Abstract
Aging, obesity, and diabetes are major risk factors for the severe progression and outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019 [COVID-19]), but the underlying mechanism is not yet fully understood. In this study, we found that the SARS-CoV-2 spike protein physically interacts with cell surface GRP78, which promotes the binding to and accumulation in ACE2-expressing cells. GRP78 was highly expressed in adipose tissue and increased in humans and mice with older age, obesity, and diabetes. The overexpression of GRP78 was attributed to hyperinsulinemia in adipocytes, which was in part mediated by the stress-responsive transcription factor XBP-1s. Management of hyperinsulinemia by pharmacological approaches, including metformin, sodium–glucose cotransporter 2 inhibitor, or β3-adrenergic receptor agonist, decreased GRP78 gene expression in adipose tissue. Environmental interventions, including exercise, calorie restriction, fasting, or cold exposure, reduced the gene expression of GRP78 in adipose tissue. This study provides scientific evidence for the role of GRP78 as a binding partner of the SARS-CoV-2 spike protein and ACE2, which might be related to the severe progression and outcome of COVID-19 in patients with older age, obesity, and diabetes. The management of hyperinsulinemia and the related GRP78 expression could be a therapeutic or preventative target.
Introduction
The outbreak of the novel β-coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, coronavirus disease 2019 (COVID-19), has rapidly spread worldwide and, to date, has resulted in over 169,000,000 human infections and more than 3,500,000 deaths. The development of SARS is the major factor for serious progression and mortality in COVID-19 patients (1). Emerging studies have shown that there is an increased risk of poor outcomes with increasing age, obesity, visceral adiposity, and diabetes (2–4), but the linked molecular mechanisms have not yet been explained. In this severe pandemic, further scientific information and therapeutic targets are required.
While adipose tissue plays an important role in the regulation of energy homeostasis, its abnormalities have harmful effects on systemic healthy states. The aging- or obesity-associated pathological expansion of adipose tissue, especially in the visceral region, contributes to the development of various metabolic diseases and their complications (5–8). Hyperinsulinemia, a chronic state of high insulin levels, is commonly found in older or obese patients (9,10) and causes detrimental cellular stress in adipose tissue, such as reactive oxygen species, endoplasmic reticulum (ER) stress, hypoxia, and inflammation (11–14). Recently, adipose tissue has been taken into account as a major reservoir for viral shedding/spread, immune activation, and cytokine amplification in SARS-CoV-2 infection (COVID-19) (15–17). However, little is known about the molecular mechanisms.
SARS-CoV-2, like a previous SARS-related coronavirus (SARS-CoV), has been known to utilize angiotensin-converting enzyme 2 (ACE2) as a major host binding receptor (18). Recent studies have suggested the involvement of other binding molecules in COVID-19, such as glucose-regulated protein 78 (GRP78) (19–22). GRP78, also known as BIP/HSPA5, is well known as a molecular chaperone localized in the ER, where it plays important roles in protein folding and assembly. Under stressed conditions, GRP78 is overexpressed and translocated to the cell surface and acts as a receptor for various endogenous and exogenous ligands (23,24). GRP78 can bind many viruses and affect multiple stages of the viral life cycle, including entry, replication, egress, and subsequent spread. This property is applicable not only to Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 coronaviruses but also potentially to SARS-CoV-2 (19,21,22,25). However, scientific evidence linking GRP78 and COVID-19 and the expression profiles and possible roles of GRP78 in COVID-19 have not been fully explored.
In this study, based on the experimental results of protein interactions and transcriptome analyses, we offer a perspective on the possible involvement of adipose tissue in the SARS-CoV-2 infection (COVID-19) of patients with older age, obesity, and diabetes by GRP78 and provide potential therapeutic and preventative approaches by managing hyperinsulinemia and the related GRP78 expression in adipose tissue.
Research Design and Methods
Material Information
For the immunoblot assay, the following antibodies were used: anti-FLAG (Sigma no. F3165, M2), anti-His (CST no. 12698, Santa Cruz no. sc-8036), anti-C9 (Santa Cruz no. sc-57432), anti-GRP78 (CST no. 3177), anti–XBP-1s (CST no. 12782), anti-ACE2 (Abcam no. ab108252), and anti-SARS-CoV-2 spike (CST no. 56996). For cellular treatments, the following recombinant proteins were used: GRP78 (Abcam no. ab78432) and SARS-CoV-2 spike protein (R&D no. 10549-CV). For the HEK293 overexpression assay, the following plasmids were used: pCMV14–3×-Flag–SARS-CoV-2 (Addgene no. 145780), pcDNA3.1-Empty, pcDNA3.1-GRP78 (Addgene no. 145780), and pcDNA3.1-hACE2 (Addgene no. 145033). For plasmid transfection, Lipofectamine 3000 was used per the manufacturer’s instructions. For extracellular crosslinking, DTSSP [3,3'-dithiobis (sulfosuccinimidyl propionate); Thermo Scientific no. 21578] was used. For the coimmunoprecipitation assay, Dynabeads Protein G (Invitrogen no. 10003D) and TNE buffer (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40) with protease inhibitor (Nacali no. 25955) and phosphatase inhibitors (2 mmol/L orthovanadate, 1 mmol/L NaF) were used.
Transcriptome and ChIP-Seq Database Analysis
The gene expression data of various human and mouse tissues were obtained from the Genotype Tissue Expression (GTEx) and mouse Encyclopedia of DNA Elements (ENCODE) projects. The gene expression data of adipose tissues were obtained from the Gene Expression Omnibus (GEO). The following data sets were used: visceral and subcutaneous adipose tissue (VAT and SAT, respectively) related, GTEx and GSE92488; age related, GTEx and GDS6247; obesity related, GDS3602 and GSE71586; diabetes related, GDS3665; insulin neutralization related, GSE35581, 3T3-L1 adipocytes; insulin and tumor necrosis factor-α (TNF-α) treatment related, GSE87853, 3T3-L1 adipocytes; XBP-1s overexpression related, GDS5065; GRP78/XBP-1 correlation-related transcriptome, GTEx; metformin related, GSE107894; thiazolidinedione (TZD) related, GSE13070; CL316,243 related, GSE98132; exercise related, GSE68161; calorie restriction related, GSE60596; fasting related, GSE154612 and GDS4918; and cold exposure related, GDS3804. The sodium–glucose cotransporter 2 inhibitor (SGLT2i)-related transcriptome was from previous research (26). The chromatin immunoprecipitation sequencing (ChIP-Seq) data of XBP-1 were obtained from and analyzed by ChIP-Atlas: GSM2292560 and GSM3449388.
