Abstract
Enteroviruses, including the Coxsackievirus Bs (CVB), have been implicated as causal agents in human type 1 diabetes. Immunization of at-risk individuals with a CVB vaccine provides an attractive strategy for elucidating the role of CVBs in the disease etiology. Previously, we have shown that an inactivated whole-virus vaccine covering all CVB serotypes (CVB1–6) is safe to administer and highly immunogenic in preclinical models, including nonhuman primates. Before initiating clinical trials with this type of vaccine, it was also important to address 1) whether the vaccine itself induces adverse immune reactions, including accelerating diabetes onset in a diabetes-prone host, and 2) whether the vaccine can prevent CVB-induced diabetes in a well-established disease model. Here, we present results from studies in which female NOD mice were left untreated, mock-vaccinated, or vaccinated with CVB1–6 vaccine and monitored for insulitis occurrence or diabetes development. We demonstrate that vaccination induces virus-neutralizing antibodies without altering insulitis scores or the onset of diabetes. We also show that NOD mice vaccinated with a CVB1 vaccine are protected from CVB-induced accelerated disease onset. Taken together, these studies show that CVB vaccines do not alter islet inflammation or accelerate disease progression in an animal model that spontaneously develops autoimmune type 1 diabetes. However, they can prevent CVB-mediated disease progression in the same model.
Introduction
Type 1 diabetes is a common autoimmune disease caused by the destruction of the insulin-producing pancreatic β-cells. Genetic and environmental factors are contributory, but their precise roles remain unclarified (1). Among the possible environmental triggers, viral infections have been widely studied, and mounting evidence suggests that enteroviruses, especially the Coxsackievirus B (CVB) serotypes, may contribute to the development of type 1 diabetes (1–3).
A few schools of thought exist regarding the mechanisms through which CVBs may cause type 1 diabetes. Results from some studies support the notion that CVBs could be involved in initiating the disease process. For instance, the TEDDY (The Environmental Determinants of Diabetes in the Young) study found that prolonged enterovirus B infections were associated with the development of islet autoimmunity but not type 1 diabetes (3). Similar results were seen in the DIPP (Diabetes Prediction and Prevention) study, where associations were also documented between enterovirus infections and islet autoimmunity (4–6). An alternative hypothesis is that CVBs accelerate an on-going autoimmune process. Data from the DAISY (Diabetes Auto Immunity Study in the Young) study imply that enterovirus infections in autoantibody-positive individuals increase the speed of progression to diabetes (7). This observation has been supported by animal models in which CVB infection accelerates the onset of diabetes in prediabetic animals (8–10). It is, of course, feasible that both hypotheses hold true and that enteroviruses may contribute to the development of type 1 diabetes in both manners.
To determine the causal role of CVBs in human type 1 diabetes, vaccine development initiatives have been undertaken (9,11–14). A nonadjuvanted inactivated vaccine comprising the six CVB1–6 serotypes was recently shown to be highly immunogenic in mice and nonhuman primates in preclinical studies (13). Furthermore, this vaccine did not alter weight gain or blood glucose levels in both models and had no effect on temperature and hematological readouts in rhesus macaques, demonstrating an excellent safety profile (13).
The recent introduction of new vaccines in the human population has shown that adverse events may occur. These include associations between vaccination and the occurrence of autoimmune diseases (15,16). As the current CVB vaccine is based on inactivated whole-virus particles and CVB virus infections have been associated with both the initiation and progression of the processes that lead to type 1 diabetes (3,5–7,17), it is also paramount to ensure that vaccination itself does not affect the onset of autoimmune diabetes in a similar manner to infectious virus.
Here, we present the results from preclinical studies testing whether vaccination of young, CVB-naïve female NOD mice (a model prone to develop autoimmune diabetes [18]) with a multivalent CVB vaccine accelerates disease onset or increases diabetes incidence. Further to this, we also examined whether this type of vaccine can provide protection against the acceleration in diabetes onset seen after CVB infection of NOD mice that are in the prediabetic phase.
Research Design and Methods
Animal Husbandry and Monitoring of Animal Health
NOD mice were bred in-house and housed in specific pathogen-free conditions at Karolinska Institutet, Stockholm, Sweden. The Stockholm Southern Animal Ethics Board granted approval for all experiments, which were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and national laws in Sweden. Animals were housed in ventilated cages and provided with water and food ad libitum. A maximum of five mice were housed per cage, and no mice were singly housed. Extended health monitoring of mice was performed, including examining changes in health status, including weight changes, alterations in natural behavior, porphyria, movement and posture, piloerection, respiration, and the skin. Animals were randomly assigned to treatment groups. Weight and blood glucose measurements were monitored weekly until the experimental end point of diabetes onset, a health score of ≥0.4, or when the animals had reached the defined end point of the experiment. The researchers were not blinded to the experimental groups during the experiments. At the experimental end point, mice were anesthetized with isoflurane, a terminal heart puncture was performed for drawing blood, and the animals were then euthanized by cervical dislocation.
Vaccine Production
CVB1–6 and CVB1 vaccines were produced by formalin inactivation of the CVB1–6 or CVB1 serotypes (13). The vaccine was then formulated in Medium M199 (Gibco, Thermo Fisher Scientific, Vanda, Finland) containing 0.1% Tween 80 by mixing 1 μg of each inactivated virus serotype per dose for the CVB1–6 vaccine or 1.8 μg for the CVB1 vaccine.
