Abstract
C-terminal tensin-like (CTEN) belongs to the tensin gene family, which encodes proteins that localize to focal adhesions and modulate integrin function. Accumulating studies have reported that CTEN expression can be upregulated or downregulated in different types of cancers, suggesting that CTEN has both oncogenic and tumor suppressor functions. In this study, by analyzing the expression level of CTEN in the human breast cancer (BRCA) samples from the clinically annotated genomic database, The Cancer Genome Atlas, we found that CTEN was downregulated in different BRCA subclasses, including luminal, human epidermal growth factor receptor 2 positive and triple-negative BRCA. Consistently, the protein level of CTEN was also reduced in BRCA based on the Proteomic Tumor Analysis Consortium. In contrast, vascular endothelial growth factor A (VEGFA), a signal protein that stimulates the formation of blood vessels, was upregulated in BRCA. CTEN overexpression in human umbilical vein endothelial cells and MCF7 significantly suppressed the expression of VEGFA, inhibited cell proliferation, migration, and tube formation in vitro. Mechanistically, CTEN bind to casitas B-lineage lymphoma (c-Cbl), an E3 ubiquitin-protein ligase, and decreased the β-catenin expression. In turn, the downregulation of β-catenin reduced the expression of VEGFA. Rescuing β-catenin expression effectively ameliorated the effect of CTEN overexpression in cell proliferation, migration, and tube formation. In conclusion, CTEN inhibited tumor angiogenesis by targeting VEGFA through c-Cbl-mediated down-regulation of β-catenin and may serve as a tumor suppressor in BRCA.
Keywords: CTEN, breast cancer, angiogenesis, VEGFA, c-Cbl, β-catenin
Introduction
Breast cancer (BRCA) is the most common cancer diagnosed among women. According to global cancer statistics of 2018, BRCA is the second leading cause of cancer-related death in women after lung cancer in the Western world.1,2 In fact, the survival rate of BRCA varied greatly worldwide, ranging from about 80% in developed countries, such as North America and Japan, to below 40% in less developed countries.1,3 The low survival rate may be explained by the lack of adequate diagnosis and treatment facilities in low-income countries.4,5 Despite great progress in the early detection and effective therapy of BRCA,6,7 elucidation of molecular mechanisms involved in BRCA is still a key problem for the complete remission of BRCA. Therefore, the identification of novel targets that are involved in the progression and regulation of BRCA tumorigenesis is necessary.
C-terminal tensin-like (CTEN or TNS4) belongs to a member of the tensin focal adhesion family, which contains 3 other members (TNS1, TNS2, and TNS3), and involves in various biological processes, such as cell adhesion, proliferation, differentiation, migration, and invasion.8-10 Compared with the other 3 members in the tension family, CTEN also localizes to the focal adhesion site but lacks the N-terminal actin-binding domain. 11 It only contains the Src homology 2 and phosphotyrosine binding at its C-terminal region and displays extensive sequence homology with other tension members. 9 CTEN was originally found to be abundantly expressed in the normal prostate and placenta. 11 Recently, accumulating studies have revealed that CTEN expression can be upregulated or downregulated in different cancer types, suggesting that CTEN has both oncogenic and tumor suppressor functions. 8 For example, CTEN upregulation was found in lung, colon, stomach, pancreas, and gastric cancer and highly associated with poor prognosis,12-19 suggesting CTEN serves as an oncogene in these cancer types. In contrast, downregulation of CTEN was found in prostate and kidney cancers, despite its low expression in the normal kidney,11,20,21 indicating an inhibitory role of CTEN in tumor tumorigenesis. In BRCA, CTEN protein expression was found to play an oncogenic role and association with poor prognostic. 22 However, the role of CTEN in BRCA still needs to be further elucidated.