Cellular and Mouse Experiments
ACE2-overexpressing HEK293T or Calu3 cells were cultured with SARS-CoV-2 spike protein for 24 h, followed by washing (ice-cold PBS, 3 times), cell lysis, and immunoblotting. For the binding assay, HEK293T cells overexpressed with ACE2 and with/without GRP78 were treated with SARS-CoV-2 spike protein (0.5–1 µg/ml) at 4°C for 1 h, followed by DTSSP crosslinking according to the manufacturer's instructions. 3T3-L1 adipocyte experiments were performed as follows. Briefly, 3T3-L1 adipocytes were differentiated with standard differentiation medium for 2 days (1 μmol/L insulin, 1 μmol/L dexamethasone, and 0.5 μmol/L 3-isobutyl-1-methylxanthine in DMEM with 10% FBS and 1% penicillin-streptomycin) and then replaced with maintenance medium (DMEM with 10% FBS and 1% penicillin-streptomycin). Fully differentiated adipocytes (days 7–9) were treated with 1 nmol/L and/or 100 nmol/L insulin with/without XBP-1 splicing inhibitor (STF-083010, 100 μmol/L) for 48 h and then snap-frozen in liquid nitrogen for Western blot analysis. Mouse adipose tissues from the interventions of 24-h fasting/feeding cycle and SGLT2i treatment (26) were snap-frozen in liquid nitrogen for Western blot analysis. Protein samples were prepared in SDS sample buffer containing dithiothreitol (DTT) and heated to 95°C for 5 min prior to loading on SDS-PAGE gels. After electrophoresis, the gel was transferred to polyvinylidene difluoride, and the membrane was blotted with specific antibodies. Densitometry was performed using ImageJ (National Institutes of Health).
Statistics Analysis
For the results shown in Fig. 2C, G, H, and I, Fig. 3A and E, Fig. 4A, B, and D–H, and Supplementary Fig. 1B, statistical analyses were made by using Student’s t test. For the results shown in Fig. 1C–E, Fig. 2D and F, Fig. 3F, and Supplementary Fig. 1C, statistical analyses were made using Tukey-Kramer test. For the results of Fig. 4F, statistical analysis for each pair was made using Wilcoxon's paired tests. For the results shown in Fig. 2E and Supplementary Fig. 1A, statistical analyses were made using Wilcoxon/Kruskal-Wallis test (rank sums) followed by post hoc tests for each pair using Wilcoxon's paired tests. All data were expressed as mean ± SEM values. Significant differences were considered at the following levels: *P < 0.05; **P < 0.01; ***P < 0.001.
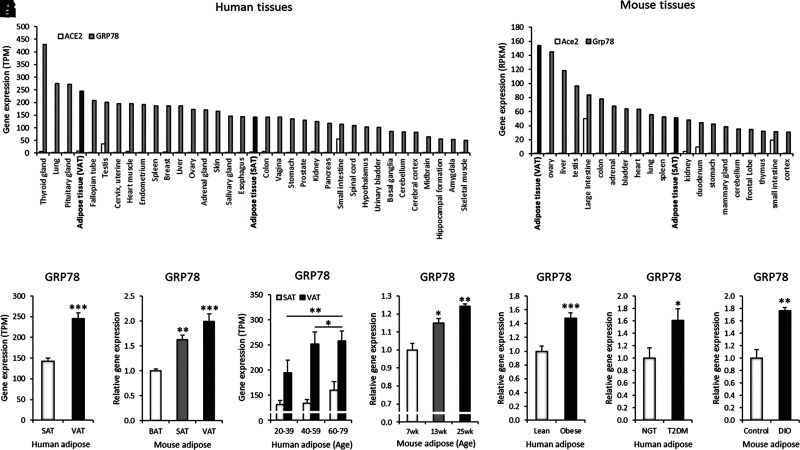
Figure 2.
GRP78 is highly expressed in adipose tissue and is elevated with aging, obesity, and diabetes. A and B: Gene expression of ACE2 and GRP78 in human and mouse tissue (human GTEx and mouse ENCODE transcriptomes; dark gray colors indicate GRP78 gene expression in adipose tissue). TPM, transcripts per million; RPKM, reads per kilobase per million. C: GRP78 gene expression in the VAT and SAT of human subjects (GTEx, SAT n = 442 and VAT n = 355). D: GRP78 gene expression in the VAT, SAT, and brown adipose tissue (BAT) of a mouse model (GSE92488, n = 3). E: GRP78 gene expression in human VAT and SAT in the indicated age-groups: GTEx, 20–39 years of age, n = 53 (VAT) and n = 70 (SAT); 40–59 years of age, n = 181 (VAT) and n = 223 (SAT); 60–79 years of age, n = 121 (VAT) and n = 149 (SAT). F: GRP78 gene expression in mouse VAT from the indicated age-groups (GDS6247, n = 3 each). G: GRP78 gene expression in the subcutaneous adipocytes of obese subjects compared with that of lean control subjects (GDS3602, n = 10 each). H: GRP78 gene expression in the VAT of subjects with diabetes compared with that of age- and BMI-matched control subjects (GDS3665, n = 5 each). NGT, normal glucose tolerant; T2DM, type 2 diabetes mellitus. I: GRP78 gene expression in the VAT of a diet-induced obesity (DIO; 12 weeks of high-fat diet) mouse model (GSE71586, n = 3 each). Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
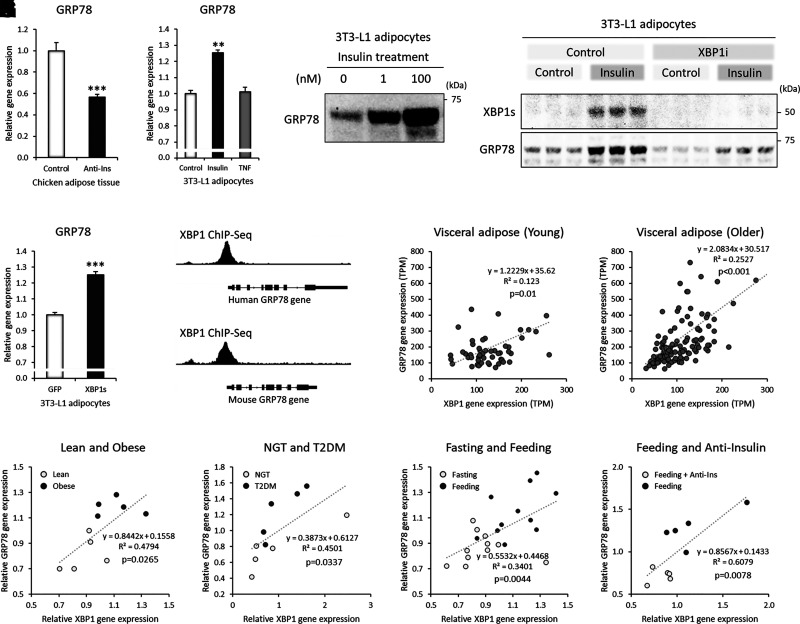
Figure 3.
