Abstract
Meteorological parameters, have been identified as an important factor involved in the transmission of vector-borne diseases. Mosquitoes are extremely sensitive to weather conditions. The aim of this study was investigate the correlation between meteorological parameters and the abundance of mosquitoes in Kashan County. Mosquitoes were collected using four different traps, including hand catch, animal baited bed net trap (usually a cow), human baited bed net trap and BG-Sentinel trap with CO2 from May to December 2019. A total number of mosquitoes collected were 1756 out of which 1621 (92.31%) were Culex, 22 (1.25%) Culiseta and 113 (6.44%) Anopheles in nine species. Most mosquitoes were collected by BG-Sentinel trap with CO2 (63.78%). Monthly distribution of the mosquitoes indicated different monthly peaks. Their high density were recorded in September and were low in December. The spearman's correlation of the mosquito abundance and the meteorological parameters shows that correlation of the number of total collected mosquitoes with relative humidity and precipitation (Rainfall) was weak negative, and there was week correlation with wind speed, and positive strong correlation with temperature. Data collected with various trap types and mosquito correlation with meteorological parameters in this study can be used for mosquito surveillance and control programs. However, meteorological parameters affect the abundance of mosquitoes, but their impact is complex and most of these variables are species specific.
Keywords: Abundance, Mosquito, Rainfall, Temperature
Highlights
-
•
Populations of mosquitoes were larger during the dry months.
-
•
The mean monthly temperature below 30 °C was most suitable for mosquitoes.
-
•
There was a non-significant relationship between wind speed and mosquitoes.
Abundance; Mosquito; Rainfall; Temperature.
1. Introduction
Emerging and re-emerging diseases are a great threat to human and animal health (Feldmann et al., 2002). Urbanization, increases in populations, degradation of the natural environment and international trade and trave has been caused that arthropod vectors of disease spread from tropical areas into temperate areas (Rogers and Packer, 1993). Climate through the effect on the time and intensity of vector-borne diseases, affects their distribution (Epstein et al., 1998; Gubler et al., 2001). Pathogens of malaria, filariasis, Zika, Chikungunya diseases, West Nile and Dengue fever were transmitted by mosquitoes to humans (Gould and Higgs, 2009; Gasperi et al., 2012). Anopheles mosquito species are responsible for the transmission of malaria, but the majority of mosquito species from the genera of Culex and Aedes are responsible for the transmission of arboviruses to humans (Tabachnick, 1991). Climate change will have short- and long-term impacts on disease transmission and a direct impact on the epidemiology of vector-borne diseases (Githeko et al., 2000).
It is commonly said that the abundance of mosquitoes is related to climate (Watson et al., 1996, 1998). Immature stages in the aquatic environment and as adults of mosquitoes are extremely sensitive to weather conditions and therefore there are concerns about mosquito-borne disease (Epstein, 2000). In warmer climates and when the water temperature rises, larval to mature is short, adult female mosquitoes are blood feeding more frequently, thus increase pathogen transmission (Githeko et al., 2000).
The main weather factors are the temperature, relative humidity and precipitation (Almeida Costa et al., 2010; Khan et al., 2018). The quality and quantity of mosquito breeding sites are affected by precipitation (Tian et al., 2015; Jemal and Al-Thukair, 2018).
Temperature and precipitation, limit distribution of mosquitoes and range of diseases transmitted by them because the biology of the vector is affected (Epstein et al., 1998; Gubler et al., 2001). To detect, and monitor mosquitoes, surveillance programs are required (Paphitou et al., 2017). Global warming have significant effects on vector-borne diseases. Biology of mosquitoes is very diverse and climatic variability is led to increased variability in mosquito communities and diseases transmitted by them (Rogers and Packer, 1993; Subak, 2003). Climatic changes may affect longevity, activity and spread of vector mosquitoes (Roiz et al., 2014; Almeida Costa et al., 2010). A higher temperature increase the replication and spread of arboviruses (Kilpatrick et al., 2008), as a result, the time of mosquito infection to transmission of the virus to humans is shortened (Takahashi, 1976). Moreover, higher temperatures change human behavior via increase outdoor activities and humans and mosquitoes will be more in contact (Konno, 1969; Bi et al., 2007). Environmental changes cause to alter interactions among vectors, reservoirs, pathogens, and human (Institute of Medicine, 2003). As a result, certain vector-borne diseases may increase in one area and decrease in another (Weicheld, 2015).
Temperature affects the hatching time of mosquito eggs (Hawley et al., 1989; Hanson and Craig, 1994; Impoinvil et al., 2007; Mohammed and Chadee, 2011). Temperature stress during the stages of larvae and pupae have carry-over effects on fecundity and survival of adults (Ezeakacha and Yee, 2019). Temperature also has a direct impact on mosquito adult stage. When temperature decreased, female longevity of Culex spp. increased (Ciota et al., 2014). Drakou et al. (2020) reported that temperature affects host searching activity of Cx. pipiens, Ae. detritus and Ae. caspius population.