In this study, by analyzing the messenger RNA (mRNA) and protein profile in the BRCA samples of human patients from the clinically annotated genomic databases, The Cancer Genome Atlas (TCGA) and the Proteomic Tumor Analysis Consortium (CPTAC), we found that the mRNA and protein levels of CTEN were downregulated in all subclasses of BRCA. By employing gain-of-function and different functional assays, we demonstrated that CTEN inhibited tumor angiogenesis and growth by downregulating vascular endothelial growth factor A (VEGFA) through down-regulation of β-catenin in BRCA.
Material and Methods
Analysis of Gene Expression in BRCA Patient
The mRNA and protein expression of CTEN and VEGFA in BRCA and its normal tissue were analyzed in the UALCAN web (http://ualcan.path.uab.edu/index.html) with default settings. 23 Specifically, the mRNA expression was analyzed in the dataset from TCGA, and the protein expression was analyzed in the dataset from the Clinical Proteomic Tumor Analysis Consortium. No patient consent was used in this study because we used existing data in the available database. All experiments were approved by The Research Ethics Committee of Jiangyin People's Hospital (Approval No. 2018004).
Plasmids and Antibodies
The plasmids expressing CTEN or β-catenin coding region were constructed by Gibson assembly using the lentivirus vector (pCDHL). The plasmids for CTEN, β-catenin or casitas B-lineage lymphoma (c-Cbl) knockdown experiments were constructed by annealing and ligation method in the SHC201 vector. All the antibodies were from Abcam. Specifically, anti-CTEN (ab99887, 1:1000 dilution), anti-VEGFA antibody (VG-1) (ab1316, 1:500 dilution), anti-β-actin antibody (ab8226, 1:5000 dilution), anti-c-Cbl antibody (YE323) (ab32027, 1:1000 dilution), anti-c-Cbl (phospho Y731) antibody (ab52855, 1:2000 dilution), anti-β-catenin antibody (ab32572, 1:500 dilution), anti-CD31 (ab28364, 1:50 dilution). The short hairpin RNA sequences for knockdown experiments are as following: CTEN#1: GAA GTG GCA GAA GTA CTG CAA; CTEN#2: CAG TGT CTG ATG TCA GCT ATA; β-catenin#1: TTG TTA TCA GAG GAC TAA ATA; β-catenin#2: ATC TGT CTG CTC TAG TAA TAA; c-Cbl: CCA GTG AGT TGG GAG TTA TTA.
Cells and Cell Culture
Human umbilical vein endothelial cells (HUVECs) and MCF7 cell lines were purchased from China National Cell Resource Center. The cells were cultured with minimum essential medium or Dulbecco's modified eagle's medium supplemented with 10% fetal bovine serum and 1 × penicillin–streptomycin (Corning) in a 37 °C incubator with an atmosphere of 5% CO2 and 95% air.
Cell Proliferation
Cell proliferation was determined using the cell counting kit-8 (CCK-8 kit) method according to the manufacturer's instructions. Briefly, 5000 cells were seeded in a 96-well plate and cultured overnight. The cell viability was measured at the following steps: aspirate the old medium before the test, add a complete medium with 10% of the test reagents, and continue to incubate for 1 h in the incubator, and measure absorbance at 450 nm.
Cell Migration Assay
Transwell assay was performed to determine the ability of cell migration. Briefly, cells were plated into the upper chamber of the transwell with a serum-free medium. The medium with 10% fetal bovine serum was added into the lower chamber, and then cultured at 37 °C for 12 h. After removing the inner membrane adherent cells, the migrated cells were fixed and stained with crystal violet. Cells from 5 dependent fields were counted and analyzed.
Tube Formation
Firstly, 0.289 mL of chilled Corning Matrigel Matrix (10 mg/mL) per well was added into the ice-cold 24-well culture plates. The HUVECs were seeded into the well at the desired confluence and then were incubated for 18 h in a 37 °C incubator with an atmosphere of 5% CO2 and 95% air. Cell morphology was captured under a 20× microscope.