GRP78 is upregulated by hyperinsulinemia, which is mediated by XBP-1s in adipocytes. A: GRP78 gene expression in chicken adipose tissue after 5 h of insulin neutralization with porcine anti-insulin serum (Anti-Ins) during feeding (GSE35581, n = 5 each). B: GRP78 gene expression after chronic insulin or TNF-α (TNF) treatment in 3T3-L1 adipocytes (GSE87853, control, and TNF, n = 3 each; insulin, n = 2). C: Western blot image of GRP78 protein after 48 h of insulin treatment in 3T3-L1 adipocytes. D: Western blot image of GRP78 protein after 48 h of insulin (10 nmol/L) treatment with/without XBP-1 splicing inhibitor (XBP-1i; 100 μmol/L). E: GRP78 gene expression after XBP-1s overexpression in 3T3-L1 adipocytes (GDS5065, n = 4). F: XBP-1 ChIP-Seq peaks (promoter regions of the XBP-1 gene spanning exon 1) in the human and mouse GRP78 genes (human LNCaP cells, GSM3449388; mouse liver, GSM2292560). G and H: Correlation between GRP78 and XBP-1 gene expression in human VAT from GTEx of young (20–39 years of age, VAT n = 53) (G) and older (60–79 years of age, VAT n = 121) (H) patients; visualized GRP78 expression ranged from 0 to 800 transcripts per million (TPM), and that for XBP-1 was from 0 to 300 TPM. I: Correlation between GRP78 and XBP-1 gene expression in the SAT of lean and obese women (GDS3602; n = 5 each). J: Correlation between GRP78 and XBP-1 gene expression in the VAT of control women (age- and BMI-matched) and women with diabetes (GDS3665, n = 5 each). NGT, normal glucose tolerant; T2DM, type 2 diabetes mellitus. K: Correlation between GRP78 and XBP-1 gene expression in the SAT of feeding (evening, 2 h after standardized meal) and fasting (evening, 26 h after standardized meal) subjects (GSE154612, n = 11 each). L: Correlation between GRP78 and XBP-1 gene expression in chicken adipose tissue after 5 h of insulin neutralization with porcine anti-insulin serum during feeding (GSE35581, n = 5 each). Data are mean ± SEM. **P < 0.01; ***P < 0.001.
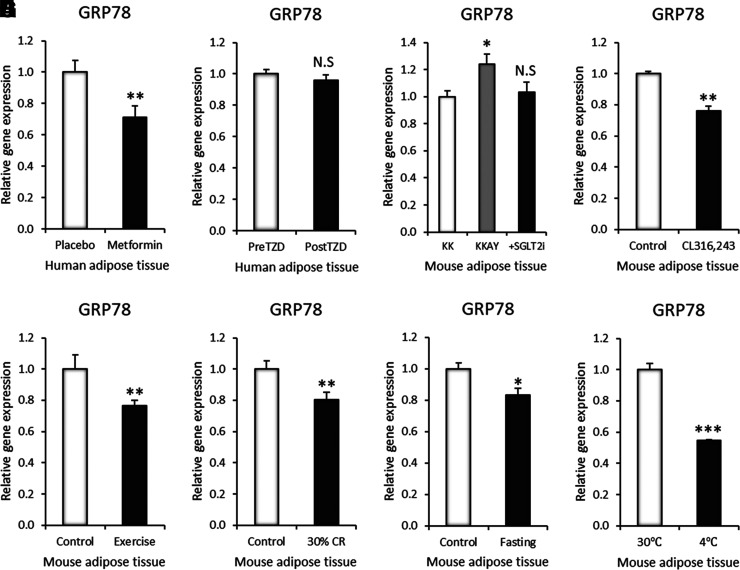
Figure 4.
GRP78 expression can be managed by pharmacological and environmental interventions in adipose tissue. A: GRP78 gene expression in SAT after 6 weeks of metformin treatment at 500 mg twice daily, increased incrementally to 2,000 mg daily at the end of 2 weeks (GSE107894, n = 14). B: GRP78 gene expression in human SAT after 3 months of TZD treatment (GSE13070, n = 40). C: GRP78 gene expression in mouse VAT after 5 weeks of SGLT2i (dapagliflozin) treatment (n = 4). D: GRP78 gene expression in mouse VAT after 3 days of CL316,243 treatment (GSE98132, n = 3). E: GRP78 gene expression in mouse SAT after 11 days of wheel running exercise (GSE68161, n = 7). F: GRP78 gene expression in mouse VAT after 10 weeks of 30% calorie restriction (CR) (GSE60596, n = 3). G: GRP78 gene expression in mouse VAT after 24 h of fasting (GDS4916, n = 5). H: GRP78 gene expression in mouse SAT after 1 week of 30°C thermoneutrality or 4°C cold exposure (GSE13432, n = 3). Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
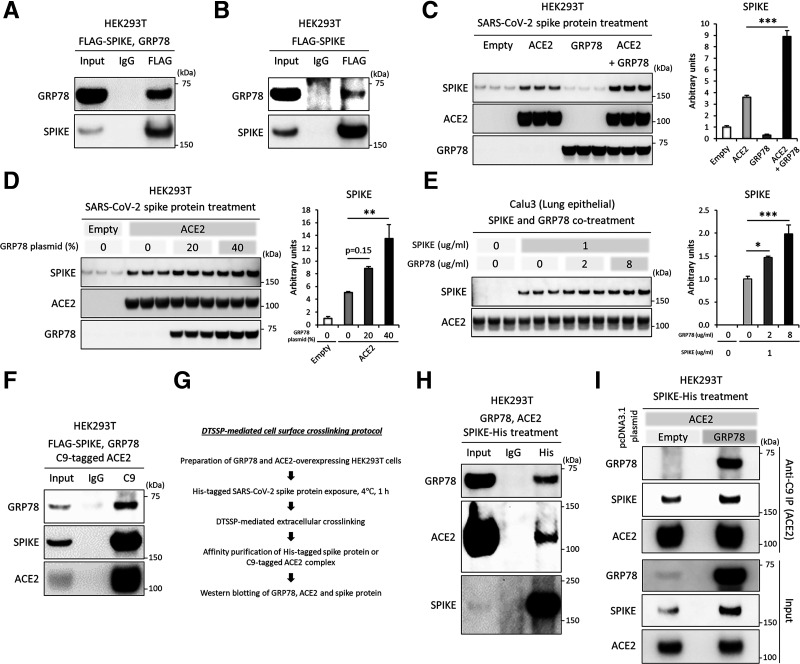
Figure 1.