Humidity affects longevity, blood feeding behavior, mating, dispersal and oviposition of mosquitoes. The results one study to examine risk factors in the Japanese encephalitis (JE) transmission, indicated that monthly mean maximum and minimum temperatures, rainfall, humidity were positively related to mosquito density in Changsha in South China (Tian et al., 2015). Therefore climatic factors and their relationship with mosquito activity are valuable for surveillance programs and mosquito control management.
Depending on the classification of the tribe Aedini, there are 70 species and 8 (or 12) genera of Iranian mosquitoes (Azari-Hamidian et al., 2020). Mosquitoes transmit two protozoa, two bacteria, four filaria, and seven arbovirus in Iran (Azari-Hamidian et al., 2019; Pouriayevali et al., 2019). Malaria is a major endemic infectious disease in Iran. Anopheles species is responsible for the transmission of malaria and seven Anopheles species have been reported as vectors of malaria in Iran (Vatandoost et al., 2019). Anopheles superpictus sensu lato (s.l.) is most abundant and distributed species among Anopheles in Kashan County (Asgarian et al., 2021). This research aims to provide insight into the impact of climatic variables on native adult mosquito abundance and activity in Kashan County.
2. Materials and methods
2.1. Study area
Mosquitoes were collected throughout Kashan County (33.9850° N, 51.4100° E). Kashan is located in the north of Isfahan Province, central Iran, includes four districts, Central, Qamsar, Niasar, and Barzok Districts. Due to the impact of the central desert of Iran on Kashan County, it has a dry climate. The average high temperature is approximately 26.2 °C and low average is about 12.1 °C. The average low relative humidity is 27% and average high is 56.4 %. Kashan rainfall is Mediterranean, so Kashan rainfall season corresponds to the cold season and early spring and its dry season coincides with the summer season. One of the effective factors in Kashan climate is local winds that often blow from the desert towards Kashan (I.R. of Iran Meteorological Organization, 2014).
Meteorological data of various climatic variables, including precipitation, relative humidity (mean, maximum and minimum), wind speed and temperature (mean, maximum, minimum) during the study period, and daily weather conditions for our study were procured from the records of Kashan meteorological organization.
This research was approved by the Ethical Committee of Tehran University of Medical Sciences (TUMS) (No.: IR.TUMS.VCR.REC.1397.1001).
2.2. Mosquito collection
Mosquitoes were collected using four different traps, including Hand Catch (HC), Animal Baited Bed Net Trap (ABBNT) (usually a cow), Human Baited Bed Net Trap (HBBNT), and BG-Sentinel Trap (BG) with CO2 from May to December 2019. In Hand Catch method, mosquitoes resting on indoor and outdoor surfaces were collected by using mouth- or battery-powered aspirators.
Collected mosquitoes were transmitted to the lab and placed in a freezer -20 °C for at least 20 min to die. Mosquitoes were identified based on a valid diagnostic key to Iran at the species level (Azari-Hamidian and Harbach, 2009). Mosquito trapping sites were selected based on observed mosquito activity, access to trapping site, private property and risk of trap vandalism or theft. Locations for trapping sites were determined using Global Positioning System (GPS). Arch Map 10.5 software was used to create a geographic database of adult mosquito sampling sites and mapping the sites of collection and distribution of the most important species of medicine (Figure 1).
Figure 1.
Adult mosquito sampling sites in Kashan County (2019).
Mosquito larvae were collected using a standard 350 ml capacity mosquito dipper from April to late December 2019. Larvae were transparent in lactophenol and individually mounted in Berlese's fluid on a microscope slide and identified by morphological characters and valid keys (Shahgudian, 1960; Zaim and Cranston, 1986; Azari-Hamidian and Harbach, 2009) Some mosquitoes specimens were deposited in the museum of medical entomology, TUMS.
2.3. Statistical analysis
The relationships of temperature, relative humidity, wind speed and precipitation (Rainfall), with abundance of adult mosquitoes were investigated on monthly by using Spearman's correlation method. The data were analyzed in IBM SPSS Statistics 26. P < 0.05 were considered as statistically significant.
3. Results
3.1. Mosquito collection results
A total number of adults of mosquitoes collected using various traps from May to December 2019 were 1756 out of which 1621 (92.31%) were Culex, 22 (1.25%) Culiseta and 113 (6.44%) Anopheles in nine species including two of the genus Anopheles, six Culex, and one Culiseta. Overall, Cx. pipiens (57.63%) was the predominant species followed by Cx. theileri (25.17%) and An. superpictus s. l. (6.15%). Most mosquitoes were collected by BG- Sentinel trap with CO2 (63.78%) and Cx. pipiens (65.44%) was the most abundant species in this trap (Table 1).