Tumor Growth in Nude Mice
Dorsal thighs of BALB/c nude mice (6-7 weeks of age, 20-24 g) were randomly divided into 2 group 5 (8 mice each). Five million cells that overexpress CTEN of empty vector were suspended in 100 μl serum-free medium and subcutaneously injected into dorsal thighs of BALB/c nude mice (6-7 weeks of age, 20-24 g). After about 30 days following the initial inoculation of indicated cells, the mice were anesthetized and euthanized. The tumors were removed, imaged, and weighed. These experiments were performed in a double-blind way. Tumor lengths (L) and widths (W) were measured every 3 days using a digital caliper, and tumor volumes were calculated using the equation volume (mm3) = L × W2/2. All animals were housed in an environment of 12/12 h light/dark cycle and controlled ambient temperature (24 ± 1 °C). Water and food are freely available. All animal studies were approved by the Jiangyin People's Hospital Animal Care and Use Committee. The study is compliant with all relevant ethical regulations involving the manipulation of experimental animals.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from cells using the Trizol-based method according to the manufacturer's instructions. The first-strand complementary DNA was reverse transcribed from 2 µg total RNA with Oligo dT primer and random primer, and then was used as a PCR template. Primers for amplification are as follows: CTEN sense: 5′-CTC CGC TTC TGT GGT ATG-3′; antisense: 5′-TTC TCC TGA GGC TCT GTC-3′; vascular endothelial growth factor (VEGF) sense: 5′-GGC AGA ATC ATC ACG AAG T-3′; antisense: 5′-CAC AGG ATG GCT TGA AGA T-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): sense, 5′-GAA GGT GAA GGT CGG AGT C-3′; antisense, 5′-GAA GAT GGT GAT GGG ATT TC-3′. The expression of mRNAs was assessed based on the threshold cycle (Ct), and relative expression levels were calculated as 2−[(Ct of mRNA) − (Ct of GAPDH)] after normalization to GAPDH expression.
Western Blot
Total proteins were extracted from cultured cells using the radioimmunoprecipitation assay buffer. Then, the soluble fraction was centrifuged, and the concentration was determined by bicinchoninic acid. Total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, after which they were transferred onto a polyvinylidene fluoride membrane. Primary antibodies were incubated with the membrane after block with 5% non-fat milk. Horseradish peroxide-conjugated secondary antibody was then added, and the signal was collected by Tanon 5500. Relative protein levels were quantified by scanning densitometry, and the relative gray value of proteins was corrected for background.
Statistical Analysis
All statistical analyses were performed using the Prism Graphpad statistical software package. P ≤ .05 was set as the threshold for statistical significance. The data were expressed as mean ± standard deviation. T-test and one-way analysis of variance were used for the statistical analysis.
Results
CTEN is Downregulated and VEGFA is Upregulated in BRCA
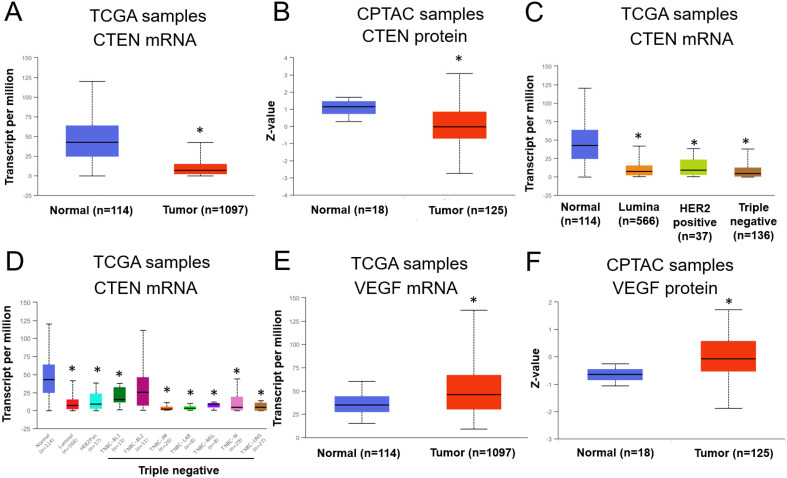
CTEN has been reported to have both oncogenic and tumor suppressor function, mainly depending on the cancer type. 8 To examine the potential role of CTEN in BRCA, we first analyzed the mRNA profile in the BRCA samples of human patients from the clinically annotated genomic database, TCGA. We found that the transcripts of CTEN were significantly less than the normal tissue (Figure 1A), suggesting that the mRNA level of CTEN was downregulated in BRCA. Consistently, the protein level of CTEN was also reduced in BRCA based on the CPTAC (Figure 1B). Moreover, CTEN expression was further evaluated in different subclasses of BRCA, including luminal, HER2 positive, and different types of triple-negative BRCA. Interestingly, the expression of CTEN was all downregulated in different subclasses of BRCA (Figure 1C and 1D), suggesting that CTEN may have a negative effect on the tumor growth in BRCA.