Vaccination Strategies
Female age-matched NOD mice (4.9–7.1 weeks old) were randomly assigned to untreated, mock-vaccinated, or vaccinated treatment groups. Animals were left untreated, vaccinated with nonadjuvanted CVB1–6 vaccine on two or three occasions, 2–3 weeks apart, vaccinated with CVB1 vaccine on three occasions, 2–3 weeks apart, or mock-vaccinated with vaccine buffer alone (M199 Medium + 0.1% Tween 80 + 0.001% formalin, v/v). Each vaccination was performed by subcutaneous (interscapular) injection (150 μL). Serum samples were collected from the tail vein when indicated in the text. Animals were euthanized 6 weeks later (Fig. 1), monitored for diabetes incidence up until the age of 30 weeks (Fig. 2), or infected with virus (Fig. 3) as described under cvb1 infection.
Figure 1.
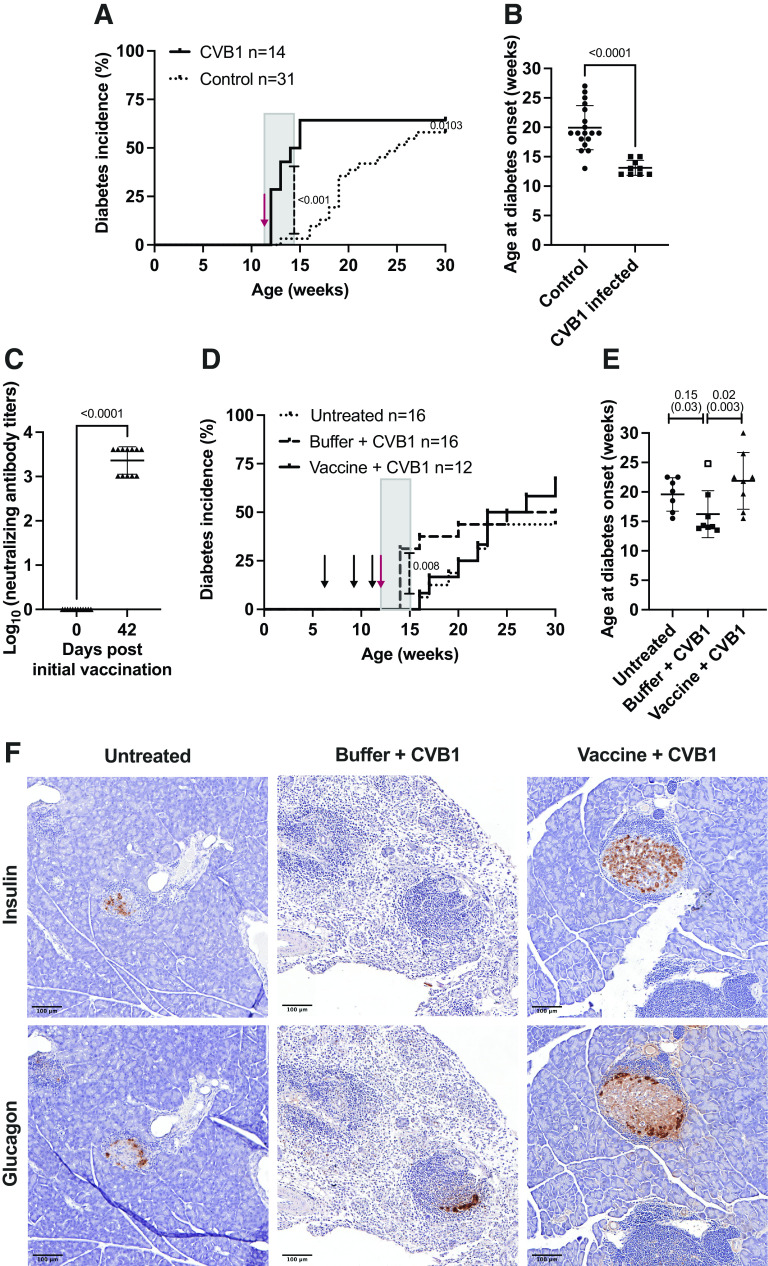
CVB1–6 vaccine does not increase pancreatic islet inflammation (insulitis) in prediabetic NOD mice. Female NOD mice (mean age, 5.5 weeks; range, 5.1–6.3 weeks) were mock-vaccinated with buffer (n = 13) or vaccinated with CVB1–6 vaccine (n = 8) by interscapular injection on three occasions (on days 0, 14, and 28 [n = 3] or on days 0, 21, and 35 [n = 5]). Mice were monitored until 12 weeks of age (6–8 weeks after the first vaccination). A: Average virus-neutralizing antibody titers in the serum of CVB1–6 vaccinated mice against the six CVB serotypes on day 41/42 after the first vaccination dose. Sera from mock-vaccinated mice had no virus-neutralizing capacity (data not shown). Shown are the mean ± SD neutralizing antibody titers, with individual mice represented by a single symbol. The blue symbols represent neutralizing antibody titer data that were also published previously (13). B–D: Sections of formalin-fixed paraffin-embedded pancreas were scored in a blinded manner for islet immune cell infiltration as described in research design and methods. B: Representative images of islets with different scores as described in research design and methods and esm methods. Scale bar = 100 μm. C: The total score per pancreas was divided by the total number of islets scored. Shown are the mean ± SD scores, with each score from an individual animal represented by a single symbol; buffer (n = 13) or CVB1–6 vaccine (n = 8). No statistically significant difference was found between the groups using an unpaired t test. D: Data show the percentage of islets from each mouse that fall into each insulitis category assessed as illustrated in B. Islets were scored as intact (0), peri-insulitis (1), insulitis (2), or destroyed (3). No statistically significant differences were found between the groups using two-way ANOVA with the Šidák multiple comparison test.