Table 1.
The collected mosquito species by varius traps, Kashan County, central Iran, 2019.
| Species | Hand Catch |
Human Baited Bed Net Trap |
Animal Baited Bed Net Trap |
BG-Sentinel Trap |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| An. maculipennis s. l. | 2 | 1.04 | 1 | 0.26 | 0 | 0 | 2 | 0.18 | 5 | 0.29 |
| An. superpictus s. l. | 3 | 1.56 | 30 | 7.92 | 0 | 0 | 75 | 6.70 | 108 | 6.15 |
| Cs. longiareolata | 22 | 11.45 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 1.25 |
| Cx. deserticola | 12 | 6.25 | 0 | 0 | 0 | 0 | 46 | 4.11 | 58 | 3.30 |
| Cx. hortensis | 13 | 6.77 | 0 | 0 | 0 | 0 | 19 | 1.70 | 32 | 1.82 |
| Cx. mimeticus | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 2.05 | 23 | 1.31 |
| Cx. perexiguus | 30 | 15.63 | 0 | 0 | 0 | 0 | 24 | 2.14 | 54 | 3.08 |
| Cx. pipiens | 45 | 23.44 | 219 | 57.78 | 15 | 23.08 | 733 | 65.44 | 1012 | 57.63 |
| Cx. theileri | 65 | 33.86 | 129 | 34.04 | 50 | 76.92 | 198 | 17.68 | 442 | 25.17 |
| Total | 192 | 100 | 379 | 100 | 65 | 100 | 1120 | 100 | 1756 | 100 |
A total of 9789 larvae were collected from urban and, rural areas of four districts of Central, Qamsar, Niasar, and Barzok in Kashan County, which included 13 species from Anopheles 772 (7.89%), Culiseta 1706 (17.42%) and Culex 7311 (74.69%). Culex pipiens had the highest abundance with 3658 (37.36%). We have already reported the results of larval sampling (Asgarian et al., 2021).
Culex pipiens is distributed in many areas of the country. Culex quinquefasciatus is found in southern areas of Iran to Yazd County in central Iran, and reported as sympatric with Cx. pipiens in the central regions. Sequencing alignment of Ace.2 gene of Cx. quinquefasciatus and Cx. pipiens showed 6.5% variation in 46 bp, especially in the intron locus of gene. Culex pipiens complex from Iran are located in two separate clades with sister branches using a phylogenetic sequencing tree (Dehghan et al., 2013).
3.2. Influence of weather on mosquito abundance
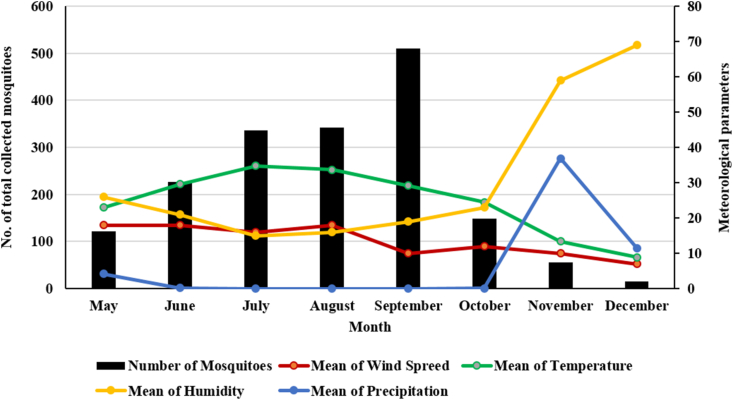
Monthly distribution of the adult of mosquitoes from May to December 2019 indicated different monthly peaks. Their highest density was recorded in September and the lowest was in December (Table 2). Average of monthly temperature was decreased from 33.7 °C in August to 29.2 °C in September which was coincided with a period of low wind speed (Figure 2).
Table 2.
Monthly collection of different adults of mosquito genera, Kashan County, central Iran, 2019.
| Month | Anopheles | Culex | Culiseta | Total |
|---|---|---|---|---|
| May | 0 | 122 | 0 | 122 |
| Jun | 11 | 216 | 0 | 227 |
| Jul | 12 | 322 | 2 | 336 |
| Aug | 28 | 308 | 6 | 342 |
| Sep | 35 | 468 | 7 | 510 |
| Oct | 22 | 122 | 5 | 149 |
| Nov | 5 | 48 | 2 | 55 |
| Dec | 0 | 15 | 0 | 15 |
| Total | 113 | 1621 | 22 | 1756 |
Figure 2.