GRP78 physically binds with the SARS-CoV-2 spike protein, which promotes the binding to and accumulation in ACE2-expressing cells. A: Western blot images of GRP78 (anti-GRP78) and SARS-CoV-2 spike protein (anti-Flag) after the coimmunoprecipitation of HEK93T cells overexpressed with Flag-tagged SARS-CoV-2 spike protein and GRP78. B: Western blot images of GRP78 (anti-GRP78) and SARS-CoV-2 spike protein (anti-Flag) after the coimmunoprecipitation of HEK93T cells overexpressed with Flag-tagged SARS-CoV-2 spike protein. C and D: Western blot images and densitometry of the SARS-CoV-2 spike protein (anti-His), ACE2 (anti-C9), and GRP78 (anti-GRP78) after treatment with His-tagged SARS-CoV-2 spike protein for 24 h in HEK293T cells overexpressed with empty, C9-tagged ACE2, and/or GRP78 plasmids. E: Western blot images and densitometry of the SARS-CoV-2 spike protein (anti-His) and ACE2 (anti-ACE2) after treatment with His-tagged SARS-CoV-2 spike protein for 24 h in Calu3 cells. F: Western blot images of GRP78 (anti-GRP78), SARS-CoV-2 spike protein (anti-Spike), and ACE2 (anti-C9) after the coimmunoprecipitation of HEK93T cells overexpressed with Flag-tagged SARS-CoV-2 spike protein and GRP78. G: Brief description of the DTSSP-mediated extracellular crosslinking protocol. H and I: Western blot images of GRP78 (anti-GRP78), SARS-CoV-2 spike protein (anti-His), and ACE2 (anti-ACE2 [H], anti-C9 [I]) after the coimmunoprecipitation of HEK293T cells overexpressed with Empty, C9-tagged ACE2, and/or GRP78 plasmids and treated with His-tagged SARS-CoV-2 spike protein for 1 h at 4°C. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Data and Resource Availability
The data sets and source data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
GRP78 Physically Interacts With the SARS-CoV-2 Spike Protein, Which Promotes Binding and Accumulation in ACE2-Expressing Cells
Viral infections begin with the binding of viral particles to host cell surface receptors. The spike protein of SARS-CoV-2, an envelope-protruding glycoprotein of the virus, is responsible for entry into a host cell by attaching to and fusing with the membrane of a host cell. First, we investigated whether GRP78 could physically interact with the SARS-CoV-2 spike protein. We overexpressed SARS-CoV-2 spike and human GRP78 proteins in HEK293T cells and then estimated the interaction by coimmunoprecipitation. The coexpression assays showed the physical binding of the SARS-CoV-2 spike protein with GRP78 (Fig. 1A). The expression of endogenous GRP78 protein is much lower than that of exogenously overexpressed GRP78, but it can still physically bind with SARS-CoV-2 spike protein (Fig. 1B).
Next, we evaluated whether GRP78 could act as an independent receptor or binding partner for ACE2. HEK293T cells were overexpressed with GRP78 and/or ACE2 and were then treated with the SARS-CoV-2 spike protein in the culture medium for 24 h, followed by immunoblotting to evaluate the cellular accumulation of the SARS-CoV-2 spike protein. Overexpression of ACE2, but not GRP78 alone, significantly accumulated SARS-CoV-2 spike protein compared with the empty vector control (Fig. 1C). Notably, the coexpression of ACE2 with GRP78 markedly enhanced the accumulation compared with that of ACE2 alone in HEK293T cells (Fig. 1C), and the level was dependent on GRP78 expression (Fig. 1D). We also assessed whether exogenously treated GRP78, as a mimetic condition of soluble GRP78 in circulation, could enhance the accumulation of SARS-CoV-2 spike protein in lung epithelial cells. The treatment of recombinant human GRP78 protein with Calu3 cells, a widely used ACE2-expressing lung epithelial cell line for SARS-CoV-2 infection, enhanced the cellular accumulation of SARS-CoV-2 spike protein in a dose-dependent manner (Fig. 1E).
Finally, we investigated whether GRP78 could form a protein complex with ACE2 and the SARS-CoV-2 spike protein in HEK293T cells. A coexpression assay showed the physical binding of ACE2 not only with the SARS-CoV-2 spike protein but also with GRP78 (Fig. 1F). Independent experiments with DTSSP-mediated extracellular crosslinking confirmed that exogenously treated SARS-CoV-2 spike protein physically interacts with cell surface GRP78 and ACE2 (Fig. 1G and H). The expression of GRP78 promoted the binding of the SARS-CoV-2 spike protein to ACE2 (Fig. 1I). The results suggest that cell surface GRP78 could facilitate the binding of SARS-CoV-2 spike protein to ACE2-expressing host cells.
