontinued specified medications after hospitalization
| Before propensity matching | After propensity matching* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Exposure | n/N (%) | RR (95% CI) | P | Relative change, % | n/N (%) | RR (95% CI) | P | Relative change, % |
| Respiratory complications | |||||||||
| GLP-1 | Continued† | 36/93 (39) | 0.87 (0.65–1.18) | 0.37 | −13 | 31/67 (46) | 0.86 (0.61–1.21) | 0.39 | −14 |
| Discontinued | 76/171 (44) | 36/67 (54) | |||||||
| DPP-4 | Continued† | 78/263 (30) | 0.69 (0.56–0.85) | <0.001 | −31 | 77/253 (30) | 0.67 (0.53–0.84) | <0.001 | −33 |
| Discontinued | 173/401 (43) | 116/253 (46) | |||||||
| Pioglitazone | Continued† | 15/42 (36) | 0.70 (0.44–1.11) | 0.11 | −30 | 13/30 (43) | 0.93 (0.53–1.63) | 0.80 | −7 |
| Discontinued | 32/63 (51) | 14/30 (47) | |||||||
| Any Med‡ | Continued† | 149/487 (31) | 0.69 (0.59–0.80) | <0.001 | −31 | 146/482 (30) | 0.66 (0.56–0.78) | <0.001 | −34 |
| Discontinued | 342/771 (44) | 221/482 (46) | |||||||
| Mortality | |||||||||
| GLP-1 | Continued† | <10/93 (—) | — | — | — | <10/67 (—) | — | — | — |
| Discontinued | 14/167 (8) | <10/67 (—) | |||||||
| DPP-4 | Continued† | 23/263 (9) | 0.42 (0.27–0.64) | <0.001 | −59 | 22/253 (9) | 0.45 (0.28–0.72) | <0.001 | −55 |
| Discontinued | 84/401 (21) | 49/253 (19) | |||||||
| Pioglitazone | Continued† | <10/42 (—) | — | — | — | <10/30 (—) | — | — | — |
| Discontinued | 11/63 (17) | <10/30 (—) | |||||||
| Any Med‡ | Continued† | 37/487 (8) | 0.48 (0.34–0.69) | <0.001 | −52 | 37/487 (8) | 0.51 (0.35–0.73) | <0.001 | −49 |
| Discontinued | 120/771 (16) | 73/487 (15) | |||||||
P values indicate significance between continued and discontinued groups.
Propensity matching balanced cohorts according to age, sex, race, ethnicity, BMI, and the presence of essential (primary) hypertension, ischemic heart disease, cerebrovascular disease, heart failure, chronic kidney disease, or overweight and obesity in the EMR within 6 months before COVID-19 diagnosis.
Patients with the specified medication listed in the EMR after hospitalization within 28 days of a diagnosis of COVID-19 or positive test results for SARS-CoV-2 were included in the continued group, while those without the specified medications were assigned to the discontinued group.
The Any Med cohort consisted of patients with T2DM with a diagnosis of COVID-19 or positive test results for SARS-CoV-2 who had GLP-1R agonists, DPP-4 inhibitors, and/or pioglitazone in the EMR.
Discussion
Of the 68,959,064 patients in the database, 1.6% of patients without T2DM and 6.5% of patients with T2DM died within 28 days after the first record of COVID-19. This reflects a fourfold increase in mortality for patients positive for COVID-19 with a comorbid condition of T2DM. Similar data were reported in a large study conducted in England, where 31.4% of all in-hospital COVID-19 deaths occurred in patients diagnosed with T2DM (9). In the current study, risk for mortality varied across the life span and was increased in each decade in patients with a comorbid diagnosis of T2DM. For those aged ≥30 years, a diagnosis of T2DM was associated with a 2.9-fold relative increase in risk for mortality. This finding is consistent with the twofold increase in mortality reported for adult (mean age 52.6 ± 17.4 years) patients positive for COVID-19 with T2DM (44). For patients <30 years of age, we found a stunning 22.8-fold relative increase in mortality for those with T2DM. With the incidence of diabetes (in particular T2DM) increasing for younger adults around the globe (45), this vulnerable population is at far greater risk of mortality. Finally, the incidence of mortality was found to peak in the ninth decade of life at 14.2% for patients without T2DM and 18.8% for patients with T2DM. This observation also is consistent with the literature showing severity of disease and death to be markedly increased in elderly patients, particularly in those with a comorbid diagnosis of T2DM (46,47).