Figure 2.
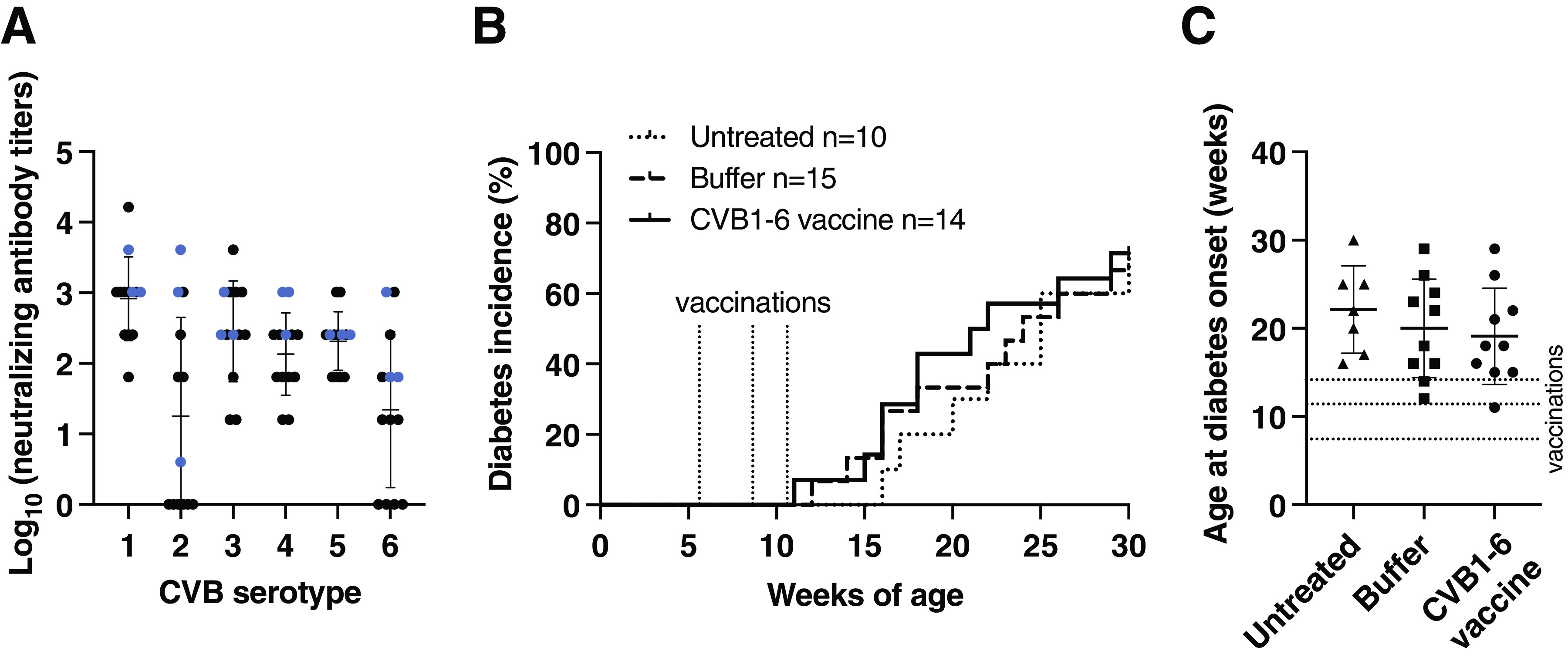
Diabetes onset is not altered in NOD mice immunized with a CVB1–6 vaccine. A–C: Female NOD mice (mean age, 5.7 weeks; range, 4.9–7.1 weeks) were left untreated (n = 10), mock-vaccinated (n = 15), or vaccinated (n = 14) with the CVB1–6 vaccine by interscapular injection on days 0 and 21 (n = 6 for buffer, n = 10 for CVB1–6 vaccine) or days 0, 21, and 35 (n = 9 for buffer, n = 4 for CVB1–6 vaccine). A: Average neutralizing antibody titers in the serum of CVB1–6 vaccinated mice against the six CVB serotypes on day 42 after the first vaccination dose. Sera from untreated and mock-vaccinated mice had no neutralizing capacity. Shown are the mean ± SD virus-neutralizing antibody titers, with individual mice represented by a single symbol. The blue symbols represent virus-neutralizing antibody titer data that were also previously published (13). Cumulative diabetes incidence (B) and average age at diabetes onset (C) in the three groups. The dotted lines in B and C show the average age at vaccination. The mean ± SD age at diabetes onset is shown in C, and the ages at which individual animals developed diabetes are displayed as single symbols. No statistically significant differences were found between the groups using the Gehan-Breslow-Wilcoxon test (B) or one-way ANOVA with the Tukey multiple comparison test (C).
Figure 3.