GRP78 Is Highly Expressed in Adipose Tissue and Is Elevated With Increasing Age, Obesity, and Diabetes
Aging, obesity, and diabetes are major risk factors for the severe progression and outcomes of COVID-19, but the relationships with GRP78 expression have not yet been explored. To understand the expression profiles of GRP78, we analyzed transcriptome data sets of human and mouse tissues. The GRP78 gene was highly expressed in both human and mouse adipose tissue, and the level was much higher than that of the ACE2 gene (Fig. 2A and B). VAT showed significantly higher gene expression levels of GRP78 than SAT in both human and mouse adipose tissue (Fig. 2C and D). The gene expression of GRP78 was significantly increased with aging in human adipose tissue (Fig. 2E and Supplementary Fig. 1A); the inductions were more prominent in VAT (1.32-fold in 20–39 vs. 60–79 years old) than in SAT (1.21-fold in 20–39 vs. 60–79 years old). There were no significant sexually dimorphic differences in GRP78 gene expression in human adipose tissue (Supplementary Fig. 1B). GRP78 gene expression was significantly increased with increasing age in mouse adipose tissue (Fig. 2F). Obesity and diabetes were associated with increased GRP78 expression in both human and mouse adipose tissue (Fig. 1G–I).
GRP78 Is Upregulated by Hyperinsulinemia, Which Is Mediated by XBP-1s in Adipocytes
Next, we investigated the regulatory mechanism of GRP78 in adipocytes. Aging, obesity, and diabetes are known to be associated with elevated circulating insulin levels, called hyperinsulinemia (9,10). Neutralization of insulin by anti-insulin serum in vivo reduced the gene expression level of GRP78 in chicken adipose tissue (Fig. 3A). Chronic insulin exposure of 3T3-L1 adipocytes increased the gene expression level of GRP78, but TNF-α, an inflammatory cytokine, did not affect the expression level (Fig. 3B). Insulin treatment significantly increased the protein expression of GRP78 in a dose-dependent manner in 3T3-L1 adipocytes (Fig. 3C). Increasing glucose concentrations in the culture media from 5.5 mmol/L (99 mg/dL) to 25 mmol/L (450 mg/dL) had no effect on the protein expression of GRP78 (Supplementary Fig. 1C). The expression of GRP78 was correlated with the cellular stress-responsive transcription factor XBP-1s (Fig. 3D). The pharmacological inhibition of XBP-1 splicing, which blocks IRE1α-mediated XBP1 mRNA splicing, markedly reduced insulin-mediated XBP-1s protein expression and decreased GRP78 protein levels in 3T3-L1 adipocytes (Fig. 3D). Overexpression of XBP-1s alone was enough to increase the gene expression of GRP78 (Fig. 3E). ChIP-Seq analyses showed the direct binding of XBP-1 in the promoter regions of the human and mouse GRP78 genes spanning exon 1 (Fig. 3F). The gene expression of GRP78 was correlated with that of XBP-1 in human VAT (Fig. 3G and H, Supplementary Fig. 2A and B); similarly, correlations were observed in human SAT (Supplementary Fig. 2C–F). The correlation levels were more significant in the older age-group (60–79 years of age; Fig. 3H) than in the younger age-group (20–39 years of age; Fig. 3G). The expression of the XBP-1 and GRP78 genes was also correlated in the SAT of lean and obese subjects (Fig. 3I). A similar result was observed in the VAT of control subjects (normal glucose tolerant) and subjects with diabetes (Fig. 3J). Notably, increased gene expression of GRP78 during feeding was correlated with that of XBP-1 in human SAT (Fig. 3K), indicating the involvement of insulin. Furthermore, decreased GRP78 gene expression by acute insulin deprivation (anti-insulin serum) during feeding was significantly correlated with XBP-1 gene expression in chicken adipose tissue (Fig. 3L), suggesting a direct insulin effect on the correlative expression. These results suggested that hyperinsulinemia induced the overexpression of GRP78 in adipocytes, which was in part mediated by the stress-responsive transcription factor XBP-1s.
GRP78 Expression Can Be Managed by Pharmacological and Environmental Interventions in Adipose Tissue
We next estimated the potential management of GRP78 gene expression by generally prescribed and easily applicable interventions for hyperinsulinemia. There are various pharmacological drugs that control hyperinsulinemia, such as metformin, peroxisome proliferator–activated receptor γ (PPARγ) agonists, and SGLT2i (27). Metformin is the most widely prescribed antidiabetic drug. The administration significantly reduced the expression level of GRP78 in the SAT of human subjects (Fig. 4A). TZDs, such as pioglitazone and rosiglitazone, are antidiabetic drugs that activate adipose PPARγ action and enhance insulin sensitivity. Treatment with TZD did not affect the expression of GRP78 in the SAT of human subjects (Fig. 4B). SGLT2i are a new class of antidiabetic agents that block reabsorption of glucose in the kidney, thereby lowering circulating glucose and insulin levels. Treatment with an SGLT2i abrogated the induction of the GRP78 gene and protein expression in the adipose tissue of an obese diabetic mouse model (KKAY) (Fig. 4C and Supplementary Fig. 3A). Chronic activation of the β3-adrenalgic receptor by a specific agonist enhances the thermogenic action of adipose tissue and improves insulin sensitivity (28,29). Treatment with a β3-adrenalgic receptor agonist, CL316,243, reduced the expression of GRP78 in the adipose tissue of the mouse model (Fig. 4D).
Circulating insulin levels are closely related to lifestyle habits and can be handled by various environmental management practices (30). Exercise enhances the utilization of excess energy sources mainly in the muscle, thereby effectively reducing circulating glucose and insulin levels (31). Exercise intervention reduced the gene expression level of GRP78 in the adipose tissue of the mouse model (Fig. 4E). Calorie restriction inhibits excess energy intake, thereby lowering circulating glucose and insulin levels (32). The implementation of calorie restriction decreased GRP78 gene expression in the adipose tissue of the mouse model (Fig. 4F). Fasting enhances fat oxidation in adipose tissue and reduces circulating glucose and insulin levels (33). Fasting intervention decreased GRP78 gene and protein expression in the adipose tissue of the mouse model (Fig. 4G and Supplementary Fig. 3B). Cold exposure enhances catecholamine-associated thermogenic activation in adipose tissue, which enhances energy expenditure and insulin sensitivity (34). Cold acclimation reduced the gene expression level of GRP78 in the adipose tissue of the mouse model (Fig. 4H).