Figure 1.
CTEN is downregulated and VEGFA is upregulated in breast cancer. (A) The mRNA expression of CTEN in human BRCA sample cohort from the TCGA database. (B) The protein expression of CTEN in human BRCA sample cohort from CPTAC database. (C and D) CTEN mRNA expression in different subtypes of human BRCA from TCGA database. (E) The mRNA expression of VEGFA in human BRCA sample cohort from the TCGA database. (F) The protein expression of VEGFA in human BRCA sample cohort from CPTAC database. *P < .05 versus normal tissue.
Abbreviations: BRCA, breast cancer; CPTAC, Proteomic Tumor Analysis Consortium; CTEN, C-terminal tensin-like; mRNA, messenger RNA; TCGA, The Cancer Genome Atlas; VEGFA, vascular endothelial growth factor A.
To investigate whether CTEN affected the tumor angiogenesis, we checked the expression levels of different genes that related to angiogenesis in the human BRCA samples from TCGA. We found VEGFA, a potent angiogenic factor, was reported to be upregulated in many tumors and contribute to tumor angiogenesis,24,25 was upregulated in the BRCA samples (Figure 1E and 1F). Thus, the expression levels of CTEN and VEGFA are inversely correlated with each other (Figure 1), suggesting there may be a direct or indirect relationship between CTEN and VEGFA. Next, we examined whether CTEN can regulate VEGFA expression (Figure 2).
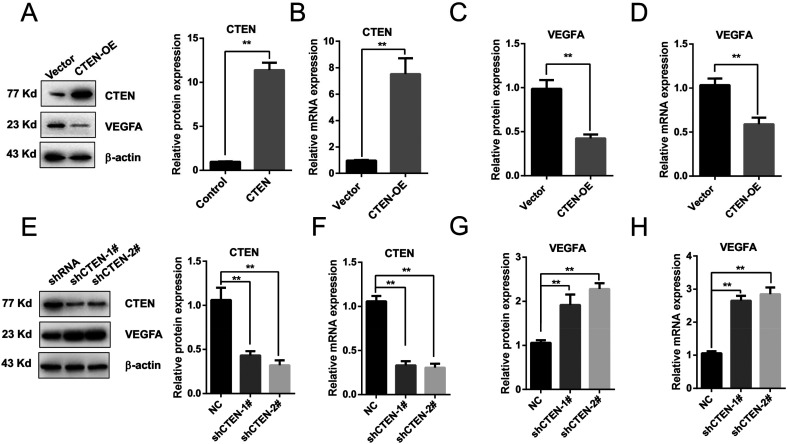
Figure 2.
CTEN overexpression suppresses VEGFA expression in HUVECs. (A) Western blot results showing overexpression of CTEN in HUVEC cells. n = 5. (B) Quantitative real-time PCR showing CTEN mRNA expression. n = 5. (C) Relative VEGFA protein levels were quantified by Western blot. n = 5. (D) Quantitative real-time PCR showing VEGFA mRNA expression. n = 5. (E) Western blot results of CTEN and VEGFA in cells knockdown of CTEN by 2 oligos. n = 5. (F) mRNA expression level of CTEN quantified by real-time RT-PCR. n = 5. (G and H) Expression level of VEGFA quantified by Western blot and real-time RT-PCR. n = 5. **P < .01.