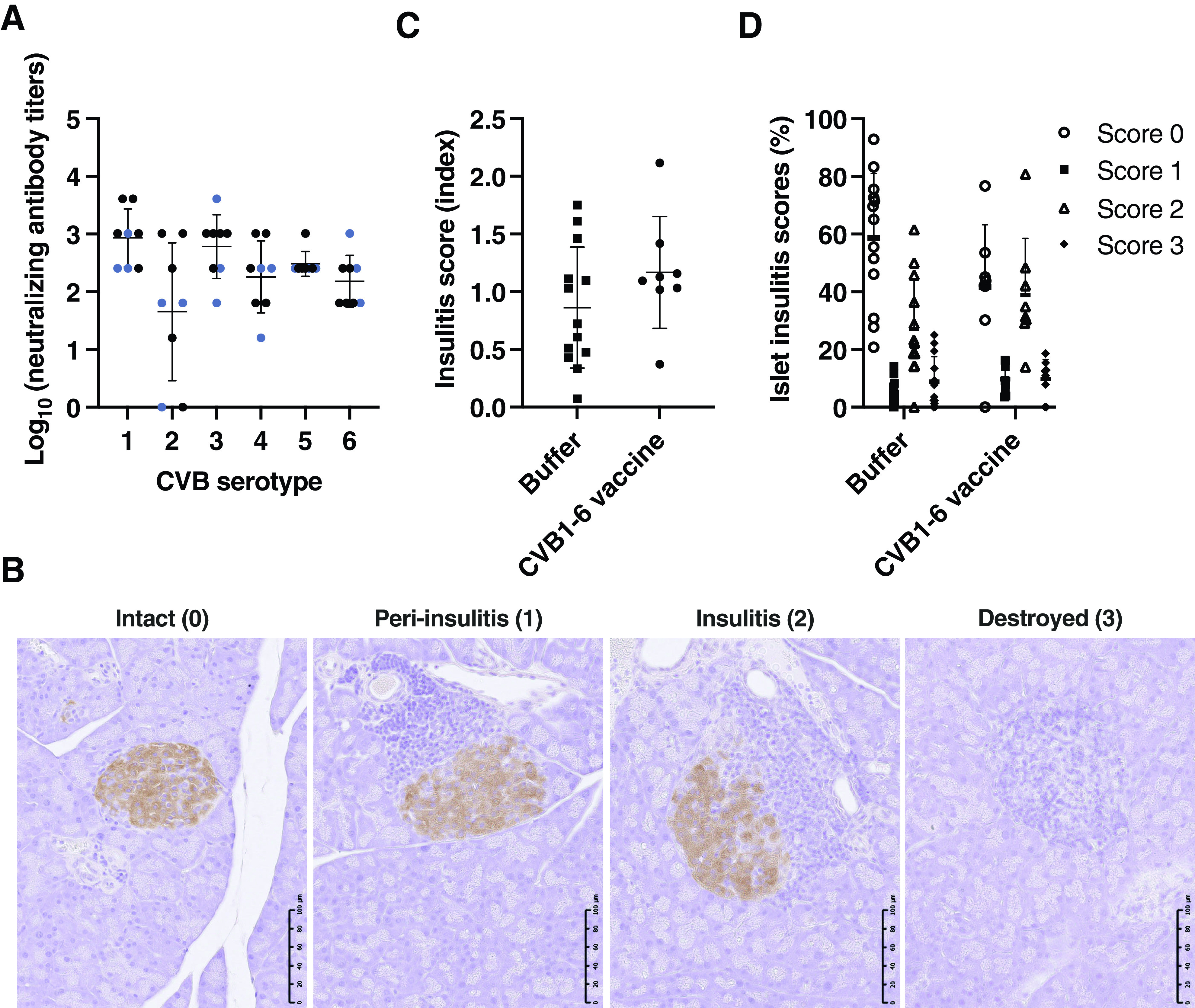
CVB1 vaccine protects against CVB1-accelerated disease in NOD mice. A and B: Female NOD mice were left untreated (control; n = 31) or infected with CVB1 (107 PFU by i.p. injection; total volume, 200 μL) between 10.5 and 13.5 weeks of age (n = 14), and diabetes incidence was followed up to 30 weeks of age. A: Diabetes incidence curves of the two groups. The red arrow indicates the mean age at infection. The gray box shows the 2-week period after virus infection. P < 0.001 when comparing the diabetes incidence curves during this period by the Gehan-Breslow-Wilcoxon test. P = 0.0103 comes from the comparison of the two curves up to 30 weeks of age by the Gehan-Breslow-Wilcoxon test. B: Age at diabetes onset. Individual mice are represented by a single symbol, and the horizontal line shows the mean ± SD age at diabetes onset. P < 0.0001 by unpaired t test. C–E: Female mice (6.3–6.9 weeks old) were left untreated (n = 16), mock-vaccinated with vaccine buffer and infected with CVB1 virus (buffer + CVB1; n = 16), or vaccinated with CVB1 vaccine and infected with CVB1 virus (vaccine + CVB1; n = 12). Vaccinations were performed on days 0, 21, and 35, and the mice were infected with virus (107 PFU by i.p. injection; total volume, 200 μL) on day 42 (12.3–12.9 weeks of age). Diabetes incidence was followed up to 30 weeks of age. C: Neutralizing antibody titers on days 0 and 42 in mice vaccinated with the CVB1 vaccine as measured by standard plaque reduction assay. Neutralizing antibodies were not detected in the mock-vaccinated and untreated groups (data not shown). Individual mice are represented by a single symbol, and the horizontal line shows the mean ± SD neutralizing antibody titer. P < 0.005 by unpaired t test. D: Diabetes incidence curves in the untreated, buffer + CVB1, and vaccine + CVB1 groups. The black arrows indicate the approximate vaccination ages, and the red arrow indicates the approximate age when the mice were infected. The gray box shows the 2-week period after virus infection. P = 0.008 when comparing the diabetes incidence curves by the Gehan-Breslow-Wilcoxon test. E: Age at diabetes onset. Individual mice are represented by a single symbol, and the horizontal line shows the mean ± SD age at diabetes onset. Groups were compared by the Kruskal-Wallis test with the Dunn test for multiple comparisons. In brackets are the P values generated when one mouse, which was borderline diabetic from 15 weeks of age but did not develop overt diabetes until 25 weeks of age, was excluded (open square; buffer + CVB1). See Supplementary Fig. 3B for the blood glucose values. F: Representative images of sequential pancreas sections stained with insulin and glucagon from mice that developed diabetes in the untreated, mock-vaccinated (buffer) + CVB1, and vaccine + CVB1 groups. Positive areas are stained brown. Scale bars are present in the bottom left-hand corner of each image. Scale bar = 100 μm.