This study is the first to provide evidence that patients with T2DM treated with GLP-1R agonists, and to a lesser extent pioglitazone and DPP-4 inhibitors, within the 6 months preceding the diagnosis of COVID-19 demonstrated better outcomes than patients not treated with these medications. Matched patients receiving GLP-1R agonists exhibited a relative reduction of 33.0% for hospital admissions, 38.4% for respiratory complications, and 42.1% for mortality within 28 days following the first record of COVID-19. Interestingly, when matched to the GLP-1R agonist–treated cohort, selected patients in the No-G/D/P cohort appeared slightly more resilient than those matched to the DPP-4, pioglitazone, or Any Med cohorts with lower admission rates, fewer respiratory complications, and reduced mortality. Patients on DPP-4 inhibitors had an 18.0% relative reduction in respiratory complications but did not have significant reductions in hospital admissions or mortality. Patients treated with pioglitazone showed a relative reduction of 29.2% for hospital admissions but did not have significant reductions in respiratory complications or mortality. Combined, patients in the Any Med cohort exhibited a relative reduction of 16.2% for hospital admissions, 27.2% for respiratory complications, and 15.1% for risk of mortality within 28 days following the first record of COVID-19.
Among patients in the DPP-4 cohort who were hospitalized, those who continued DPP-4 treatment had reduced mortality compared with those who discontinued DPP-4 treatment during hospitalization (9% vs. 19%, respectively). This is consistent with another recent multicenter retrospective study that showed that treatment with the DPP-4 inhibitor sitagliptin during hospitalization for COVID-19 was associated with decreased mortality and improved clinical outcomes compared with standard-of-care treatment in patients with T2DM (48). In addition to anti-inflammatory effects, DPP-4 inhibitors may reduce the entry of SARS-CoV-2 into cells because ACE2, the binding site for SARS-CoV-2, has high homology with DPP-4 (49).
The mean baseline HbA1c for all patients with T2DM and a diagnosis of COVID-19 or positive SARS-CoV-2 test results was 7.7% (Table 2). This is above the target of 7% and indicates that on average, patients with T2DM and COVID-19 have impaired glycemic control. There was no significant difference in the mean baseline HbA1c between those alive at 28 days compared with those deceased at 28 days (7.7% vs. 7.8%, respectively). This is unexpected as worse glycemic control has been associated with worse clinical outcomes in patients with diabetes who develop COVID-19. Because HbA1c was similar in those deceased at 28 days to those alive at 28 days, this finding suggests that other factors beyond glycemic control contribute to mortality in patients with T2DM and COVID-19.
The mean baseline HbA1c was significantly greater in the GLP-1 cohort compared with the No-G/D/P cohort (8.4% vs. 7.5%) and in the DPP-4 cohort compared with the No-G/D/P cohort (8.0% vs. 7.5%) (Table 3). This may be a limitation of the study as differences in HbA1c may have contributed to differences in hospitalizations, respiratory complications, and mortality between cohorts. Considering that the mean baseline HbA1c was higher in the GLP-1 cohort compared with the No-G/D/P cohort, the expected effect would have been worse outcomes in the GLP-1 cohort. Despite having worse baseline glycemic control, the GLP-1 cohort had significant reductions in hospitalizations, respiratory complications, and mortality compared with the No-G/D/P cohort. With regard to the DPP-4 cohort, considering that the mean baseline HbA1c was higher in the DPP-4 cohort compared with the No-G/D/P cohort, the expected effect would have been worse outcomes in the DPP-4 cohort. Despite having worse baseline glycemic control in the DPP-4 cohort, there was no significant difference in hospitalizations in the DPP-4 cohort compared with the No-G/D/P cohort. Additionally, the DPP-4 cohort had significant reductions in respiratory complications compared with the No-G/D/P cohort suggesting that factors other than glycemic control may be responsible for the protective effects of GLP-1 analogs and DPP-4 inhibitors in patients with T2DM and COVID-19.
