Abstract
Background
Diabetic wounds are characterized by delayed healing and impaired angiogenesis. Aloe vera and human umbilical vein endothelial cells (HUVECs) are reported to facilitate wound healing, and the former also has hypoglycemic property. Matrix metalloproteinases are enzymes that play a role in diabetic wound pathogenesis.
Objective
To investigate whether oral Aloe vera can enhance the efficacy of HUVEC transplantation and inhibit the expression of matrix metalloproteinases in wound healing of diabetic mice.
Materials and methods
BALB/c nude mice were randomly assigned into five groups: normal control group, diabetic group (DM), DM transplanted with HUVECs, DM treated with oral Aloe vera, and DM treated with combined HUVECs and oral Aloe vera. Diabetes was induced by streptozotocin. Bilateral full-thickness excision cutaneous wounds were created. At days 7 and 14 post-wounding, the following parameters were determined: blood glucose, wound area, wound perfusion, capillary vascularity, re-epithelialization rate and tissue VEGF levels. Tissue expressions of MMP-2 and MMP-9 were compared between the DM mice and those treated with oral Aloe vera.
Results
Over days 7 and 14, Aloe vera exerted glucose-lowering effect in diabetic mice. Higher wound closure rate, blood flow and capillary vascularity, and lower MMP-2 and MMP-9 expressions were observed at both time points in DM treated with Aloe vera group compared with DM group (P < 0.05). Moreover, combined therapy of HUVECs and oral Aloe vera was more effective than Aloe vera or HUVECs alone in increasing VEGF levels, capillary vascularity and wound perfusion. Blood glucose levels were negatively correlated with angiogenesis (P = 0.000.
Conclusion
It is suggested that oral Aloe vera enhances the efficacy of HUVEC transplantation on diabetic wound angiogenesis, partly through improving glycemic control. Oral Aloe vera also promotes diabetic wound healing via inhibition of MMP-2 and MMP-9 expressions.
Keywords: Diabetic wound healing, Aloe vera, Human umbilical vein endothelial cells (HUVECs), Matrix metalloproteinases (MMPs), Angiogenesis
Diabetic wound healing; Aloe vera; Human umbilical vein endothelial cells (HUVECs); Matrix metalloproteinases (MMPs); Angiogenesis.
1. Introduction
Diabetes is a chronic disease considered to be one major global public health concern. As stated by the International Diabetes Federation, the global diabetes prevalence is projected to rise from 463 million people in 2019 to 578 million by 2030 and 700 million by 2045 [1]. Among the most common complications of diabetes are foot ulcers where 15% of diabetic patients have foot ulcers that lead to amputation by 84% [2]. Long-term hyperglycemia can cause damage to many organs, especially nerves and blood vessels. This damage is partly due to an increased formation of advanced glycation end products (AGEs) that triggers endothelial cell dysfunction and impaired angiogenesis [3].
It is evident that damaged endothelial cells can be repaired by endothelial progenitor cells (EPCs) which are critical to angiogenesis. EPCs are immature endothelial cells with subtypes from different origins. It has been shown that the amount and function of circulating EPCs are decreased in type 1 and type 2 diabetic patients [4], and this decrease was inversely related to HbA1C levels [5]. The function of EPCs in type 2 diabetic patients with good glycemic control was also better than those with poorly-controlled diabetes [6]. Interestingly, EPCs are effective in promoting angiogenesis and the healing process of diabetic and non-diabetic wounds [7, 8].
Human umbilical vein endothelial cells (HUVECs) are differentiated endothelial cells having the ability to proliferate, migrate and form new capillary networks. Also, HUVECs have been reported to secrete multiple cytokines and growth factors that accelerate angiogenesis and wound healing [9, 10]. Furthermore, relative to EPCs, HUVECs are easily obtainable. For regenerative medicine research, HUVECs are, therefore, among one of the most interesting options in place of EPCs to boost wound healing.
One of the biomarkers which involves in wound healing are matrix metalloproteinases (MMPs). MMPs are zinc-dependent enzymes that degrade the extracellular matrix [11, 12]. MMP-9 was found to increase in chronic wounds, which directly contributes to delayed healing through its actions on collagen degradation and impaired epithelialization [13]. Besides the role in tissue remodeling, MMP-2 and MMP-9 also exhibited an anti-angiogenic effect in vitro, which subsequently impaired wound healing [14].
Aloe vera (L.) Burm. f. is known as a herbal medicine with various pharmacological properties, including blood glucose-lowering, anti-inflammatory, antibacterial, antioxidant and wound healing promotion activities [15, 16]. A number of studies in rodent models have proven the therapeutic efficacy of Aloe vera gel on diabetic and non-diabetic wounds. Aloe vera was reported to enhance the wound healing process by reducing wound inflammation, stimulating fibroblast proliferation, collagen synthesis, wound contraction, re-epithelialization and angiogenesis, as well as increasing the production of growth factors such as transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) in wounds [17, 18, 19, 20]. In vitro studies revealed that β-sitosterol and aloesin extracted from aloe possessed angiogenic effect by stimulating the proliferation and migration of HUVECs, thus facilitating wound healing [21, 22].
As aforementioned, HUVECs and Aloe vera are beneficial to diabetic wound healing and to the promotion of angiogenesis. Moreover, administration of aloe through oral route can lower blood glucose levels. Therefore, it is interesting to study the effects of oral Aloe vera on the efficacy of transplanted HUVECs in diabetic wound healing, and to identify whether Aloe vera has the ability to reduce MMP-2 and MMP-9 expressions. To our knowledge, little information is available regarding such issues.
2. Materials and methods
2.1. Animals
A total of fifty male BALB/c nude mice (7–8 weeks old; body weight 20–25 g) were purchased from the Nomura Siam International Co., Ltd., Thailand. The mice were given an acclimation period of at least 1 week prior to the experimentation. They were housed in the Animal House of the Faculty of Medicine at Chulalongkorn University, in single cages at 25 ± 2 °C room temperature with a 12-h light dark cycle and fed ad libitum. Approval of the experimental protocol was granted by the Institutional Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University (approval no. 07/2561). All animal procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council of Thailand.
The animals were randomly divided into 5 groups (n = 10 each): normal control group (CON), diabetic group (DM), DM transplanted with HUVECs (DM + HUVECs), DM treated with oral Aloe vera (DM + AV), and DM treated with combined HUVECs and oral Aloe vera (DM + HUVECs + AV). Each group was subdivided into 2 subgroups for parameter studies at days 7 and 14 post-wounding. In the first part of the study, the following parameters were determined in all five groups: blood glucose, wound area, wound perfusion, capillary vascularity, re-epithelialization rate and tissue VEGF levels. In the second part, tissue expressions of MMP-2 and MMP-9 were compared between DM mice and those treated with oral Aloe vera.