CVB1 Infection
Female NOD mice (10.5–13.5 weeks old) were randomly assigned to control (n = 31) or CVB1 infection (n = 14; 107 plaque-forming units [PFU] CVB1 by intraperitoneal [i.p.] injection, total volume 200 μL) groups (Fig. 3A and B). In other experiments (Fig. 3C–E), female NOD mice (6.3–6.9 weeks old) were assigned to untreated (n = 16), mock-vaccination (n = 16), or CVB1 vaccine (n = 12) groups and vaccinated as described in vaccination strategies. Mice in the mock- and CVB1-vaccine groups were infected with CVB1 (107 PFU by i.p. injection, total volume, 200 μL) 1 week after the final vaccination (∼12–13 weeks old). In both experimental set ups, diabetes incidence was followed up until 30 weeks of age/diabetes onset.
Blood Glucose Measuring and Monitoring of Diabetes Incidence
Blood glucose concentrations were measured in blood drawn from the tail vein using a Bayer Contour XT blood glucose meter (Bayer, Basel, Switzerland). Diabetes was defined as a blood glucose value ≥18 mmol/L. If the blood glucose value was between 13 and 18 mmol/L, the mouse was checked the next day, and if it remained >13 mmol/L, the mouse was deemed diabetic.
Neutralizing Antibody Measurements
CVB1–6 neutralizing antibody titers were measured by a standard virus plaque reduction assay using green monkey kidney (GMK) cells (National Institute for Health and Welfare, Helsinki, Finland; mycoplasma negative [4,17,19]). In short, serum was serially diluted starting with a 1:4 dilution and was mixed with 100 PFU of the respective CVB serotypes used to produce the vaccine (for details regarding the viruses, see Stone et al. [13] and Hämäläinen et al. [20]). The serum-virus suspensions were incubated for 1 h at 37°C and then overnight at room temperature. GMK cells were grown to 95% confluency in 12-well plates, and the virus-serum mixture was added to these cells and incubated at 37°C for 1 h, then replaced with a semisolid medium (minimum essential medium supplemented with 0.67% carboxymethylcellulose [Merck, Sigma-Aldrich, Espoo, Finland]). Plates were incubated for 2 days at 37°C, and then the cells were fixed and stained with formaldehyde-crystal violet solution. Plaque numbers were counted with the researchers blinded to the treatment groups, and serum samples that had a reduction in plaque numbers of ≥80% compared with an untreated virus control were deemed to be positive for neutralizing antibodies. This assay has a technical detection limit of 1:4 and serum sample positivity for neutralizing antibodies was set to a dilution ≥1:16.
Histology and Immunohistochemistry
Mouse pancreases were collected, formalin-fixed in 4% paraformaldehyde overnight, and embedded in paraffin. Organs were cut into 5-μm-thick sections. For the insulitis scoring (Fig. 1), each pancreas was sectioned in two to three levels with >20 sections difference between each level (100 μm), and for the histological assessment in Fig. 3 and Supplementary Fig. 4, sections from one level of the pancreas were used. Sections were deparaffinized and stained with primary antibodies against insulin (1:20,000; A0564, Dako, Ely, U.K.) or glucagon (1:12,000; EP3070, Abcam, Cambridge, U.K.; both validated in formalin-fixed paraffin-embedded murine pancreas sections) and counterstained with hematoxylin using standard immunohistochemical techniques, as previously described (9,21).
Insulitis Scoring
Pancreas sections stained with insulin and glucagon were assessed in a blinded manner by light microscopy by two investigators and ranked for insulitis according to the following ranking method: 0—healthy islet: normal morphology with no mononuclear cells surrounding or infiltrating the islets; 1—peri-insulitis: mononuclear cells surrounding the islets on their periphery; 2—insulitis: infiltration of mononuclear cells into the islet; 3—infiltrated islet: no signs of insulin staining (denoted destroyed islet). See Fig. 1B for an example of islets with different scores. An insulitis score for each mouse was obtained by calculating the scores for each pancreas and dividing this total score by the number of islets examined. Data are presented as mean ± SD insulitis score for each treatment group.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA). Insulitis scores (index), CVB1 neutralizing antibody titers, and age at diabetes onset (CVB1-infected mice) were analyzed by an unpaired t test. The percentage of islets with differing insulitis scores was assessed by two-way ANOVA with the Šidák multiple comparison test. Age at diabetes onset (CVB1–6 vaccinated mice) was analyzed by one-way ANOVA with the Tukey multiple comparison test. Diabetes survival curves were assessed by the Gehan-Breslow-Wilcoxon test. In the studies examining virus-accelerated diabetes onset, the differences in the survival curves were assessed 2 weeks after infection when the acceleration in disease onset is expected to occur by the Gehan-Breslow-Wilcoxon test, as previously described (22,23). Age at diabetes onset in the CVB1 vaccine studies was assessed by the Kruskal-Wallis test with the Dunn test for multiple comparisons. Data are expressed as mean ± SD. A P value ≤0.05 was considered statistically significant.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. No applicable resources were generated or analyzed during the current study.