Limitations
The main limitation of this study is its retrospective nature. Causal relationships could not be inferred, and we could not control variables such as the duration of use of medications before hospital admission, dosage, and treatments received after admission. Furthermore, analyses only included patients with a diagnostic code for T2DM in the EMR within the 6 months preceding the first record of COVID-19. Therefore, patients without a visit for T2DM within that time frame or with undiagnosed diabetes or prediabetes were not captured in these analyses. While this limited the sample size, it also increased the certainty that all patients included in the analyses were diagnosed with T2DM, leading to more conservative results. This limitation does, however, prevent analysis of the duration of T2DM. The length of time each patient has had T2DM is an important variable that should be assessed in prospective analyses. Additionally, outside a randomized controlled trial, it is not possible to know whether underlying variables may have influenced the results, such as having an established relationship with a prescribing physician or access to medical care. The design of the current study may have accounted for such factors based on the wide geographical and socioeconomic inclusion of the database and propensity matching; however, a prospective trial is necessary to fully determine whether there are protective effects of GLP-1R agonists, DPP-4 inhibitors, or pioglitazone on adverse outcomes in patients with T2DM and COVID-19. Another limitation of the study is the method of collection and analysis available. To provide real-time electronic health records of patients with COVID-19, the TriNetX COVID-19 Research Network only allows access to aggregated counts and statistical summaries of deidentified information. Furthermore, all analyses are conducted using the browser-based real-time analytics feature of TriNetX as previously described (42,43), which may limit the flexibility of statistical methods such as the use of propensity matching. Notably, the size of the pioglitazone cohort after propensity matching is much smaller than the other cohorts, making the interpretation of the analyses less reliable than the other cohorts.
Conclusions
The results of this study suggest that treatment with GLP-1R agonists before hospitalization and DPP-4 inhibitors before and/or during hospitalization may be expedient interventions to reduce mortality in patients with COVID-19 and T2DM. This hypothesis warrants further evaluation to determine whether the suggestion of a protective effect bears out in a prospective observational study or adequately powered randomized controlled trial. Subsequent assessment also should consider whether prolonged use is necessary or whether benefit can be derived from acute administration. The DPP-4 data are particularly interesting in this regard. Clinical trials also could be considered in patients without T2DM and across the life span to determine whether these agents possess intrinsic protective properties that could represent a novel therapeutic approach against SARS-CoV-2 infection.
Article Information
Acknowledgments. The authors thank Catharin Paules, Penn State College of Medicine/Milton S. Hershey Medical Center, for advice pertaining to analyses and for reviewing an early draft of the manuscript.
Funding. Penn State Clinical and Translational Science Institute provides access to the TriNetX network and is supported by the National Center for Advancing Translational Sciences and its Clinical and Translational Science Award (grant UL1-TR-002014). This work also was supported by National Institution on Drug Abuse Merit Award R37-DA 009815 (to P.S.G.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.E.N. shaped the design of the study, conducted the database search and all analyses, produced the first draft of the manuscript, and reviewed/edited the manuscript. N.T.R.-K. shaped the design of the study, contributed to the interpretation of the results, contributed to the composition of the discussion, and reviewed/edited the manuscript. K.B. collaborated in interpretation of the results and in the revision of the manuscript. P.A.H. contributed to the interpretation of the results and in the revision of the manuscript. D.L.L. collaborated in writing of the methods and reviewed/edited the manuscript. J.L.K. contributed to the interpretation of the results and in the revision of the manuscript. L.J.P. contributed to the interpretation of the results and reviewed/edited the manuscript. P.S.G. conceived the study, shaped its design, contributed to the discussion, and reviewed/edited the manuscript. J.E.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A non–peer-reviewed version of this article was submitted to the SSRN preprint server (https://ssrn.com/abstract=3725612) on 30 November 2020.