The collected mosquito adults and meteorological variables by month, Kashan County, central Iran, 2019.
The spearman's correlation of the abundance of adult mosquitoes and the meteorological parameters showed that there was a strong negative and significant relationship between Anopheles abundance with relative humidity (−0.74) and rainfall (−0.84), while its relationship with temperature was a positive correlation but not statistically significant, and it was no correlation with wind speed. A strong positive correlation and significant was observed between mosquito Culex with temperature (0.85), while its relationship with relative humidity and rainfall was a negative relationship and significant. Also a weak positive correlation was observed between Culex and wind speed (0.35), but the relationship was not significant. Culiseta had negative correlation with the relative humidity, rainfall and wind speed, whereas, its relationship was positive with temperature, but not significant (Table 3).
Table 3.
The spearman's correlation values between adult mosquito abundance and climatic variables, Kashan County, central Iran, 2019.
| Parameters |
Anopheles |
Culex |
Culiseta |
|||
|---|---|---|---|---|---|---|
| r- value | p-value∗ | r- value | p-value∗ | r- value | p-value∗ | |
| Temperature | 0.64 | 0.08 | 0.85 | 0.00 | 0.39 | 0.33 |
| Relative humidity | -0.74 | 0.03 | -0.92 | 0.00 | -0.54 | 0.16 |
| Wind speed | 0.08 | 0.83 | 0.35 | 0.38 | -0.13 | 0.75 |
| Precipitation | -0.84 | 0.00 | -0.90 | 0.00 | -0.67 | 0.06 |
R = 1.0–0.9 (Very Strong Correlation), r = 0.89–0.7 (Strong Correlation), r = 0.69–0.4 (Moderate Correlation), r = 0.39–0.1 (Weak Correlation), ∗p > 0.05 (Non-significant); P < 0.05 (significant).
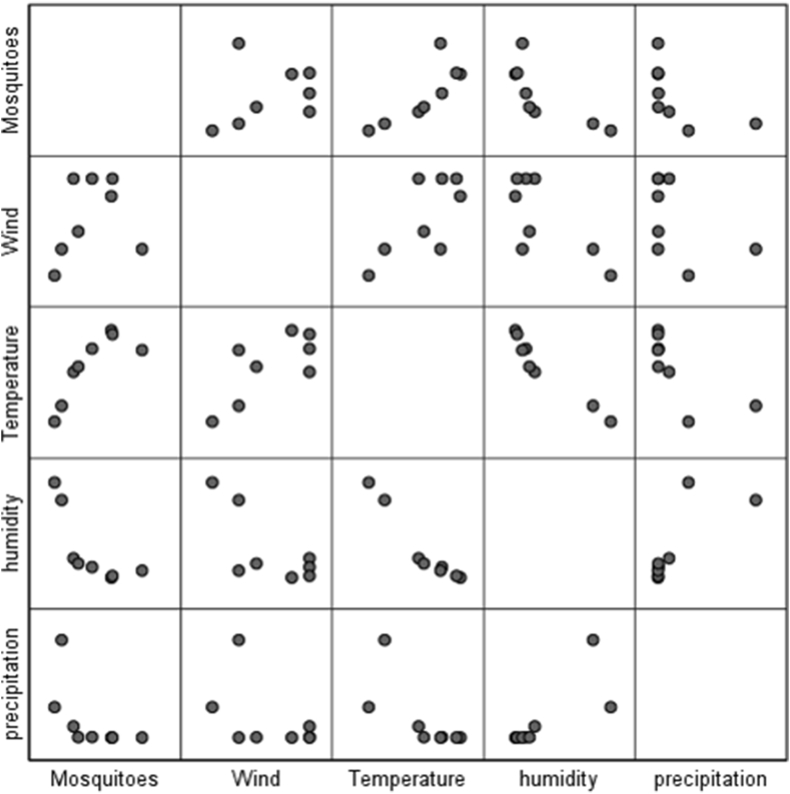
Generally, our results showed that correlation of the number of total collected mosquitoes with relative humidity and precipitation (Rainfall) was weak negative, and there was week correlation with wind speed, and positive strong correlation with temperature (Figure 3).
Figure 3.
Scatter plot to show correlation of meteorological parameters with number of total collected mosquitoes, Kashan County, central Iran, 2019.