Results
A CVB1–6 Vaccine Does Not Aggravate Insulitis in NOD Mice
First, we studied whether the CVB1–6 vaccine alters pancreatic islet inflammation in age-matched female NOD mice. Young mice that had no previous exposure to CVBs were vaccinated three times—on days 0, 14, and 28 (n = 3) or on days 0, 21, and 35 (n = 5)—with the CVB1–6 vaccine or with vaccine buffer (n = 13), and their pancreases were assessed at ∼12 weeks of age. As seen before (13), vaccinated mice had CVB1–6 neutralizing antibodies by day 41 or 42 after the initial vaccination dose (Fig. 1A), which were absent on day 0 (data not shown). Neutralizing antibody data, shown in blue, were presented in a previous study (13), but the pancreases were not assessed for insulitis in this study. Moreover, the CVB1–6 vaccine had no negative effects on animal weight and blood glucose levels up to 6 weeks after the initial vaccination (the end point of the study) (Supplementary Fig. 1A–F). Pancreatic islet inflammation was assessed, and the average number of islets scored per animal was 30 ± 14 (range 9–64). All animals showed signs of pancreatic islet inflammation, but no significant differences in pancreatic insulitis scores between mock- and CVB1–6-vaccinated mice were observed (Fig. 1B–D). These results imply that the CVB1–6 vaccine does not alter immune cell infiltration in the pancreatic islets of Langerhans.
Diabetes Onset Is Not Affected in NOD Mice Vaccinated With a CVB1–6 Vaccine
Next, we examined the safety of the CVB1–6 vaccine with regards to diabetes development in NOD mice. To address whether the vaccine changed the onset of diabetes young animals were left untreated (n = 10), mock-vaccinated (n = 15), or vaccinated with CVB1–6 vaccine (n = 14) two or three times on days 0, 21, and 35. Blood glucose levels were monitored until 30 weeks of age or until diabetes onset, when the mice were removed. Vaccine immunogenicity was confirmed by CVB1–6 neutralizing antibody responses (Fig. 2A), which were absent at day 0 in all mice and at day 42 in untreated and mock-vaccinated mice (data not shown). No detrimental outcomes on weight (Supplementary Fig. 2) or general health status were seen. CVB1–6 vaccination did not alter the incidence of diabetes compared with the mock-vaccinated and untreated groups, and the kinetics of diabetes onset did not differ between the groups (Fig. 2B). Likewise, no differences were seen in the mean age at diabetes onset when animals from the three groups were compared (Fig. 2C). Taken together, these data indicate that the CVB1–6 vaccine does not alter the development of autoimmune diabetes in NOD mice.
A CVB Vaccine Protects Against CVB1-Accelerated Diabetes Onset in NOD Mice
CVB infections have been implicated in type 1 diabetes in humans and have also been shown to accelerate the onset of diabetes in prediabetic mice (7–10). As such, we next decided to examine whether vaccination can prevent the accelerating effect that CVB infection has on the development of diabetes in NOD mice. First, we confirmed that CVB infection accelerates the onset of diabetes in prediabetic female NOD mice in our colony. Prediabetic animals were left untreated or infected with CVB1, and the incidence of diabetes was monitored up to 30 weeks of age. CVB1-infected mice developed diabetes faster than the control group (Fig. 3A), and the mean age at diabetes onset was significantly lower in infected animals (13.1 weeks old) compared with the controls (19.9 weeks old) (Fig. 3B).
We subsequently wanted to see whether a CVB vaccine could protect against this virus-mediated acceleration in diabetes onset. Female NOD mice were left untreated, mock-vaccinated and then infected with CVB1 (mock + CVB1), or vaccinated and then infected with CVB1 (vaccine + CVB1). Diabetes incidence was monitored until the mice were 30 weeks old. To ensure the vaccine was immunogenic, virus-neutralizing antibodies were measured in serum collected prior to infection (day 42). A good vaccine neutralizing antibody response was induced in mice vaccinated with the CVB1 vaccine (Fig. 3C), which was absent in mock-vaccinated animals (data not shown). As expected, an acceleration in diabetes onset was seen in the mock-vaccinated (buffer) + CVB1 group compared with untreated mice (Fig. 3D). In comparison, the CVB1 vaccine protected against CVB1-mediated acceleration in diabetes onset, and the survival curve in the vaccine + CVB1 group mirrored that of the untreated animals (Fig. 3D). Significant differences between the curves were detected in the 2 weeks after infection, when most of the acceleration occurs. Moreover, the mean age at diabetes onset was lower in the mock-vaccinated (buffer) + CVB1 group (16.3 weeks old) (Fig. 3E) than in the vaccine + CVB1 group (21.9 weeks old) (Fig. 3E) and the untreated group (19.6 weeks old) (Fig. 3E).