2.2. Induction of type 1 diabetes
Following 6-to-8-h fasting, diabetes was induced in mice by intraperitoneal injection of streptozotocin (45 mg/kg) (Sigma Chemicals, St. Louis, MO, USA) dissolved in 0.05 M citrate buffer (pH 4.5) for 5 consecutive days. Two weeks after diabetic induction, blood samples taken from mouse tail tip by pricking method were measured for glucose levels two hours postprandially using glucometer. Mice with blood glucose values above 200 mg/dL were considered diabetic and recruited in the study. The experiment was started eight weeks thereafter to ensure the development of endothelial dysfunction in mice [23].
2.3. Preparation of HUVECs for wound implantation
HUVECs (ATCC, Manassas, Virginia, USA) were obtained from the Stem Cell and Cell Therapy Research Unit, Faculty of Medicine, Chulalongkorn University. The HUVECs were cultivated in endothelial cell growth medium 2 (Lonza Group Ltd, Basel, Switzerland) mixed with 1% penicillin-streptomycin (P/S) in 5% CO2 incubator at 37 °C.
2.4. Preparation of Aloe vera
Aloe vera powder used in this study was extracted from its fresh leaves (Naturex Inc., South Hackensack, USA). The Aloe powder was freshly dissolved in 0.2 mL of distilled water and given orally to the mice at a dose of 200 mg/kg twice a day. The administration was done after wound creation until days 7 and 14 post-wounding.
2.5. Creation of full-thickness excisional wound model
Ten weeks following diabetic induction, which included 2 weeks after diabetic induction and eight weeks after diabetic verification, the animals were anesthetized by intraperitoneal injection of sodium pentobarbital (55 mg/kg). The skin was disinfected and full-thickness excision wounds (0.6 × 0.6 cm2) were inflicted with scissors on the left and right sides of the back of the animal [24]. The wound bed was applied with fibrin gel (Shanghai RAAS Blood Products Co., Ltd., Shanghai, China) or fibrin gel containing 1×106 cells of HUVECs according to the experiment groups. Co-loading of fibrin gel with HUVECs was done by adding 40 μL of fibrin gel to 1×106 cells of HUVECs and mixed together using vortex mixer before applying to each wound. The wound was then covered with Tegaderm (3M Company, St. Paul, MN, USA). Parameter studies were conducted on the seventh and fourteenth days after wound infliction.
2.6. Detection of blood glucose levels
Blood samples were obtained from the tail tip of each mouse by pricking method. The 2-h postprandial blood glucose levels were measured before wound creation (day 0) and at days 7 and 14 post-wounding using test strips and glucometer.
2.7. Analysis of wound closure
At days 0, 7 and 14 post-wounding, both wounds of each mouse were photographed with a digital camera (Fujifilm XA2, Tokyo, Japan). Wound sizes were analyzed using digital image software (Image-Pro Plus 6.1; Media Cybernetics, Rockville, MD, USA). The sizes of both wounds were then averaged for each mouse. The percentage of wound closure was calculated using the following formula: % wound closure = (area of original wound – area of final wound) × 100/area of original wound.
2.8. Measurement of wound blood perfusion
Blood perfusion was measured at eight sites with equal spacing around the wound and digitally recorded using laser doppler flowmeter (PeriFlux System 5000; Perimed B, Stockholm, Sweden), where the probe was placed perpendicular to and 1 mm away from the wound edge. The values obtained were then averaged for each mouse. Wound blood perfusion was expressed as perfusion unit (PU).
2.9. Assessment of wound angiogenesis
The animals were put under anesthesia by intraperitoneal sodium pentobarbital (55 mg/kg) and placed in a supine position. Jugular vein cannulation was done to allow an injection of 0.1 mL of 5% fluorescein isothiocyanate-labeled dextran (Sigma Chemicals, St. Louis, MO, USA). The assessment of wound angiogenesis was performed on wound bed. The images of the blood vessels were visualized and recorded using a confocal fluorescence microscopy (100× magnification; Nikon eclipse E800, Nikon, Japan). Capillaries were identified by the vessels with a diameter of less than 15 μm. Image Pro II 6.1 Program was used to analyze the percentage of capillary vascularity (%CV), which was then calculated as follows: %CV = (pixel number within capillaries/total pixel number of the entire frame) x 100 [24].
2.10. Histopathological study for re-epithelialization
Before the animals were sacrificed with an overdose of sodium pentobarbital, the left-side wound samples were collected (0.6 × 0.6 cm2) and fixed in 10% formaldehyde for 24 h. Tissue samples were cut at the center of the wound and then embedded in paraffin, cut into 4 μm-thickness sections and stained with hematoxylin-eosin. Re-epithelialization was determined using a light microscope at 10× magnification. Analysis of re-epithelialization was conducted using Image-Pro Plus 6.1 (Media Cybernetics, Rockville, MD, USA). The percentage of re-epithelialization was calculated by the following formula: % re-epithelialization = (distance covered by epithelium/distance of the wound edges) ×100 [25].
2.11. Determination of tissue VEGF levels
The right-side wound sample was collected from each mouse (0.6 × 0.6 cm2) without surrounding skin tissue and frozen at –80๐C for protein extraction. Briefly, a 50-mg tissue sample was homogenized in RIPA lysis buffer (Sigma Chemicals, St. Louis, MO, USA) with protease inhibitor (Sigma Chemicals, St. Louis, MO, USA). The sample was sonicated and centrifuged at 10,000 rpm for 10 min. The supernatant was collected and analyzed for total tissue protein and VEGF by enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, Minnesota, USA).
2.12. Immunohistochemistry of MMP-2 and MMP-9 expressions
Tissue expressions of MMP-2 and MMP-9 were compared between the DM and DM + AV groups using immunohistochemical technique. The paraffin-embedded tissue samples of the left-side wound were cut into 2-μm-thick sections. Deparaffinization, rehydration and antigen retrieval were then performed using PT Link PT100 Slide Stainer (Dako, Santa Clara, CA, USA). Next, endogenous peroxidase activity was inactivated with 3% hydrogen peroxide for 5 min at room temperature. To assess MMP-2 and MMP-9 expressions, the sections were incubated for 1 h at room temperature with primary antibodies, including anti-MMP2 antibody produced in rabbit (Sigma Chemical Co., St. Louis, Missouri, USA) at 1:50 dilution and anti-MMP9 antibody produced in rabbit (Sigma Chemical Co., St. Louis, Missouri, USA) at 1:50 dilution, respectively. As secondary antibody for both proteins, goat anti-rabbit immunoglobulins/hrp (Dako, Santa Clara, CA, USA) was used at room temperature for 30 min. Slides were stained with 3,3′-diaminobenzidine at room temperature for 5 min, and finally counterstained with hematoxylin for 10 min. The images were photographed with light microscope at 400× and analyzed using Image Pro Plus 6.1 Program. The percentage of MMP-2 and MMP-9 expressions was calculated as follows: (pixel number within the brown color area/total pixel number of the entire frame) × 100.