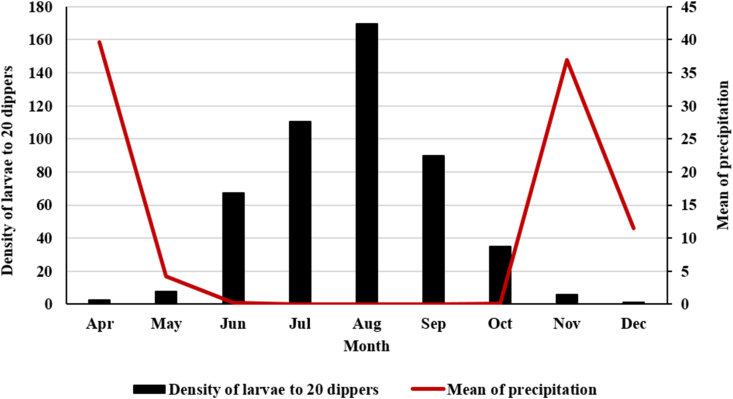
Most larvae were caught in dry months. In August, the larval habitats of the mosquitoes were in the best conditions to maintain the mosquito population, so the number of larvae caught in August was higher. The collected larvae and precipitation have been shown by month in Figure 4.
Figure 4.
The collected mosquito larvae and mean of precipitation by month, Kashan County, central Iran, 2019.
4. Discussion
Abundance and association of mosquitoes with the meteorological parameters has been studied in some countries and meteorological parameters, have been identified as an important factor involved in the transmission of vector-borne diseases (Watts et al., 1987; Ram et al., 1998; Wu et al., 2007). Temperature, relative humidity, wind speed and precipitation affect on mosquito survival, abundance and distribution.
Our findings indicate that mosquito abundance was low in temperatures above 30 °C, and was consistent with the results of other researchers. Ciota et al., (2014) demonstrate that temperature significantly affect larvae and adult survival, mosquito size, blood feeding, and fecundity of populations of the Culex mosquitoes and temperatures greater than 30 °C increased mosquito mortality (Ciota et al., 2014). Researchers in eastern province of Saudi Arabia found a positive correlation between mosquitoes with relative humidity and rainfall but observed negative correlation with temperature (Jemal and Al-Thukair, 2018). Roiz et al. (2014) highlighted the climate-mosquito abundance relationship are complex and these effects are non-linear. In Ekiti State, Nigeria, temperature and Rainfall had a significant effect on the distribution and abundance of the mosquito vectors (Simon-Oke and Olofintoye, 2015). In Jeddah, Saudi Arabia monthly distribution of Culex, Aedes and Anopheles during 2016 and 2017 had different seasonal peaks. The average temperature below 30 °C was suitable for adult mosquito abundance in the area of study. Correlation of Culex, and Anopheles abundance with relative humidity, temperature and rainfall was not significant. Aedes had a moderately positive correlation, and not statistically significant with temperature and relative humidity. There was a moderately positive correlation between dengue fever mosquito Aedes with wind speed, but negative with Aedes and Anopheles (Khan et al., 2018).
In our study during June to August and also October, the maximum temperature on some days was over 40 °C that can be lethal for the mosquitoes. The mean monthly temperature below 30 °C (29.2 °C) in September was most suitable for adult mosquito survival and abundance, which seem to be more suitable for their host searching activity. In September, some larval habitats in Central and Niasar Districts were dried, reducing the population of adult mosquitoes in October.
Bashar and Tuno (2014) studied the relationship between climatic factors and Anopheles mosquito abundance in Bangladesh. They reported a positive association between relative humidity with mosquito abundance, but there was no significant correlation with temperature and rainfall. In an endemic village near the Malaysia-Thailand border was not found any correlation between rainfall and Anopheles mosquitoes (Rahman et al., 1993).
The precipitation affects mosquito populations. Precipitation creates breeding sites for mosquitoes and larval development. On the other hand, high precipitation may decrease mosquito populations. In our study, climate difference was caused by differences in the density patterns of mosquitoes, so that populations of mosquitoes were larger during the dry months.
A significant negative relationship was observed between monthly relative humidity and number of sampled Anopheles and Culex, but relationship with Culiseta was not significant. In April, November and December, rainfall increased, due to floods and rapid water currents, mosquito larval habitats were destroyed, reducing the abundance of adult mosquitoes, especially in the case of An. superpictus s. l., An. maculipennis s. l., Cx. deserticola, Cx. hortensis, Cx. mimeticus, and Cx. perexiguus species whose their larval habitats is on the banks of calm flowing rivers. Also, the average monthly temperature in November and December was 13.4 and 8.9 °C, respectively, so low temperature also reduced the abundance of mosquitoes. Densities of Culex spp. especially Cx. pipiens were highest in July–September. Drought conditions may be a benefit for Cx. pipiens because of the concentration of organic matter in urban basins and ponds (Epstein, 2001).