The protective capacity of the vaccine was further illustrated when pancreas integrity was compared between the untreated, mock-vaccinated (buffer) + CVB1, and vaccine + CVB1 groups at the onset of diabetes (Fig. 3F). Vaccinated animals had healthy exocrine tissue morphology at the time of diabetes onset in a similar manner to untreated animals, whereas there was significant exocrine tissue destruction in the mock-vaccinated (buffer) group, as shown by the representative images in Fig. 3F. Differences were also seen between these groups in the animals that did not develop diabetes by 30 weeks of age. There was evidence of exocrine tissue loss in the pancreas of mock-vaccinated animals, as illustrated by the presence of islets in fat tissue (Supplementary Fig. 4E and F), although some exocrine tissue had remained healthy or regenerated in these animals (Supplementary Fig. 4C and D). In contrast, normal pancreas histology was seen in the untreated and vaccinated groups (Supplementary Fig. 4A, B, G, and H). Collectively, these studies show that a CVB vaccine protects against CVB1-accelerated diabetes in NOD mice.
Discussion
Preclinical studies are an important part of initial vaccine efficacy and safety assessments. These studies serve to identify elements that require further assessment and can also help to design vaccination schedules. Additionally, they may uncover adverse events, including undesired immune reactions that can, for example, lead to autoimmune diseases. Such diseases have occurred, albeit rarely, after immunization with other vaccines (15,16). Our studies demonstrate that a multivalent CVB vaccine does not accelerate the onset of diabetes in NOD mice, a commonly used animal model for type 1 diabetes. We confirmed that early vaccination with this vaccine induces virus-neutralizing antibodies and showed that immunity to CVBs is achieved without altering islet inflammation or changing the average time to diabetes onset. These results are in line with our previous observation that vaccination of prediabetic NOD mice with a monovalent CVB1 vaccine did not increase the production of insulin autoantibodies (9). This also suggests that inactivation of the viruses abolishes the diabetogenic properties of the CVBs, which have previously been observed in the NOD mouse (8–10) and which are suspected in humans (4,7).
Human cohort studies focused on understanding the triggers of type 1 diabetes have produced results suggesting that CVBs could be critically involved at different stages of the disease. In the TEDDY and DIPP studies, enterovirus infections were associated with the development of islet-specific autoantibodies (3–6). In contrast, the DAISY study reported that enterovirus infections accelerated the speed of progression to overt diabetes in autoantibody-positive individuals (7). Different animal models exist that may replicate how CVBs could contribute to type 1 diabetes development in humans, as alluded to in the cohort studies. Direct infection of the β-cell by CVBs is a possible mechanism through which β-cell autoimmunity could be induced. In our previous studies using the SOCS-1-tg mouse model, where the β-cells are susceptible to CVB infection leading to diabetes (21,24), we showed that CVB vaccines can prevent virus-induced diabetes (13,14). It is also possible to mimic virus acceleration of an on-going autoimmune process by infecting prediabetic NOD mice with CVBs (8–10). In this study, we report for the first time that a CVB vaccine is also capable of preventing virus-mediated acceleration in diabetes onset. Type 1 diabetes appears to be a highly heterogenous disease, and it is feasible that both virus-induced autoimmunity and acceleration in the rate of diabetes onset in autoantibody-positive individuals after virus infection could occur in different groups. The ability of CVB vaccines to prevent both forms of virus-mediated diabetes in relevant preclinical models provides excellent proof-of-concept evidence for the use of such a vaccine to elucidate the multiple potential roles of CVBs in human type 1 diabetes.
Based on the aforementioned studies (among others) that suggest enteroviruses may have an important role in type 1 diabetes and from promising results using the mono- and current multivalent CVB vaccine in preclinical studies (9,13,14,25), the production and clinical testing of a similar multivalent CVB vaccine was recently initiated (11,12,26). Our previous work with experimental CVB vaccines demonstrates that such vaccines show strong potential for use in the prevention of CVB infections and diseases associated with these infections in humans (13,14). We also found that there were no adverse effects on glucose regulation (13) and no conspicuous infiltration of immune cells in the pancreas (Stone et al. unpublished observation) in rhesus macaques immunized with the multivalent CVB vaccine. The current study builds on these foundations by suggesting that this type of vaccine does not alter islet inflammation or diabetes onset in a preclinical mouse model for autoimmune type 1 diabetes.
In summary, this study provides data that support the use of an equivalent vaccine in human clinical trials to establish whether CVBs are involved in type 1 diabetes. Such trials will involve the immunization of young children with a genetic predisposition for the disease who are yet to experience a CVB infection. If the involvement of CVBs in type 1 diabetes is confirmed, the vaccine could provide a viable preventative measure for this disease.
Article Information
Acknowledgments. The authors would like to acknowledge S. Parvin, Karolinska Institutet, Stockholm, Sweden, for her help with processing and staining histological samples, the M.F.-T. group for scientific discussions and laboratory assistance, and the animal staff at the Preclinical Laboratory (PKL) Facility at Karolinska Institutet, Stockholm, Sweden, for their assistance with the animal studies. M. Jokela, from the Faculty of Medicine and Health Technology (Tampere University, Finland), is acknowledged for assisting with the vaccine production and M. Kekäläinen and M. Ovaskainen, from the Faculty of Medicine and Health Technology (Tampere University, Finland), for the analysis of virus antibodies. The authors also would like to acknowledge Biocenter Finland for its infrastructure support.