Abbreviations: CTEN, C-terminal tensin-like; HUVEC, human umbilical vein endothelial cell; OE, overexpression; RT-PCR, reverse transcriptase-polymerase chain reaction; VEGFA, vascular endothelial growth factor A.
CTEN Overexpression Suppresses VEGFA Expression and Inhibits Cell Proliferation, Migration, and Angiogenesis
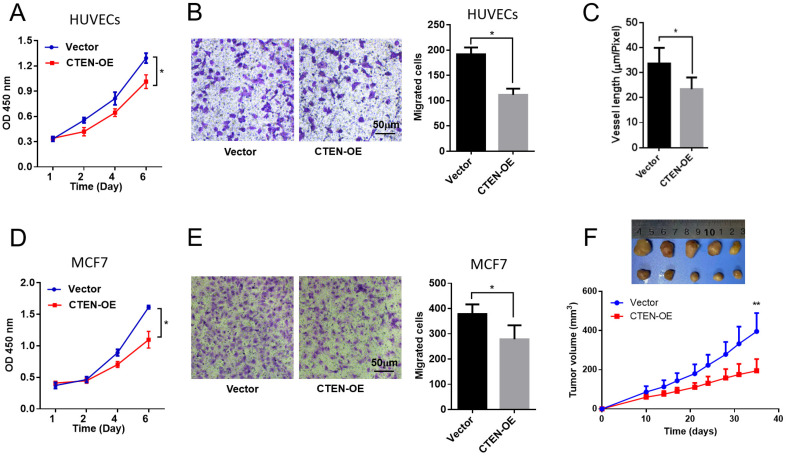
To examine the effects of CTEN-overexpression-mediated VEGFA downregulation, various function assays were employed to evaluate the cell proliferation, migration, and tube formation after CTEN overexpression in HUVECs. Firstly, a CCK-8 assay was performed to measure the ability of cell proliferation. As shown in Figure 3A, CTEN overexpression significantly suppressed cell proliferation in a time-dependent manner. Secondly, cell migration ability was detected by the cell numbers that migrated through the membrane in the transwell experiment. We found that CTEN overexpression dramatically impaired cell migration ability (Figure 3B). Thirdly, a tube formation assay was performed to evaluate the angiogenic ability after CTEN overexpression. As shown in Figure 3C, tube formation was greatly inhibited by CTEN overexpression. These results demonstrated an inhibitory effect of CTEN in cell proliferation, migration, and angiogenesis in vitro, probably by downregulating VEGFA.
Figure 3.
Increased CTEN expression suppression cell growth, migration, and tube formation in vitro and in vivo. (A) The CCK-8 kit assay showing CTEN overexpression suppressed the proliferation of HUVEC cells. n = 5. (B) CTEN overexpression inhibited migration of HUVEC cells. n = 5. (C) CTEN overexpression decreased tube formation of HUVEC cells. n = 5. (D) CTEN overexpression suppressed proliferation of MCF7 cells. n = 5. (E) CTEN overexpression decreased migration of MCF7 cells. n = 5. (F) CTEN overexpression decreased tumor growth in nude mice. n = 8. *P < .05, **P < .01.
Abbreviations: CCK-8, cell counting kit-8; CTEN, C-terminal tensin-like; HUVEC, human umbilical vein endothelial cell.
Next, we further verified these inhibitory effects of CTEN in a BRCA cell line, MCF7 cells. Consistently, CTEN overexpression significantly decreased cell proliferation and migration in MCF7 cells (Figure 3D and 3E). Furthermore, MCF7 cells with CTEN overexpression were subcutaneously injected into nude mice. We found that compared with the control group, mice in the CTEN overexpression group developed a significantly smaller tumor size (Figure 3F). In addition, the VEGF expression and the vessel formation were also checked in the xenograft tumors with CTEN overexpression. We found that the VEGF expression was reduced after CTEN overexpression in the xenograft tumors (Supplemental Figure 1A). Immunostaining of CD31, a marker of angiogenesis, was also decreased after CTEN overexpression, suggesting a decrease in vessel formation (Supplemental Figure 1B).