2.13. Statistical analysis
Results are presented as mean ± SEM. Data were analyzed by one-way ANOVA, and multiple comparisons among groups were assessed using the Least Significant Difference (LSD). Correlation analysis was performed using the two-tailed Pearson's correlation. All statistical analyses were conducted using SPSS program (version 22). Differences were considered statistically significant at P < 0.05.
3. Results
At the end of the study, there were 9 mice left in the DM group as one died during the experiment due to its diabetic condition.
3.1. Effect of Aloe vera on blood glucose levels
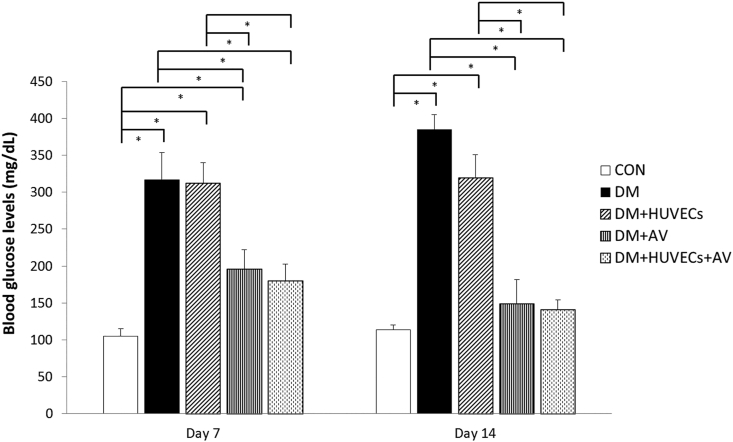
At days 7 and 14 post-wounding, blood glucose levels of the diabetic mice treated with Aloe vera (DM + AV and DM + HUVECs + AV) were significantly lower than those not receiving Aloe vera (DM and DM + HUVECs).
At day 7 post-wounding, no significant difference in blood glucose level was observed between the DM + HUVECs + AV and the CON groups. The DM + AV group had a near-normal glucose level despite having a significantly higher level than that of the CON group. Particularly, at day 14 post-wounding, glucose levels of Aloe vera-treated diabetic mice (DM + AV and DM + HUVECs + AV) were comparable to the CON group (Figure 1).
Figure 1.
Blood glucose levels at days 7 and 14 post-wounding. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
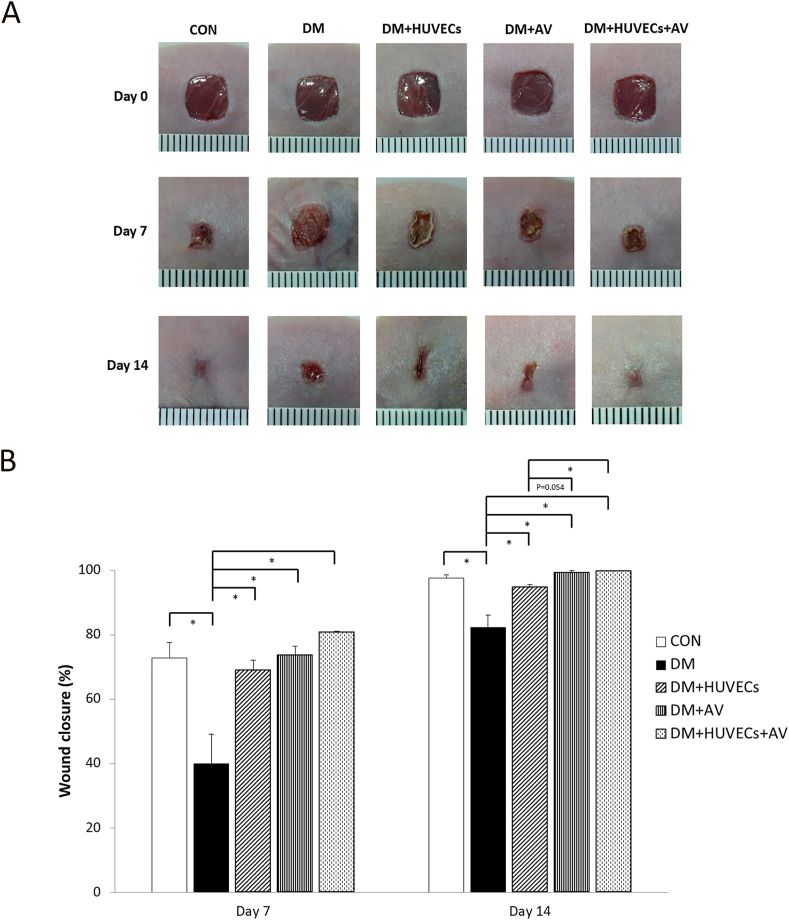
3.2. Effect of Aloe vera and HUVECs on wound closure
Photographs of the wounds at days 0, 7 and 14 are displayed in Figure 2A. Figure 2B shows that the percentage of wound closure in the untreated DM group was lower than the CON group at days 7 and 14 after wounding. All of the treated groups had significantly higher wound closure rates than the untreated DM at both time points. Moreover, on day 14, wound closure was better in the DM + HUVECs + AV group compared with the DM + HUVECs group.
Figure 2.
Effect of combined treatment with HUVECs and oral Aloe vera on wound closure. (A) Photographs of full-thickness excisional skin wounds at days 0, 7 and 14 post-wounding. (B) Percentage of wound closure at days 7 and 14 post-wounding. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
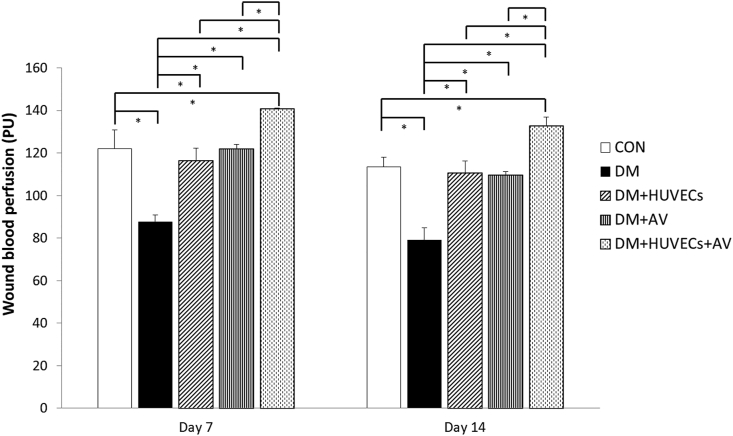
3.3. Effect of Aloe vera and HUVECs on wound blood perfusion
As illustrated in Figure 3, wound blood perfusion at both days 7 and 14 after wounding was decreased in the DM group compared with the CON group. A rise in wound blood perfusion was observed in all of the treated groups compared with untreated group. Interestingly, the DM + HUVECs + AV group had significantly higher wound perfusion than the CON, DM + AV or DM + HUVECs groups.
Figure 3.