Culex pipiens prefers to lay its eggs in containers rich in organic matter, heavy rainfall cause that these containers overflow or the amount of their organic matter decrease and make them less suitable for Cx. pipiens (Weicheld, 2015). Increasing the temperature, to some extent, increases the development, reproduction and abundance of mosquito populations. Culex pipiens maintained at warmer temperatures were more infected with West Nile virus than mosquitoes maintained at cooler temperatures (Dohm et al., 2002). The initial 1999 outbreak of West Nile virus in New York was due to warm winters followed by hot, dry summers. In later years with similar climatic conditions, there was a spread of the disease (Epstein, 2001).
In study of Nejati et al. (2020) in borderline of Iran and Pakistan, wind speed and temperature had a direct significant correlation with the total number of collected mosquito while there was a non-significant positive relationship between wind speed and Anopheles and Culex in our study.
In addition to meteorological parameters and their effect on mosquito-borne diseases, human activities and behavior also play an important role in the transmission of these diseases (Gubler et al., 2001). In Kashan County changes in living conditions, land leveling, destruction of lowlands and highlands, improvement of streams and cementation of streams is major factors in the disappearance of malaria, although climate-related natural disasters such as floods may increase or change human contact with mosquitoes. Vector-borne pathogens may be affected by climate change. More research is needed to better understand the relationships of these pathogens and their vectors and climatic variables.
5. Conclusion
Study of weather conditions and its effect on mosquito community dynamics and disease transmission is important for performancing effective surveillance and control programs (Bradford, 2005). Global warming can change pattern of rainfall and temperature and directly or indirectly affects larval habitats and the abundance of mosquitoes. Data collected with various trap types and mosquito correlation with meteorological parameters in this study can be usful for mosquito surveillance and control programs. In Kashan County, some mosquito species have the importance of health due to human-blood sucking. Because of the wide range of climates and altitudes (from 942 m in Central District to 2044 m in Barzok District) of Kashan County, monitoring and recording meteorological parameters are necessary to aware of the possibility of growing conditions new species and climate changes that are suitable for invasive species, especially Aedes aegypti and Ae. albopictus. However, meteorological parameters affect the abundance of mosquitoes, but their impact is complex and most of these variables are species specific (Roiz et al., 2014). Geographic Information Systems (GIS), and monitoring mosquito behavior in relation to environmental and climatic factors is needed for increasing our knowledge to understand these relationships.
Declarations
Author contribution statement
Seyed Hassan Moosa-Kazemi, Mohammad Mehdi Sedaghat: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tahereh Sadat Asgarian: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Tehran University of Medical Sciences (TUMS).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are grateful to Dr SM Asgarian for helping with field collections.
Contributor Information
Seyed Hassan Moosa-Kazemi, Email: moosakazemi@tums.ac.ir.
Mohammad Mehdi Sedaghat, Email: sedaghat@hotmail.co.uk.
References
- Almeida Costa E.A.P., Mendonça Santos E.M., Correia J.C., Albuquerque C.M.R. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev. Bras. Entomol. 2010;54(3):488–493. [Google Scholar]
- Asgarian T.S., Moosa-Kazemi S.H., Sedaghat M.M., Dehghani R., Yaghoobi-Ershadi M.R. Fauna and larval habitat characteristics of mosquitoes (Diptera: Culicidae) in Kashan County, central Iran. J. Arthropod. Borne Dis. 2021;15(1):69–81. doi: 10.18502/jad.v15i1.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari-Hamidian S., Abai M.R., Norouzi B. Mansonia uniformis (Diptera: Culicidae), a genus and species new to southwestern Asia, with a review of its medical and veterinary importance. Zootaxa. 2020;4772(2):385–395. doi: 10.11646/zootaxa.4772.2.10. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S., Harbach R.E. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae) Zootaxa. 2009;2078(1):1–33. [Google Scholar]