Footnotes
J.E.N., N.T.R.-K., and P.S.G. contributed equally.
J.E.N. and P.S.G. contributed equally.
This article contains supplementary material online at https://doi.org/10.2337/figshare.16594847.
This article is part of a special article collection available at https://diabetes.diabetesjournals.org/collection/diabetes-and-COVID19-articles.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19–preliminary report. Reply. N Engl J Med 2020;383:994. [DOI] [PubMed] [Google Scholar]
- 3. Beigel JH, Tomashek KM, Dodd LE, et al.; ACTT-1 Study Group Members . Remdesivir for the treatment of Covid-19–final report. N Engl J Med 2020;383:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . WHO Welcomes Preliminary Results About Dexamethasone Use in Treating Critically Ill COVID-19 Patients. Accessed 25 June 2020. Available from: https://www.who.int/news/item/16-06-2020-who-welcomes-preliminary-results-about-dexamethasne-use-in-treating- critically-ill-covid-19-patients
- 5. Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Physicians India 2020;68:19–26 [PubMed] [Google Scholar]
- 6. Chung SM, Lee YY, Ha E, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J 2020;44:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Udwadia ZF, Tripathi AR, Nanda VJ, Joshi SR. Prognostic factors for adverse outcomes in COVID-19 infection. J Assoc Physicians India 2020;68:62–66 [PubMed] [Google Scholar]
- 8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 9. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020;36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 2020;53:38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montefusco L, Ben Nasr M, D’Addio F, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab 2021;3:774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai Y, Lian P, Li J, Zhang Z, Qiao J. The active GLP-1 analogue liraglutide alleviates H9N2 influenza virus-induced acute lung injury in mice. Microb Pathog 2021;150:104645. [DOI] [PubMed] [Google Scholar]
- 14. Fandiño J, Vaz AA, Toba L, et al. Liraglutide enhances the activity of the ACE-2/Ang(1-7)/Mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A. Int J Endocrinol 2018;2018:6920620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology 2015;156:3559–3569 [DOI] [PubMed] [Google Scholar]
- 16. Sato T, Shimizu T, Fujita H, et al. GLP-1 receptor signaling differentially modifies the outcomes of sterile vs viral pulmonary inflammation in male mice. Endocrinology 2020;161:bqaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang LH, Pang XF, Bai F, et al. Preservation of glucagon-like peptide-1 level attenuates angiotensin II-Induced tissue fibrosis by altering AT1/AT 2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc Drugs Ther 2015;29:243–255 [DOI] [PubMed] [Google Scholar]
- 18. Sánchez-Aguilar M, Ibarra-Lara L, Del Valle-Mondragón L, et al. Rosiglitazone, a ligand to PPARγ, improves blood pressure and vascular function through renin-angiotensin system regulation. PPAR Res 2019;2019:1371758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang W, Xu YZ, Liu B, et al. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. ScientificWorldJournal 2014;2014:603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akhtar S, Benter IF, Danjuma MI, Doi SAR, Hasan SS, Habib AM. Pharmacotherapy in COVID-19 patients: a review of ACE2-raising drugs and their clinical safety. J Drug Target 2020;28:683–699 [DOI] [PubMed] [Google Scholar]
- 21. Lim S, Oh TJ, Dawson J, Sattar N. Diabetes drugs and stroke risk: intensive versus conventional glucose-lowering strategies, and implications of recent cardiovascular outcome trials. Diabetes Obes Metab 2020;22:6–15 [DOI] [PubMed] [Google Scholar]