Effect of combined treatment with HUVECs and oral Aloe vera on wound blood perfusion. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
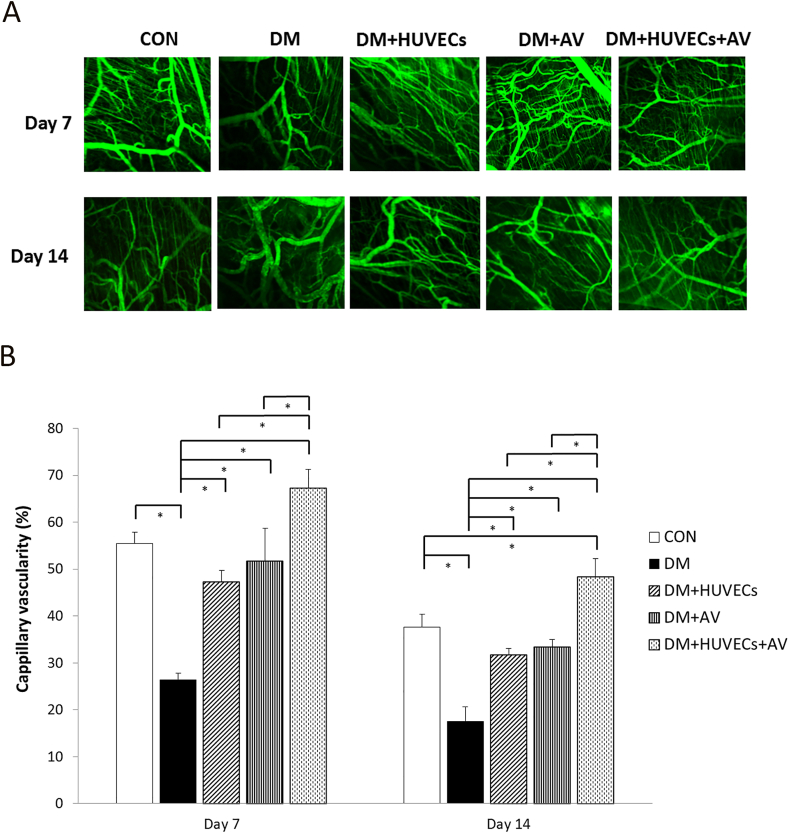
3.4. Effect of Aloe vera and HUVECs on wound angiogenesis
Figure 4A depicts the confocal images of wound angiogenesis of all the experiment groups. Figure 4B reveals that the percentage of capillary vascularity of the DM group was significantly decreased at days 7 and 14 post-wounding compared with the CON and all treatment groups. At day 7 post-wounding, no significant difference in capillary vascularity was noted among the treated mice and the CON mice. In addition, the capillary vascularity of the DM + HUVECs + AV group was more than that of the DM + AV or DM + HUVECs groups at both time points, and more than that of the CON group at day 14.
Figure 4.
Effect of combined treatment with HUVECs and oral Aloe vera on wound angiogenesis. (A) The confocal images of capillaries in the wound bed. Bar scale is 100 μm. (B) Percentage of capillary vascularity. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
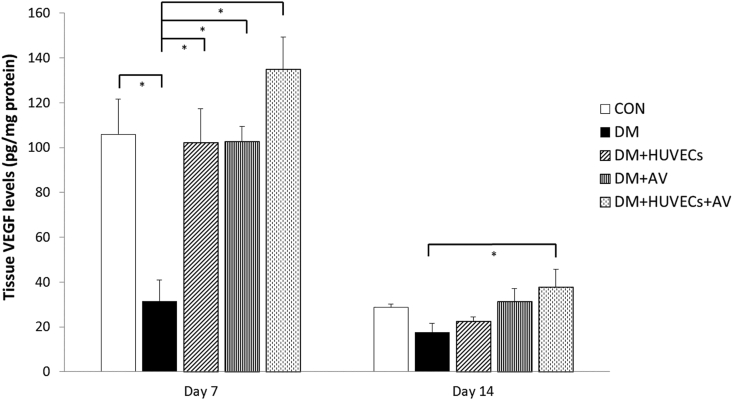
3.5. Effect of Aloe vera and HUVECs on tissue VEGF levels
On day 7, the DM group had a significantly decreased tissue VEGF level than the CON group. Such VEGF levels were elevated in all of the treated groups compared with the DM group. Moreover, the DM + HUVECs + AV had a higher VEGF level than the DM at day 14 post-wounding (Figure 5).
Figure 5.
Effect of combined treatment with HUVECs and oral Aloe vera on tissue VEGF levels. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
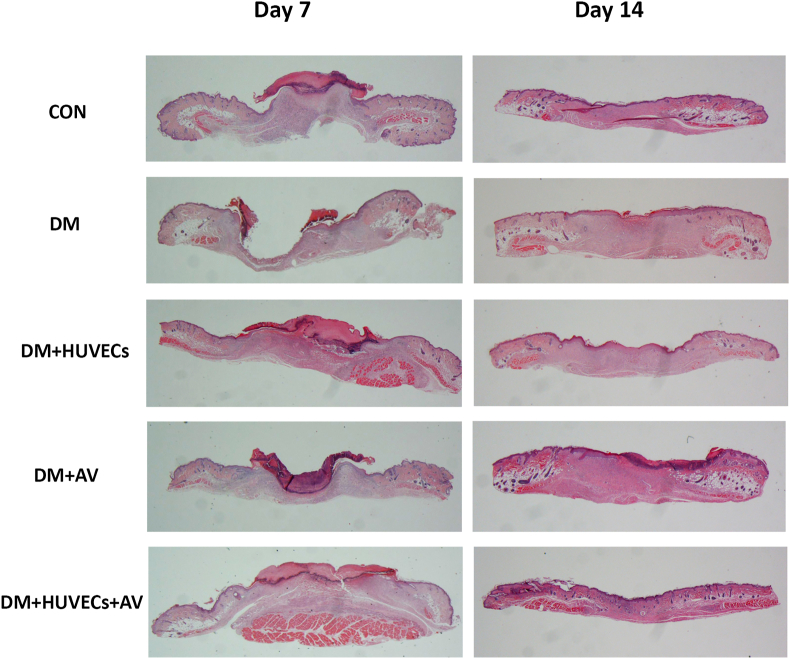
3.6. Effect of Aloe vera and HUVECs on re-epithelialization
Figure 6 showing histopathology of the lesions on days 7 and 14 notes the difference in wound healing between the untreated diabetic mice and the treated groups. On day 7, all the diabetic mice treated with different modalities had complete healing, whereas ulcer persisted in the untreated diabetic mice. On day 14, no difference in this process between groups was found since complete wound healing was detected in all mice.
Figure 6.
Photomicrographs showing histopathology of the full-thickness skin wounds on days 7 and 14 in hematoxylin and eosin-stained sections at 10×. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group.