- Azari-Hamidian S., Norouzi B., Harbach R.E. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019;194:106–122. doi: 10.1016/j.actatropica.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Bashar K., Tuno N. Seasonal abundance of Anopheles mosquitoes and their association with meteorological factors and malaria incidence in Bangladesh. Parasites Vectors. 2014;7(1):1–10. doi: 10.1186/1756-3305-7-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi P., Zhang Y., Parton K.A. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J. Infect. 2007;55(6):551–556. doi: 10.1016/j.jinf.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bradford C.M. Texas Tech University; 2005. Effects of Weather on Mosquito Biology, Behavior, and Potential for West Nile Virus Transmission on the Southern High plains of Texas. [Google Scholar]
- Ciota A.T., Matacchiero A.C., Kilpatrick A.M., Kramer L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014;51(1):55–62. doi: 10.1603/me13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan H., Sadraei J., Moosa-Kazemi S.H., Akbari Baniani N., Nowruzi F. The molecular and morphological variations of Culex pipiens complex (Diptera: Culicidae) in Iran. J. Vector Borne Dis. 2013;50(2):111–120. [PubMed] [Google Scholar]
- Dohm D.J., O'Guinn M.L., Turell M.J. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2002;39(1):221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Drakou K., Nikolaou T., Vasquez M., Petric D., Michaelakis A., Kapranas A., Papatheodoulou A., Koliou M. The effect of weather variables on mosquito activity: a snapshot of the main point of entry of Cyprus. Int. J. Environ. Res. Publ. Health. 2020;17(4):1403. doi: 10.3390/ijerph17041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P.R. Is global warming harmful to health? Sci. Am. 2000;283(2):50–57. doi: 10.1038/scientificamerican0800-50. [DOI] [PubMed] [Google Scholar]
- Epstein P.R. West Nile virus and the climate. J. Urban Health. 2001;78(2):367–371. doi: 10.1093/jurban/78.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P.R., Diaz H.F., Elias S., Grabherr G., Graham N.E., Martens W.J., MosIey-Thompson E., Susskind J. Biological and physical signs of climate change: focus on mosquito-borne diseases. Bull. Am. Meteorol. Soc. 1998;79(3):409–418. [Google Scholar]
- Ezeakacha N.F., Yee D.A. The role of temperature in affecting carryover effects and larval competition in the globally invasive mosquito Aedes albopictus. Parasites Vectors. 2019;12:123. doi: 10.1186/s13071-019-3391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Czub M., Jones S., Dick D., Garbutt M., Grolla A., Artsob H. Emerging and re-emerging infectious diseases. Med. Microbiol. Immunol. 2002;191(2):63–74. doi: 10.1007/s00430-002-0122-5. [DOI] [PubMed] [Google Scholar]
- Gasperi G., Bellini R., Malacrida A.R., Crisanti A., Dottori M., Aksoy S. A new threat looming over the Mediterranean basin: emergence of viral diseases transmitted by Aedes albopictus mosquitoes. PLoS Neglected Trop. Dis. 2012;6(9) doi: 10.1371/journal.pntd.0001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko A.K., Lindsay S.W., Confalonieri U.E., Patz J.A. Climate change and vector-borne diseases: a regional analysis. Bull. World Health Organ. 2000;78(9):1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Gould E.A., Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009;103(2):109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J., Reiter P., Ebi K.L., Yap W., Nasci R., Patz J.A. Climate variability and change in the United States: potential impacts on vector-and rodent-borne diseases. Environ. Health Perspect. 2001;109(suppl 2):223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S.M., Craig G.B., Jr. Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 1994;31:192–201. doi: 10.1093/jmedent/31.2.192. [DOI] [PubMed] [Google Scholar]
- Hawley W.A., Pumpuni C.B., Brady R.H., Craig G.B., Jr. Overwintering survival of Aedes albopictus (Diptera: Culicidae) eggs in Indiana. J. Med. Entomol. 1989;26:122–129. doi: 10.1093/jmedent/26.2.122. [DOI] [PubMed] [Google Scholar]
- Impoinvil D.E., Cardenas G.A., Gihture J.I., Mbogo C.M., Beier J.C. Constant temperature and time period effects on Anopheles gambiae egg hatching. J. Am. Mosq. Control Assoc. 2007;23:124–130. doi: 10.2987/8756-971x(2007)23[124:ctatpe]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . National Academy of Sciences; Washington DC: 2003. Microbial Threats to Health: Emergence, Detection, and Response. [PubMed] [Google Scholar]
- I.R. of Iran Meteorological Organization . 2014. Climate Profile of Kashan County.http://www.kashanmet.ir/ Available from. [Google Scholar]
- Jemal Y., Al-Thukair A.A. Combining GIS application and climatic factors for mosquito control in Eastern Province, Saudi Arabia. Saudi. J. Biol. 2018;25(8):1593–1602. doi: 10.1016/j.sjbs.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Elhossary S., Khan I.A., Al Zahrani M.H., Al Zahrani F.S., Al Bashri F.M. The impact of climatic variables with GIS application on the abundance of medically important mosquitoes (Diptera: Culicidae) in Jeddah, Saudi Arabia. Int. J. Mosq. Res. 2018;5(5):12–18. [Google Scholar]