- 22. Williams DM, Nawaz A, Evans M. Diabetes and novel coronavirus infection: implications for treatment. Diabetes Ther 2020;11:1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carboni E, Carta AR, Carboni E. Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med Hypotheses 2020;140:109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciavarella C, Motta I, Valente S, Pasquinelli G. Pharmacological (or synthetic) and nutritional agonists of PPAR-γ as candidates for cytokine storm modulation in COVID-19 disease. Molecules 2020;25:E2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract 2020;162:108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montastruc F, Romano C, Montastruc JL, et al. Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to intensive care unit in south of France. Therapie 2020;75:381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katsiki N, Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: a “promised land” in the COVID-19 era? J Diabetes Complications 2020;34:107723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solerte SB, Di Sabatino A, Galli M, Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol 2020;57:779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wadden TA, Hollander P, Klein S, et al.; NN8022-1923 Investigators . Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes 2013;37:1443–1451 [DOI] [PubMed] [Google Scholar]
- 31. Zaccardi F, Htike ZZ, Webb DR, Khunti K, Davies MJ. Benefits and harms of once-weekly glucagon-like peptide-1 receptor agonist treatments: a systematic review and network meta-analysis. Ann Intern Med 2016;164:102–113 [DOI] [PubMed] [Google Scholar]
- 32. Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 2018;14:390–403 [DOI] [PubMed] [Google Scholar]
- 33. Bramante C, Tignanelli CJ, Dutta N, et al. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. 2 September 2020. medRxiv:2020.09.01.20185850 [Google Scholar]
- 34. Hussein H, Zaccardi F, Dhalwani NN, Davies MJ, Khunti K, Gray LJ. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors (SGLT-2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) in patients with type 2 diabetes: a systematic review and network meta-analysis study protocol. BMJ Open 2018;8:e023206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussein H, Zaccardi F, Khunti K, Seidu S, Davies MJ, Gray LJ. Cardiovascular efficacy and safety of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabet Med 2019;36:444–452 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Rosenblit PD. Glucagon-like peptide-1 receptor agonists and cardiovascular risk reduction in type 2 diabetes mellitus: is it a class effect? Curr Cardiol Rep 2018;20:113. [DOI] [PubMed] [Google Scholar]
- 37. Savchenko LG, Digtiar NI, Selikhova LG, et al. Liraglutide exerts an anti-inflammatory action in obese patients with type 2 diabetes. Rom J Intern Med 2019;57:233–240 [DOI] [PubMed] [Google Scholar]
- 38. Li Q, Tuo X, Li B, Deng Z, Qiu Y, Xie H. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sci 2020;250:117531. [DOI] [PubMed] [Google Scholar]
- 39. Tan SA, Tan L. Liraglutide and semaglutide attenuate inflammatory cytokines interferongamma, tumor necrosis factor-alpha, and interleukin-6: possible mechanism of decreasing cardiovascular risk in diabetes mellitus. J Am Coll Cardiol 2019;73:186630975304 [Google Scholar]
- 40. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495:251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lebovitz HE, Banerji MA. Insulin resistance and its treatment by thiazolidinediones. Recent Prog Horm Res 2001;56:265–294 [DOI] [PubMed] [Google Scholar]
- 42. Stacey J, Mehta MD. Using EHR data extraction to streamline the clinical trial process. Clin Res (Alex) 2017;4:2–7 [Google Scholar]
- 43. Stapff M. Use of electronic health data in clinical development. Pharm Ind 2017;79:204–210 [Google Scholar]
- 44. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 2020;14:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F; Consensus Workshop Group . Type 2 diabetes in the young: the evolving epidemic: the International Diabetes Federation consensus workshop. Diabetes Care 2004;27:1798–1811 [DOI] [PubMed] [Google Scholar]
- 46. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan WJ, Liang WH, Zhao Y, et al.; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Solerte SB, D’Addio F, Trevisan R, et al. Sitagliptin Treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care 2020;43:2999–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]