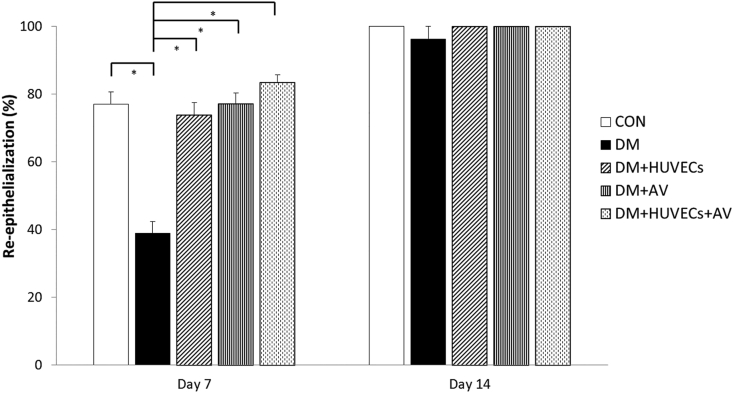
At day 7 post-wounding, there was a significant decrease in re-epithelialization rate of the DM group compared with that of the CON group. The re-epithelialization rate of all treated diabetic groups was greater than the DM group, but comparable to that of the CON group. However, no significant differences were seen among all groups on day 14 (Figure 7).
Figure 7.
Effect of combined treatment with HUVECs and oral Aloe vera on re-epithelialization. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group. Results are presented as mean ± SEM. ∗P < 0.05: significantly different between the groups.
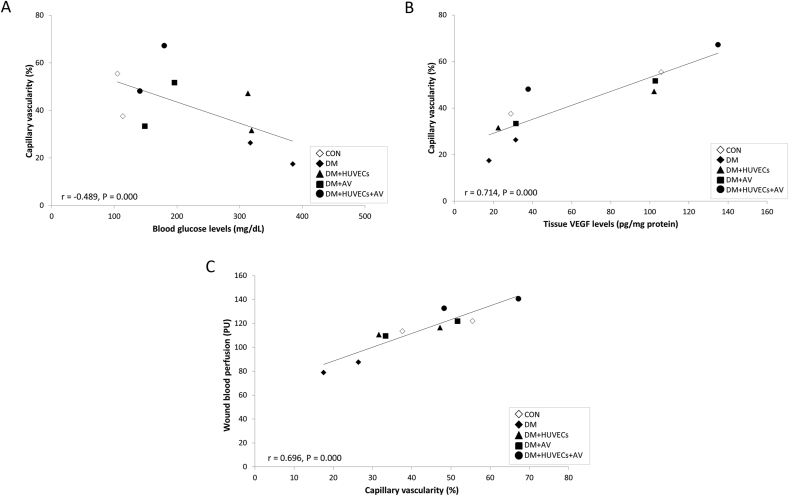
3.7. Correlation analysis of blood glucose levels and capillary vascularity
Figure 8A displays a correlation analysis showing that blood glucose levels were inversely correlated with %CV to moderate degree (r = –0.489, P = 0.000). There were also substantially positive correlations between %CV and tissue VEGF levels (r = 0.714, P = 0.000), and between %CV and wound blood perfusion (r = 0.696, P = 0.000), as shown in Figures 8B and 8C, respectively.
Figure 8.
Correlation analysis of blood glucose levels and capillary vascularity. (A) A negative correlation between blood glucose levels and %CV (r = –0.489, P = 0.000). (B) A positive correlation between %CV and tissue VEGF levels. (C) A positive correlation between %CV and wound blood perfusion. CON: normal control group; DM: diabetic group; DM + HUVECs: DM transplanted with human umbilical vein endothelial cells group; DM + AV: DM treated with oral Aloe vera group; and DM + HUVECs + AV: DM treated with combined HUVECs and oral Aloe vera group.
3.8. Effect of Aloe vera on the MMP-2 and MMP-9 expressions of diabetic mice
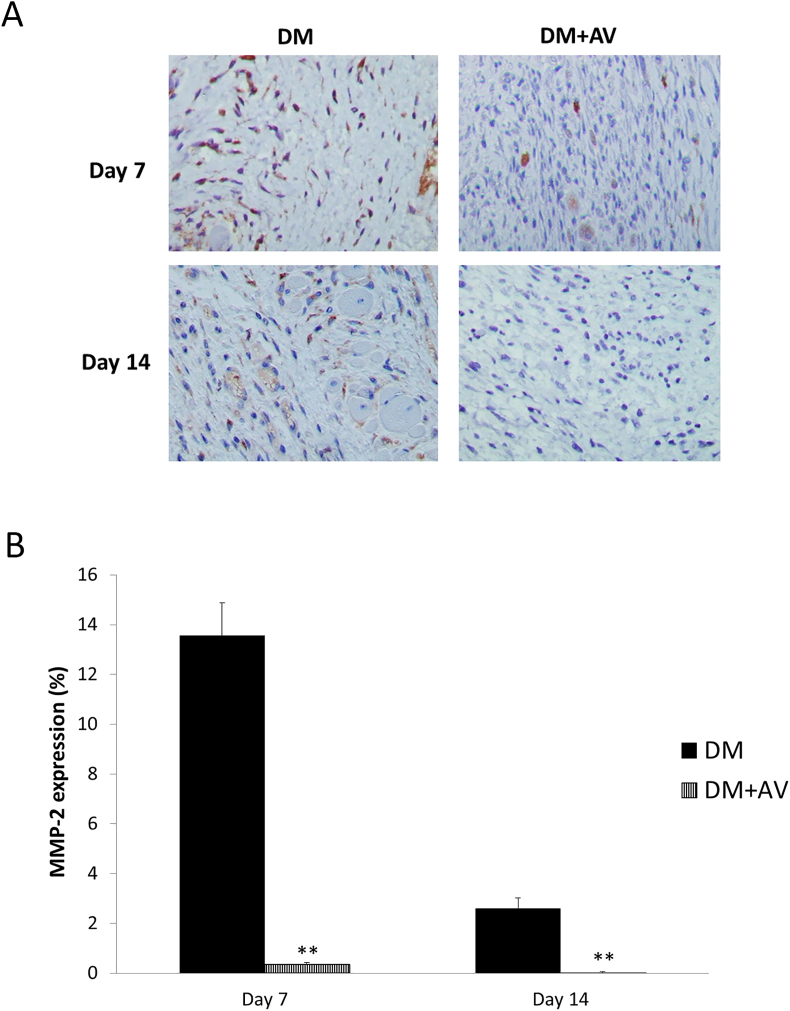
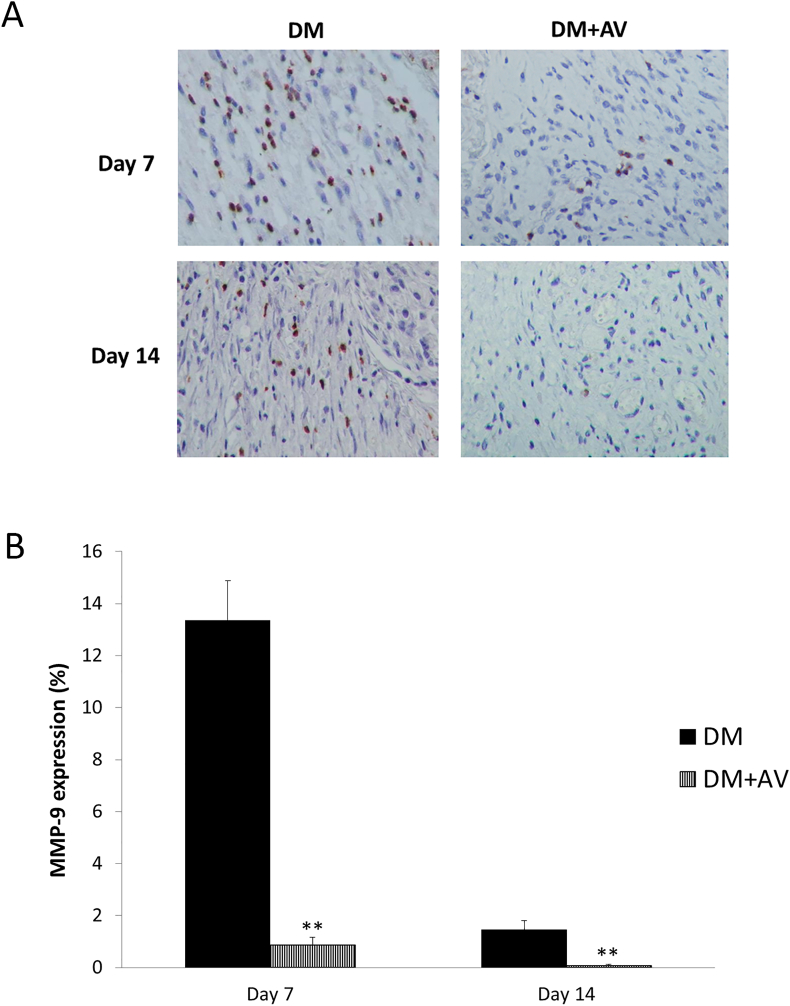
Figures 9 and 10, respectively demonstrate a marked diminution of both MMP-2 and MMP-9 expressions in the DM + AV group compared with the untreated DM at days 7 and 14 post-wounding (MMP-2: P = 0.001 and 0.009 for days 7 and 14, respectively; MMP-9: P = 0.000 and 0.009 for days 7 and 14, respectively).
Figure 9.
Effect of oral Aloe vera on tissue MMP-2 expression. (A) Immunohistochemical images of tissue MMP-2 expression. (B) Percentage of MMP-2 expression. DM: diabetic group; DM + AV: DM treated with oral Aloe vera group. Results are presented as mean ± SEM. ∗∗P < 0.01: significantly different from DM group.
Figure 10.
Effect of oral Aloe vera on tissue MMP-9 expression. (A) Immunohistochemical images of tissue MMP-9 expression. (B) Percentage of MMP-9 expression. DM: diabetic group; DM + AV: DM treated with oral Aloe vera group. Results are presented as mean ± SEM. ∗∗P < 0.01: significantly different from DM group.
4. Discussion