Discussion
In summary, we show that GRP78 physically interacts with the SARS-CoV-2 spike protein, which facilitates the binding to and accumulation in ACE2-expressing cells (Fig. 1). GRP78 is highly expressed in adipose tissue and is upregulated in patients with older age, obesity, and diabetes (Fig. 2). This overexpression is likely attributed to hyperinsulinemia and is in part mediated by the stress-responsive transcription factor XBP-1s (Fig. 3). Pharmacological or environmental interventions that are clinically feasible and easily applicable for the management of circulating insulin levels can effectively reduce the expression of GRP78 in adipose tissue (Fig. 4).
This research provides scientific evidence that cell surface GRP78 can act as a binding partner of the SARS-CoV-2 spike protein and ACE2 (Fig. 1), and the interactions might aggravate the binding and infection of SARS-CoV-2 to host cells. The roles of GRP78 as a host binding factor have been reported in other coronaviruses, including Middle East respiratory syndrome coronavirus and bat coronavirus HKU9, which also bind to spike proteins and promote attachment and entry (19,25). Similarly, other viruses, such as dengue virus, coxsackievirus A9, Japanese encephalitis virus, and Tembusu virus, utilize GRP78 as the host binding receptor or partner and facilitate entry into target cells (35–38). Of note, a recently published article from the Lee group (39) showed results very similar to those of our biochemical experiments that GRP78 directly forms a protein complex with spike protein and ACE2. They showed the possible stabilization of ACE2 by GRP78 on the cell surface; antibody-mediated reduction of cell surface GRP78 could attenuate the infection of SARS-CoV-2 pseudovirus in ACE2-expressing cells (39). Taken together, these findings suggest that cell surface GRP78 plays an important role in SARS-CoV-2 infection via physical interaction with the spike protein and ACE2 (Fig. 5).
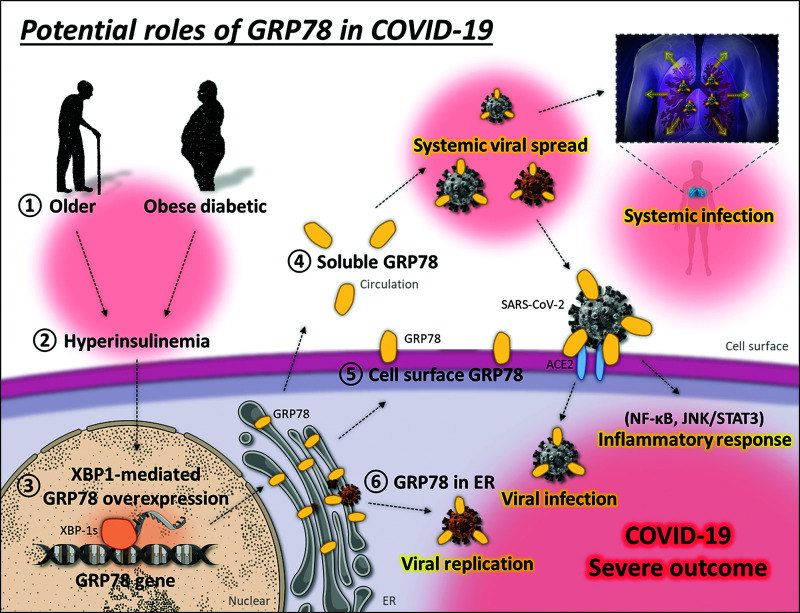
Figure 5.
Potential roles of GRP78 in COVID-19. In patients with older age, obesity, and diabetes (1), hyperinsulinemia (2) causes cellular stress and induces the XBP-1-mediated overexpression of GRP78 (3) in adipose tissue, which promotes the localization of GRP78 to the circulation (4) and cell surface (5), not only to ER (6). GRP78 physically interacts with SARS-CoV-2 spike protein, which might play a number of crucial roles in the viral lifecycle. The interaction of the spike protein with soluble and cell surface GRP78 might facilitate the binding and infection of SARS-CoV-2 to ACE2-expressing host cells. Soluble GRP78 attached to SARS-CoV-2 in circulation possibly induces the systemic viral spread and infection. Binding of SARS-CoV-2 to cell surface GRP78 or the related stimuli could activate the NF-κB or JNK/STAT3 transcriptional pathway and induce cellular inflammatory responses. SARS-CoV-2 potentially exploits ER-located GRP78 as a molecular chaperone to produce and assemble viral particles, which enables successful viral replication (brown-colored virus indicates newly replicated SARS-CoV-2). The high expression of GRP78 in patients with older age, obesity, and diabetes may contribute to the severe progression and outcome of COVID-19.
Aging, obesity, diabetes, and visceral adiposity are major risk factors for the severe progression and outcome of COVID-19. In this study, we showed that these risk factors are all associated with increased GRP78 expression in adipose tissue (Fig. 2). The observed hyperinsulinemia is likely attributable to the induction of GRP78, which is markedly correlated with the expression of XBP-1 (Fig. 3). GRP78 is found not only in cellular compartments but also in circulation and is also increased in patients with obesity and diabetes (40). GRP78 facilitates the entry of binding substances via the endocytosis pathway (41). Importantly, we found that soluble GRP78, a mimetic of the circulating form, can facilitate the accumulation of SARS-CoV-2 spike protein in lung epithelial cells in vitro (Fig. 1E). These results suggest that soluble GRP78 in circulation, possibly from adipose tissue, can bind to SARS-CoV-2 and potentially induce the systemic viral spreading, entry, and infection of ACE2-expressing cells (Fig. 5).
Although a preliminary study showed SARS-CoV-2 infection in human adipocytes (https://agencia.fapesp.br/adipose-tissue-may-be-a-reservoir-for-sars-cov-2-brazilian-researchers-suggest/33729/), it is still uncertain whether SARS-CoV-2 could infect the adipocytes of patients. Adipose tissue is widely distributed throughout the body not only in visceral (mesenteric/omental fat) and subcutaneous (subcutaneous fat) regions but also in parts of the lung (intrathoracic fat), pancreas (pancreatic fat), heart (epicardial fat), and kidney (perirenal fat). Because the virus can infect multiple organs beyond the respiratory tract, including the kidneys, heart, liver, brain, pancreas, and blood (42,43), SARS-CoV-2 also possibly invades, infects, and resides in the adipose tissue found in these organs. The COVID-19 pandemic is still a severe threat worldwide, and further clinical research is needed to address these points.