- Kilpatrick A.M., Meola M.A., Moudy R.M., Kramer L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4(6) doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno J. Cyclic outbreak of Japanese encephalitis among pigs and humans. Am. J. Epidemiol. 1969;10(2):132–133. doi: 10.1093/oxfordjournals.aje.a120643. [DOI] [PubMed] [Google Scholar]
- Nejati J., Zaim M., Vatandoost H., Moosa-Kazemi S.H., Bueno-Marí R., Azari-Hamidian S., Sedaghat M.M., Hanafi-Bojd A.A., Yaghoobi-Ershadi M.R., Okati-Aliabad H., Collantes F. Employing different traps for collection of mosquitoes and detection of dengue, Chikungunya and Zika vector, Aedes albopictus, in borderline of Iran and Pakistan. J. Arthropod-Borne Dis. 2020;14(4):376–390. doi: 10.18502/jad.v14i4.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paphitou N.I., Tourvas A., Floridou D., Richter J., Tryfonos C., Christodoulou C. The first human case of neuroinvasive West Nile virus infection identified in Cyprus. J. Infect. Public Health. 2017;10(6):891–893. doi: 10.1016/j.jiph.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Pouriayevali M.H., Rezaei F., Jalali T., Baniasadi V., Fazlalipour M., Mostafavi E., Khakifirouz S., Mohammadi T., Fereydooni Z., Tavakoli M., Azad-Manjiri S. Imported cases of Chikungunya virus in Iran. BMC Infect. Dis. 2019;19(1):1–8. doi: 10.1186/s12879-019-4637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W.A., Hassan A.A., Adanan C. Seasonality of Anopheles aconitus mosquitoes, a secondary vector of malaria, in an endemic village near the Malaysia-Thailand border. Acta Trop. 1993;55(4):263–265. doi: 10.1016/0001-706x(93)90084-o. [DOI] [PubMed] [Google Scholar]
- Ram S., Khurana S., Kaushal V., Gupta R., Khurana S. Incidence of dengue fever in relation to climatic factors in Ludhiana, Punjab. Indian J. Med. Res. 1998;108:128. [PubMed] [Google Scholar]
- Roiz D., Ruiz S., Soriguer R., Figuerola J. Climatic effects on mosquito abundance in Mediterranean wetlands. Parasites Vectors. 2014;7(1):1–13. doi: 10.1186/1756-3305-7-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.J., Packer M.J. Vector-borne diseases, models, and global change. Lancet. 1993;342(8882):1282–1284. doi: 10.1016/0140-6736(93)92367-3. [DOI] [PubMed] [Google Scholar]
- Shahgudian E.R. A key to the Anophelines of Iran. Acta Med. Iran. 1960:38–48. [PubMed] [Google Scholar]
- Simon-Oke I., Olofintoye L. The effect of climatic factors on the distribution and abundance of mosquito vectors in Ekiti State. J. Biol. Agric. Health. 2015;5:142–146. [Google Scholar]
- Subak S. Effects of climate on variability in Lyme disease incidence in the northeastern United States. Am. J. Epidemiol. 2003;157(6):531–538. doi: 10.1093/aje/kwg014. [DOI] [PubMed] [Google Scholar]
- Tabachnick W.J. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. Am. Entomol. 1991;37:14–26. [Google Scholar]
- Takahashi M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on the pattern of transmission of Japanese encephalitis virus. J. Med. Entomol. 1976;13(3):275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- Tian H.Y., Bi P., Cazelles B., Zhou S., Huang S.Q., Yang J., Pei Y., Wu X.X., Fu S.H., Tong S.L., Wang H.Y. How environmental conditions impact mosquito ecology and Japanese encephalitis: an eco-epidemiological approach. Environ. Int. 2015;79:17–24. doi: 10.1016/j.envint.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H., Raeisi A., Saghafipour A., Nikpour F., Nejati J. Malaria situation in Iran: 2002–2017. Malar. J. 2019;18(1):1–7. doi: 10.1186/s12936-019-2836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R.T., Zinyowera M.C., Moss R.H. Contribution of Working Group II to the Second Assessment of the Inter Governmental Panel on Climate Change (IPCC) Cambridge University Press; Cambridge: 1996. Impacts, adaptations and mitigation of climate change: scientific- technical analyses. [Google Scholar]
- Watson R.T., Zinyowera M.C., Moss R.H. Cambridge University Press; Cambridge: 1998. The Regional Impacts of Climate Change: an Assessment of Vulnerability. Special Report of the Inter Governmental Panel on Climate Change (IPCC) Working Group II. [Google Scholar]
- Watts D.M., Burke D.S., Harrison B.A., Whitmire R.E., Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 1987;36(1):143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- Weicheld J. 2015. Impact of Environmental Factors on Mosquito Population Abundance and Distribution in King County, Washington. M. Sc dissertation. [Google Scholar]
- Wu P.C., Guo H.R., Lung S.C., Lin C.Y., Su H.J. Weather as an effective predictor for occurrence of dengue fever in Taiwan. Acta Trop. 2007;103(1):50–57. doi: 10.1016/j.actatropica.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Zaim M., Cranston P.S. Checklist and keys to the culicinae of Iran (Diptera; Culicidae) Mosq. Systemat. 1986;18:233–245. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.