The main finding of this study is that over 7 and 14 days after wounding in diabetic mice, there were delayed wound closures, decreased blood perfusion and capillary vascularity in wounds. Oral administration of Aloe vera, apart from lowering blood glucose, was able to ameliorate all of the above parameters and inhibit the expressions of MMP-2 and MMP-9 in diabetic wounds at both time points, except for re-epithelialization and tissue VEGF levels that were increased only at day 7. Moreover, combined treatment of Aloe vera and HUVECs caused a higher level of blood flow and capillary vascularity than treatment with either HUVECs or Aloe vera alone at both time points.
This study confirmed the characteristics of diabetic wounds, including poor blood supply and impaired angiogenesis. VEGF, a crucial pro-angiogenic growth factor, was also decreased, which is consistent with previous reports [26, 27]. It is known that chronic hyperglycemia induces the overproduction of superoxide by mitochondria, which contributes to capillary endothelial cell dysfunction [28] and reduction in the number and function of the circulating EPCs [4, 29]. This leads to defective angiogenesis and blood perfusion that eventually results in incomplete and prolonged wound repair [30]. In order to mimic the effect of chronic hyperglycemia on wound healing, we therefore, created wounds eight weeks following diabetic induction in mice when endothelial dysfunction was developed [23].
As angiogenesis is a key factor for successful healing, attempts to accelerate diabetic wound healing through stimulating angiogenesis have been made. Promising results were experimentally revealed by using various therapeutic modalities such as Aloe vera [19, 20], EPC transplantation [7, 8, 29]. Likewise, the present study showed that treatment with oral Aloe vera or transplanted HUVECs significantly increased capillary vascularity and wound blood perfusion of diabetic mice at days 7 and 14 post-wounding. VEGF level was also increased but only at day 7 because normally, after full-thickness excisional wounding, its level increases maximally between day 3 to day 7, and then decreases to basal levels thereafter [31]. Based on literature data, B-sitosterol, a major substance in Aloe vera gel, is found to induce angiogenesis and wound repair by increasing VEGF mRNA expression [21]. Moreover, aloesin from Aloe vera enhanced angiogenesis in HUVECs and improved wound closure in hairless mice by modulating MAPK/Rho and Smad signaling pathways [22]. With regard to HUVECs, evidence shows that the action of HUVECs is largely mediated in a paracrine manner through the release of pro-angiogenic factors, such as VEGF, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [9, 10], which is in line with the action of EPCs [32]. Nevertheless, further clarification is required whether transplanted HUVECs may also exert their effects by directly forming tube-like structures as vascular formation in wounds.
The present study indicated the ability of Aloe vera to increase the efficacy of HUVEC transplantation in diabetic wounds. Over days 7 and 14 of the treatment, combined treatment of HUVECs and oral Aloe vera was superior to HUVECs or Aloe vera alone, and even exceeded the normal controls in improving angiogenesis and wound blood flow. Furthermore, at day 14 post-wounding, tissue VEGF was increased only in the combined treatment mice, but not those treated with HUVECs or Aloe vera.