In the current study, we show that various insulin-sensitizing drugs (metformin, SGLT2i, and β3-adrenalgic receptor agonist) and environmental interventions (exercise, calorie restriction, fasting, and chronic cold exposure) can reduce the expression of GRP78 in adipose tissue (Fig. 4). These interventions are sometimes associated with weight loss, and this might also be an important factor for reduced GRP78 expression in adipose tissue. In that acute and direct deprivations of insulin (fasting and anti-insulin treatment in Fig. 3L and M) are enough to reduce the gene expression level of GRP78 with XBP-1 (Fig. 3L and M), we speculate that better insulin sensitivity or low insulin levels reduce GRP78 expression first, and then weight loss is likely to be accompanied by chronic or long-term pharmacological and environmental effects. TZDs had little effect on GRP78 expression in the human adipose tissue (Fig. 4B). The forced PPARγ activation might act as a stress for adipocytes and be associated with unchanged GRP78 expression in adipose tissues. TZDs still have great systemic insulin-sensitizing and protective effects for many other tissues and cells; thus, their potential therapeutic/preventative roles in COVID-19 need to be defined in future studies.
Viruses such as SARS-CoV-2 contain various viral proteins, such as spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins, used for their life cycle in the host. They exploit the molecular chaperones of host cells for the synthesis of these viral components, which is essential for viral replication (25,44,45). GRP78 is well known as a molecular chaperone for a wide range of proteins and plays a central role in viral protein synthesis and maturation (19). GRP78 plays an important role in the viral protein production, folding, and/or assembly of many viruses, including dengue virus, Japanese encephalitis virus, human cytomegalovirus, Ebola virus, and hepatitis B virus (37,46–50). The inhibition of GRP78 can disrupt the entry and/or production of these viruses (19). A recent study has shown that AR12 (OSU-03012), a potent inhibitor of PDK1/AKT signaling, reduces GRP78 expression and inhibits SARS-CoV-2 replication (22). During the course of evolution, viruses have somehow acquired the ability to utilize GRP78 functions not only for entry into host cells but also for the production and assembly of viral proteins facilitating viral replication (Fig. 5).
Exaggerated inflammatory responses are associated with the severe progression and outcome of COVID-19 patients (51). Proinflammatory transcription factors, including nuclear factor κB (NF-κB) and Janus kinase (JNK)/signal transducer and activator of transcription 3 (STAT3), play central roles in the regulation of inflammatory responses and are associated with immune cell activation and cytokine amplification in many pathophysiological conditions. Cell surface GRP78 has been shown to be associated with the activation of proinflammatory transcription factors (23). Cell surface GRP78 promotes NF-κB activation in endothelial cells and increases the expression levels of ICAM-1 and VCAM-1, accelerating the development of atherosclerotic lesions (52). JNK/STAT3 activation mediated by cell surface GRP78 increases the cell growth and migration of breast cancer (53). The binding of SARS-CoV-2 to GRP78 might induce the activation of proinflammatory transcription factors and possibly contribute to deleterious inflammatory responses, such as immune activation and cytokine amplification, in COVID-19 patients (Fig. 5).
Viral infection can selectively block host protein synthesis and hijack the host ER translational function to produce viral proteins in massive quantities (19). This causes ER overload and results in ER stress-mediated GRP78 overexpression, as shown in many cases of viral infections (19,46,50). The induction of GRP78 induction physiologically protects cells from apoptosis and exerts prosurvival effects under various stress conditions, but this ironically supports the long-lasting life cycle of viruses in the host cells (19). Cell surface GRP78 can also bind and stabilize ADAM17, which is known to enhance the entry of SARS-CoV by shedding ACE2 (54). A recent study showed that GRP78 is also involved in the egress of β-coronaviruses, including SARS-CoV-2, and is released together with viruses via the lysosomal exocytic pathway (21). These pathophysiological features of GRP78 might also contribute to the severe progression and outcome of COVID-19.
The spike protein of SARS-CoV-2 can bind its receptor, ACE2, and mediates membrane fusion and viral entry. In the current study, we utilized a recombinant spike protein of SARS-CoV-2 to analyze the binding and/or accumulation in ACE2-expressing mammalian cells. Interestingly, the spike protein, when treated in the conditioned medium yet without all other proteins and membrane structures from a virion, can somehow also be absorbed by the cells. Absorbance of spike protein is well described in previous SARS-CoV research that found the binding of spike protein to ACE2 induces translocation to cytoplasmic compartments (55). Similar results were observed in SARS-CoV-2 research for localization of spike protein both on the cell surface and in intracellular compartments (39). Mechanistically, binding of SARS-CoV-2 to ACE2 induces endocytosis in several ways, including clathrin-, caveola-, flotillin-, and micropinocytosis-dependent pathways (56). Biochemical assays utilizing the recombinant spike protein might be a useful and simple tool for analyzing the binding and endocytosis of SARS-COV-2, circumventing technical and safety issues of viral use.
Collectively, we offer a perspective on the possible involvement of adipose tissue in COVID-19. In patients with older age, obesity, and diabetes, hyperinsulinemia causes adipose cellular stress and induces the XBP-1-mediated overexpression of GRP78, which may increase its localization to the cell surface and circulation (23,24,39,40). The high expression of GRP78 might aggravate viral infection, spread, replication, and inflammatory response as a binding partner of the SARS-CoV-2 spike protein and ACE2, which possibly contributes to the severe progression and outcome of COVID-19 in patients with older age, obesity, and diabetes (Fig. 5). We hope this prospective study will aid in better understanding COVID-19 and emphasize that the management of hyperinsulinemia and the related GRP78 expression might be a therapeutic or preventative approach for COVID-19. It is also worth noting that GRP78 could be a target for COVID-19 drugs to disrupt multiple steps of the viral life cycle, and further clinical and experimental studies need to be done.