Funding. Financial support was received from Barndiabetesfonden (Swedish Child Diabetes Foundation), Diabetesfonden (the Swedish Diabetes Foundation), Karolinska Institutet, including the Strategic Research Program in Diabetes, Business Finland (formerly TEKES, Therdiab project, diary no. 1843/31/2014), the Academy of Finland (grant 309455 awarded to M.M.H. and grant 288671 awarded to H.H.), the Sigrid Juséliuksen Säätiö (Sigrid Jusélius Foundation), the Reino Lahtikari Foundation and the JDRF (2-SRA-2017-A-N).
Duality of Interest. H.H. owns stocks and is the chairman of the board of Vactech Ltd, which develops vaccines against picornaviruses. H.H. and M.F.-T. serve on the scientific advisory board of Provention Bio Inc., which is developing a clinical CVB vaccine in collaboration with Vactech Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. V.M.S. and M.B. performed experiments. V.M.S., M.B., A.-B.S.-K., and M.F.-T. analyzed results. V.M.S., M.B., and M.F.-T. wrote and edited the manuscript. V.M.S., M.M.H., A.-B.S.-K., V.P.H., H.H., and M.F.-T. designed the study. M.M.H. produced and performed quality control analyses of the vaccine. All authors read, edited, and approved the final manuscript. H.H. and M.F.-T. are the guarantors of this work, and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the JDRF's Network for Pancreatic Organ Donors (nPOD) with Diabetes 12th Annual Meeting, Tampa, FL, 23–26 February 2020, at the virtual 13th Annual nPOD Meeting, 22–24 February 2021, and at the virtual 57th European Association for the Study of Diabetes Annual Meeting, 28 September–1 October 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16556424.
References
- 1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson SJ, Morgan NG. Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention. Curr Opin Pharmacol 2018;43:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vehik K, Lynch KF, Wong MC, et al.; TEDDY Study Group . Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med 2019;25:1865–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laitinen OH, Honkanen H, Pakkanen O, et al. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes 2014;63:446–455 [DOI] [PubMed] [Google Scholar]
- 5. Oikarinen S, Martiskainen M, Tauriainen S, et al. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 2011;60:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salminen K, Sadeharju K, Lönnrot M, et al. Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol 2003;69:91–98 [DOI] [PubMed] [Google Scholar]
- 7. Stene LC, Oikarinen S, Hyöty H, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes 2010;59:3174–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med 1998;4:781–785 [DOI] [PubMed] [Google Scholar]
- 9. Larsson PG, Lakshmikanth T, Laitinen OH, et al. A preclinical study on the efficacy and safety of a new vaccine against coxsackievirus B1 reveals no risk for accelerated diabetes development in mouse models. Diabetologia 2015;58:346–354 [DOI] [PubMed] [Google Scholar]
- 10. Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 2000;49:708–711 [DOI] [PubMed] [Google Scholar]
- 11. Dunne JL, Richardson SJ, Atkinson MA, et al. Rationale for enteroviral vaccination and antiviral therapies in human type 1 diabetes. Diabetologia 2019;62:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hyöty H, Leon F, Knip M. Developing a vaccine for type 1 diabetes by targeting coxsackievirus B. Expert Rev Vaccines 2018;17:1071–1083 [DOI] [PubMed] [Google Scholar]
- 13. Stone VM, Hankaniemi MM, Laitinen OH, et al. A hexavalent coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates. Sci Adv 2020;6:eaaz2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone VM, Hankaniemi MM, Svedin E, et al. A coxsackievirus B vaccine protects against virus-induced diabetes in an experimental mouse model of type 1 diabetes. Diabetologia 2018;61:476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol 2018;15:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roivainen M, Knip M, Hyöty H, et al. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol 1998;56:74–78 [DOI] [PubMed] [Google Scholar]
- 18. Mullen Y. Development of the nonobese diabetic mouse and contribution of animal models for understanding type 1 diabetes. Pancreas 2017;46:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sioofy-Khojine AB, Lehtonen J, Nurminen N, et al. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia 2018;61:1193–1202 [DOI] [PubMed] [Google Scholar]
- 20. Hämäläinen S, Nurminen N, Ahlfors H, et al. Coxsackievirus B1 reveals strain specific differences in plasmacytoid dendritic cell mediated immunogenicity. J Med Virol 2014;86:1412–1420 [DOI] [PubMed] [Google Scholar]
- 21. Flodström M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol 2002;3:373–382 [DOI] [PubMed] [Google Scholar]
- 22. McCall KD, Thuma JR, Courreges MC, et al. Toll-like receptor 3 is critical for coxsackievirus B4-induced type 1 diabetes in female NOD mice. Endocrinology 2015;156:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serreze DV, Wasserfall C, Ottendorfer EW, et al. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. J Virol 2005;79:1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flodström M, Tsai D, Fine C, Maday A, Sarvetnick N. Diabetogenic potential of human pathogens uncovered in experimentally permissive beta-cells. Diabetes 2003;52:2025–2034 [DOI] [PubMed] [Google Scholar]
- 25. Hankaniemi MM, Laitinen OH, Stone VM, et al. Optimized production and purification of Coxsackievirus B1 vaccine and its preclinical evaluation in a mouse model. Vaccine 2017;35:3718–3725 [DOI] [PubMed] [Google Scholar]
- 26. Provention Bio . Provention Bio Initiates First-in-Human Study of Coxsackievirus B Vaccine Candidate PRV-101. 15 December 2020. Accessed 5 March 2021. Available from https://investors.proventionbio.com/2020-12-15-Provention-Bio- Initiates-First-in-Human-Study-of-Coxsackievirus-B-Vaccine-Candidate- PRV-101