This synergistic effect between Aloe vera and HUVECs on vascular protection in diabetic wounds may be partly explained by the systemic effect of Aloe vera on hypoglycemia. This study demonstrated that oral administration of Aloe vera normalized blood glucose levels over 14 days of treatment, which agrees with previous studies [33, 34]. More interestingly, blood glucose was decreased as capillary vascularity was increased, showing that blood glucose levels were inversely correlated with %CV (r = –0.489, P = 0.000). There were also positive correlations between %CV and tissue VEGF level (r = 0.714, P = 0.000), and between %CV and wound blood perfusion (r = 0.696, P = 0.000). These findings imply that oral Aloe vera which combines with HUVEC transplantation helps lower blood glucose, thus improving wound angiogenesis and blood flow through an increase in VEGF and a subsequent wound healing promotion. The findings also support earlier reports that good glycemic control is associated with improved wound healing in diabetic animal models and patients with diabetic foot ulcers [35, 36, 37, 38]. Another possible explanation for Aloe vera increasing HUVEC transplantation efficacy is the direct beneficial effect of oral Aloe vera on transplanted HUVECs. From in vitro studies conducted by Moon et al. and Wahedi et al., β-sitosterol and aloesin extracted from Aloe vera were able to stimulate the proliferation and migration of HUVECs [21, 22]. Additionally, Sargowo et al. found that oral administration of Aloe vera in diabetic rats enhanced the number of circulating EPCs, thereby increasing the levels of VEGF and the vasodilator producing endothelial nitric oxide synthase (eNOS) [39]. However, the effect of oral Aloe vera on HUVEC recruitment and incorporation into new capillaries in wound and whether oral Aloe vera can increase paracrine function of HUVECs still remain unclear.
The rates of re-epithelialization and wound closure, which are impacted in diabetes, were also demonstrated in the present study to enhance at day 7 post-wounding by Aloe vera, HUVECs and the combination therapy. However, no superior effect of the combination therapy was observed.
In the second part of the study, oral administration of Aloe vera strongly inhibited MMP-2 and MMP-9 expressions in wounds of diabetic mice. Many studies have shown the role of MMPs in diabetic wound pathogenesis. For example, patients with metabolic syndrome are reported to have high levels of MMP-2 and MMP-9 compared with normal individuals [10]. This increase in MMPs induces increased angiostatin and reduced VEGF production, leading to impaired neovascularization in diabetic patients [40]. MMP-9 also disturbs the migration of keratinocytes [11]. On the contrary, in diabetic mice receiving highly selective inhibitors of MMP-2 and MMP-9 and in MMP-9 knockout mice, wound healing was augmentd by decreasing inflammation and increasing angiogenesis and re-epithelialization [41]. Results showing inhibition of MMP-2 and MMP-9 expressions in diabetic mice treated with Aloe vera were in agreement with early studies. According to Vijayalakshmi et al., Aloe vera exhibited anti-inflammatory activity by downregulation of MMP-9 in peripheral blood mononuclear cells [42]. Kudalkar et al. found that the activity of MMP-2 and MMP-9, which involved in the degradation of the extracellular matrix in gingival tissues from patients with chronic periodontitis, was inhibited by Aloe vera [43]. Consequently, this study suggested that the mechanism that may, at least in part, be responsible for promotion of the wound healing process by oral Aloe vera is its inhibitory effect on MMP-2 and MMP-9 expressions.
Although are results found that oral administration of Aloe vera accelerates the healing of full-thickness excisional wounds in diabetic mice, there were some study limitations. Healed wounds may be covered with scab, making it hard to determine the actual wound closure rate. Besides, the experiment was carried out in mouse model, hence the results obtained may not be exactly applied to humans.
5. Conclusion
In summary, oral administration of Aloe vera accelerates the healing of full-thickness excisional wounds in diabetic mice by increasing re-epithelialization, promoting VEGF, angiogenesis and subsequent blood supply. Such positive effects of oral Aloe vera are partly due to its inhibition of MMP-2 and MMP-9 expressions. Moreover, oral Aloe vera enhances the efficacy of transplanted HUVECs for restoration of microcirculation in diabetic wound healing. Further investigations are warranted to identify whether oral administration of Aloe vera can improve the autocrine and paracrine potencies of transplanted HUVECs in diabetic wound healing. It also remains to be elucidated to what extent transplanted HUVECs directly incorporate into neo-vasculature of the wound to facilitate healing. Long-term benefits of combined HUVECs and oral Aloe vera treatment on the matrix organization of wound repair requires further exploration. Therefore, Aloe vera and HUVECs may be useful as an alternative treatment for diabetic ulcers in the future.
Declarations
Author contribution statement
Supassanan Kaewsrisung: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Supakanda Sukpat: Performed the experiments.
Nipan Issarasena: Contributed reagents, materials, analysis tools or data.
Suthiluk Patumraj: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Juraiporn Somboonwong: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Thai Government Budget Grant, Chulalongkorn University (grant number GRB_BSS_60_57_30_11), and the Ratchadaphiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (grant number RA61/090).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Associate Professor Dr. Somboon Keelawat, Head of the Department of Pathology, Faculty of Medicine at Chulalongkorn University, for histopathological description advice.
References
- 1.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., Shaw J.E., Bright D., Williams R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. ninth ed. [DOI] [PubMed] [Google Scholar]
- 2.Reiber G.E., Pecoraro R.E., Koepsell T.D. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann. Intern. Med. 1992;117:97–105. doi: 10.7326/0003-4819-117-2-97. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Kang H., Ma X., Liu J., Fan Y., Deng X. High glucose-induced endothelial progenitor cell dysfunction. Diabetes Vasc. Dis. Res. 2017;14:381–394. doi: 10.1177/1479164117719058. [DOI] [PubMed] [Google Scholar]
- 5.Tepper O.M., Galiano R.D., Capla J.M., Kalka C., Gagne P.J., Jacobowitz G.R., Levine J.P., Gurtner G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 6.Churdchomjan W., Kheolamai P., Manochantr S., Tapanadechopone P., Tantrawatpan C., U-Pratya Y., Issaragrisil S. Comparison of endothelial progenitor cell function in type 2 diabetes with good and poor glycemic control. BMC Endocr. Disord. 2010;10:5. doi: 10.1186/1472-6823-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik K., Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21:1131–1150. doi: 10.1016/j.jcyt.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Sommer K., Jakob H., Kisch T., Henrich D., Marzi I., Frank J., Sander A.L. Local application reduces number of needed EPC for beneficial effects on wound healing compared to systemic treatment in mice. Eur. J. Trauma Emerg. Surg. 2021 doi: 10.1007/s00068-021-01621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y., Yang Y., Xiao L., Li S., Liao X., Liu H. Autocrine and paracrine effects of vascular endothelial cells promote cutaneous wound healing. BioMed Res. Int. 2021;2021:6695663. doi: 10.1155/2021/6695663. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D., Yu Z., Li Y., Wang Y., Li Q., Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020;51:251–263. doi: 10.1007/s10735-020-09877-6. [DOI] [PubMed] [Google Scholar]