This study is based on scientific evidence of GRP78 protein interactions, expression profiles, and regulation in adipose tissue. The biochemical experiments employed overexpression experiments or treatments with high-dose (microgram) soluble proteins to investigate the interaction between GRP78 and the SARS-CoV-2 spike protein. Future studies are required to determine whether GRP78 is a critical host factor for COVID-19, especially in patients with older age, obesity, and diabetes, as well as the relationships with hyperinsulinemia. This study does not contradict or warn against insulin treatments for patients with diabetes but suggests the potential significance of chronic hyperinsulinemia and the related GRP78 expression in patients with older age, obesity, and diabetes with COVID-19.
Article Information
Acknowledgments. We would like to thank all the members of the Department of Metabolic Medicine, Graduate School of Medicine, Osaka University.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.S. designed the study and researched the data. J.S. and I.S. wrote the manuscript. S.T. and S.N. researched the data and reviewed the manuscript. A.F., S.K., and M.O. contributed to the discussion and reviewed the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.16700761.
This article is part of a special article collection available at https://diabetes.diabetesjournals.org/collection/diabetes-and-COVID19-articles.
References
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol 2020;16:341–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020;111:154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 6. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738 [DOI] [PubMed] [Google Scholar]
- 7. Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001;294:2166–2170 [DOI] [PubMed] [Google Scholar]
- 8. Kishida K, Funahashi T, Matsuzawa Y, Shimomura I. Visceral adiposity as a target for the management of the metabolic syndrome. Ann Med 2012;44:233–241 [DOI] [PubMed] [Google Scholar]
- 9. Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest 1983;71:1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 11. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–911 [DOI] [PubMed] [Google Scholar]
- 13. Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 14. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003;112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring) 2020;28:1187–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kruglikov IL, Shah M, Scherer PE. Obesity and diabetes as comorbidities for COVID-19: underlying mechanisms and the role of viral-bacterial interactions. eLife 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28:1191–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ha DP, Van Krieken R, Carlos AJ, Lee AS. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J Infect 2020;81:452–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect 2020;80:554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghosh S, Dellibovi-Ragheb TA, Kerviel A, et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 2020;183:1520–1535.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rayner JO, Roberts RA, Kim J, et al. AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochem Pharmacol 2020;182:114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gopal U, Pizzo SV. Cell surface GRP78 signaling: an emerging role as a transcriptional modulator in cancer. J Cell Physiol 2020; 236:2352–2363 [DOI] [PubMed] [Google Scholar]
- 24. Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005;35:373–381 [DOI] [PubMed] [Google Scholar]
- 25. Chu H, Chan CM, Zhang X, et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J Biol Chem 2018;293:11709–11726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishitani S, Fukuhara A, Shin J, Okuno Y, Otsuki M, Shimomura I. Metabolomic and microarray analyses of adipose tissue of dapagliflozin-treated mice, and effects of 3-hydroxybutyrate on induction of adiponectin in adipocytes. Sci Rep 2018;8:8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerru N, Singh-Pillay A, Awolade P, Singh P. Current anti-diabetic agents and their molecular targets: a review. Eur J Med Chem 2018;152:436–488 [DOI] [PubMed] [Google Scholar]
- 28. O’Mara AE, Johnson JW, Linderman JD, et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest 2020;130:2209–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finlin BS, Memetimin H, Zhu B, et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 2020;130:2319–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McAuley KA, Williams SM, Mann JI, et al. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes Care 2002;25:445–452 [DOI] [PubMed] [Google Scholar]
- 31. Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998;49:235–261 [DOI] [PubMed] [Google Scholar]
- 32. Weiss EP, Racette SB, Villareal DT, et al.; Washington University School of Medicine CALERIE Group . Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006;84:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 2019;381:2541–2551 [DOI] [PubMed] [Google Scholar]
- 34. Hanssen MJ, Hoeks J, Brans B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015;21:863–865 [DOI] [PubMed] [Google Scholar]
- 35. Cabrera-Hernandez A, Thepparit C, Suksanpaisan L, Smith DR. Dengue virus entry into liver (HepG2) cells is independent of hsp90 and hsp70. J Med Virol 2007;79:386–392 [DOI] [PubMed] [Google Scholar]
- 36. Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol 2002;76:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nain M, Mukherjee S, Karmakar SP, et al. GRP78 is an important host factor for Japanese encephalitis virus entry and replication in mammalian cells. J Virol 2017;91:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao D, Liu Q, Han K, et al. Identification of glucose-regulated protein 78 (GRP78) as a receptor in BHK-21 cells for duck Tembusu virus infection. Front Microbiol 2018;9:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carlos AJ, Ha DP, Yeh DW, et al. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem 2021;296:100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Girona J, Rodríguez-Borjabad C, Ibarretxe D, et al. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merkel A, Chen Y, George A. Endocytic trafficking of DMP1 and GRP78 complex facilitates osteogenic differentiation of human periodontal ligament stem cells. Front Physiol 2019;10:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020;383:590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 2021;3:149–165 [DOI] [PubMed] [Google Scholar]
- 44. Chan CP, Siu KL, Chin KT, Yuen KY, Zheng B, Jin DY. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2006;80:9279–9287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Booth L, Roberts JL, Ecroyd H, et al. AR-12 inhibits multiple chaperones concomitant with stimulating autophagosome formation collectively preventing virus replication. J Cell Physiol 2016;231:2286–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buchkovich NJ, Maguire TG, Yu Y, Paton AW, Paton JC, Alwine JC. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J Virol 2008;82:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buchkovich NJ, Maguire TG, Paton AW, Paton JC, Alwine JC. The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment. J Virol 2009;83:11421–11428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014;109:171–174 [DOI] [PubMed] [Google Scholar]
- 49. Cho DY, Yang GH, Ryu CJ, Hong HJ. Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo. J Virol 2003;77:2784–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wati S, Soo ML, Zilm P, et al. Dengue virus infection induces upregulation of GRP78, which acts to chaperone viral antigen production. J Virol 2009;83:12871–12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ong EZ, Chan YFZ, Leong WY, et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe 2020;27:879–882.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crane ED, Al-Hashimi AA, Chen J, et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI Insight 2018;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yao X, Liu H, Zhang X, et al. Cell surface GRP78 accelerated breast cancer cell proliferation and migration by activating STAT3. PLoS One 2015;10:e0125634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA 2008;105:7809–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 2008;18:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Glebov OO. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J 2020;287:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]