- 11.Krishnaswamy V.R., Mintz D., Sagi I. Matrix metalloproteinases: the sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:2220–2227. doi: 10.1016/j.bbamcr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Ayuk S.M., Abrahamse H., Houreld N.N. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J. Diab. Res. 2016;2016:2897656. doi: 10.1155/2016/2897656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiss M.J., Han Y.P., Garcia E., Goldberg M., Yu H., Garner W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery. 2010;147:295–302. doi: 10.1016/j.surg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrich D., Lichtenegger F., Unglaub F., Smeets R., Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast. Reconstr. Surg. 2005;116:539–545. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 15.Babu S.N., Noor A. Bioactive constituents of the genus Aloe and their potential therapeutic and pharmacological applications: a review. J. Appl. Pharmaceut. Sci. 2020;10:133–145. [Google Scholar]
- 16.Sánchez M., González-Burgos E., Iglesias I., Gómez-Serranillos M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 2020;25:1324. doi: 10.3390/molecules25061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somboonwong J., Thanamittramanee S., Jariyapongskul A., Patumraj S. Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J. Med. Assoc. Thai. 2000;83:417–425. [PubMed] [Google Scholar]
- 18.Khorasani G., Hosseinimehr S.J., Azadbakht M., Zamani A., Mahdavi M.R. Aloe versus silver sulfadiazine creams for second-degree burns: a randomized controlled study. Surg. Today. 2009;39:587–591. doi: 10.1007/s00595-008-3944-y. [DOI] [PubMed] [Google Scholar]
- 19.Atiba A., Nishimura M., Kakinuma S., Hiraoka T., Goryo M., Shimada Y., Ueno H., Uzuka Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am. J. Surg. 2011;201:809–818. doi: 10.1016/j.amjsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Atiba A., Ueno H., Uzuka Y. The effect of aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J. Vet. Med. Sci. 2011;73:583–589. doi: 10.1292/jvms.10-0438. [DOI] [PubMed] [Google Scholar]
- 21.Moon E.J., Lee Y.M., Lee O.H., Lee M.J., Lee S.K., Chung M.H., Park Y.I., Sung C.K., Choi J.S., Kim K.W. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 1999;3:117–123. doi: 10.1023/a:1009058232389. [DOI] [PubMed] [Google Scholar]
- 22.Wahedi H.M., Jeong M., Chae J.K., Do S.G., Yoon H., Kim S.Y. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine. 2017;28:19–26. doi: 10.1016/j.phymed.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Nacci C., Tarquinio M., De Benedictis L., Mauro A., Zigrino A., Carratù M.R., Quon M.J., Montagnani M. Endothelial dysfunction in mice with streptozotocin-induced type 1 diabetes is opposed by compensatory overexpression of cyclooxygenase-2 in the vasculature. Endocrinology. 2009;150:849–861. doi: 10.1210/en.2008-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukpat S., Isarasena N., Wongphoom J., Patumraj S. Vasculoprotective effects of combined endothelial progenitor cells and mesenchymal stem cells in diabetic wound care: their potential role in decreasing wound-oxidative stress. BioMed Res. Int. 2013;2013:459196. doi: 10.1155/2013/459196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somchaichana J., Bunaprasert T., Patumraj S. Acanthus ebracteatus Vahl. ethanol extract enhancement of the efficacy of the collagen scaffold in wound closure: a study in a full-thickness-wound mouse model. J. Biomed. Biotechnol. 2012;2012:754527. doi: 10.1155/2012/754527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okizaki S., Ito Y., Hosono K., Oba K., Ohkubo H., Kojo K., Nishizawa N., Shibuya M., Shichiri M., Majima M. Vascular endothelial growth factor receptor type 1 signaling prevents delayed wound healing in diabetes by attenuating the production of IL-1beta by recruited macrophages. Am. J. Pathol. 2016;186:1481–1498. doi: 10.1016/j.ajpath.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Lin C., Yin G., Ou M., Zheng S. The effects of HIF-1α and VEGF on wound healing in diabetic mice. Biomed. Res. 2017;28:8121–8124. [Google Scholar]
- 28.Volpe C.M.O., Villar-Delfino P.H., dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L., Dai S.C., Luan X., Chen J., Cannavicci A. Dysfunction and therapeutic potential of endothelial progenitor cells in diabetes mellitus. J. Clin. Med. Res. 2018;10:752–757. doi: 10.14740/jocmr3581w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okonkwo U.A., Chen L., Ma D., Haywood V.A., Barakat M., Urao N., DiPietro L.A. Compromised angiogenesis and vascular integrity in impaired diabetic wound healing. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He T., Smith L.A., Harrington S., Nath K.A., Caplice N.M., Katusic Z.S. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 33.Pothuraju R., Sharma R.K., Onteru S.K., Singh S., Hussain S.A. Hypoglycemic and hypolipidemic effects of Aloe vera extract preparations: a review. Phytother Res. 2016;30:200–207. doi: 10.1002/ptr.5532. [DOI] [PubMed] [Google Scholar]
- 34.Noor A., Gunasekaran S., Vijayalakshmi M.A. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with Aloe vera extract. Pharmacogn. Res. 2017;9(Suppl 1) doi: 10.4103/pr.pr_75_17. S99–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Sullivan J.B., Hanson R., Chan F., Bouchier-Hayes D.J. Tight glycaemic control is a key factor in wound healing enhancement strategies in an experimental diabetes mellitus model, Ir. J. Med. Sci. 2011;180:229–236. doi: 10.1007/s11845-010-0630-z. [DOI] [PubMed] [Google Scholar]
- 36.Inouye K.A., Bisch F.C., Elsalanty M.E., Zakhary I., Khashaba R.M., Borke J.L. Effect of metformin on periimplant wound healing in a rat model of type 2 diabetes. Implant Dent. 2014;23:319–327. doi: 10.1097/ID.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 37.Katsuhiro M., Hui Teoh S., Yamashiro H., Shinohara M., Fatchiyah F., Ohta T., Yamada T. Effects on glycemic control in impaired wound healing in Spontaneously Diabetic Torii (SDT) Fatty rats. Med. Arch. 2018;72:4–8. doi: 10.5455/medarh.2018.72.4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang J., Wang S., He Y., Xu L., Zhang S., Tang Z. Reasonable glycemic control would help wound healing during the treatment of diabetic foot ulcers. Diab. Ther. 2019;10:95–105. doi: 10.1007/s13300-018-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sargowo D., Handaya A.Y., Widodo M., Lyrawati D., Tjokroprawiro A. Aloe gel enhances angiogenesis in healing of diabetic wound. Indones. Biomed. J. 2011;3:204–215. [Google Scholar]
- 40.Chung A.W., Hsiang Y.N., Matzke L.A., McManus B.M., van Breemen C., Okon E.B. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ. Res. 2006;99:140–148. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 41.Gao M., Nguyen T.T., Suckow M.A., Wolter W.R., Gooyit M., Mobashery S., Chang M. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc. Natl. Acad. Sci. 2015;112:15226–15231. doi: 10.1073/pnas.1517847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijayalakshmi D., Dhandapani R., Jayaveni S., Jithendra P.S., Rose C., Mandal A.B. In vitro anti inflammatory activity of Aloe vera by down regulation of MMP-9 in peripheral blood mononuclear cells. J. Ethnopharmacol. 2012;141:542–546. doi: 10.1016/j.jep.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 43.Kudalkar M.D., Nayak A., Bhat K.S., Nayak R.N. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: an in-vitro study. Ayu. 2014;35:85–89. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.