CTEN Reduced VEGFA Expression by Binding to c-Cbl and Decreasing β-Catenin Expression
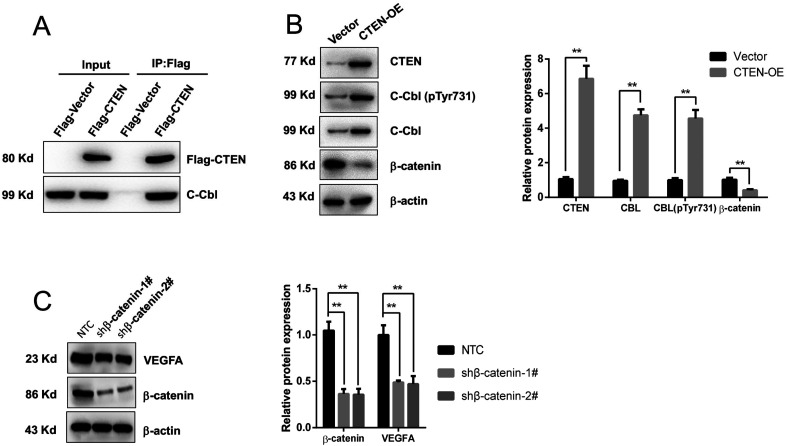
Next, we further investigated the molecular mechanism underlying CTEN-overexpression-mediated VEGFA downregulation. c-Cbl, an E3 ubiquitin-protein ligase, was found to negatively regulate angiogenesis through the Wnt signal pathway. 26 To investigate whether there is an interaction between CTEN and c-Cbl, an immunoprecipitation assay was employed to examine the interaction between CTEN and c-Cbl. As shown in Figure 4A, c-Cbl was found to interact with CTEN in HUVECs. The immunoprecipitation was performed by using an anti-hemagglutinin antibody and then blotted with an anti-c-Cbl antibody. Interestingly, CTEN overexpression significantly increased the expression level of c-Cbl in HUVECs (Figure 4B).
Figure 4.
CTEN reduced VEGFA expression by binding to c-Cbl and decreasing β-catenin expression. (A) Immunoprecipitation assay showing the interaction of CTEN with CBL. (B) Western blot results showing that CTEN overexpression increased c-Cbl expression and phosphorylation. n = 4. (C) Knockdown of β-catenin by 2 oligos reduced the VEGFA expression. n = 5. **P < .01.
Abbreviations: c-Cbl, casitas B-lineage lymphoma; CTEN, C-terminal tensin-like; VEGFA, vascular endothelial growth factor A.
The c-Cbl ubiquitin ligase was reported to regulate the degradation of β-catenin by its tyrosine phosphorylation at a 731 site (Tyr-731). 26 In fact, the phosphorylation of Tyr-731 in c-Cbl was dramatically elevated after CTEN overexpression, while the expression level of β-catenin was significantly downregulated (Figure 4B), probably via c-Cbl-mediated β-catenin ubiquitination. Importantly, VEGFA can be transcriptionally regulated by β-catenin. 27 Knocking down β-catenin expression in HUVECs significantly decreases VEGFA expression (Figure 4C).
Similar results were observed in MCF7 cells (Supplemental Figure 2). Therefore, we hypothesis that CTEN overexpression downregulated VEGFA expression by binding with c-Cbl and decreasing β-catenin expression, thus inhibited tumor angiogenesis.
Rescuing β-Catenin Expression or c-Cbl Knockdown Effectively Eliminated the Effects of CTEN Overexpression in Cell Proliferation, Migration, and Tube Formation of HUVECs
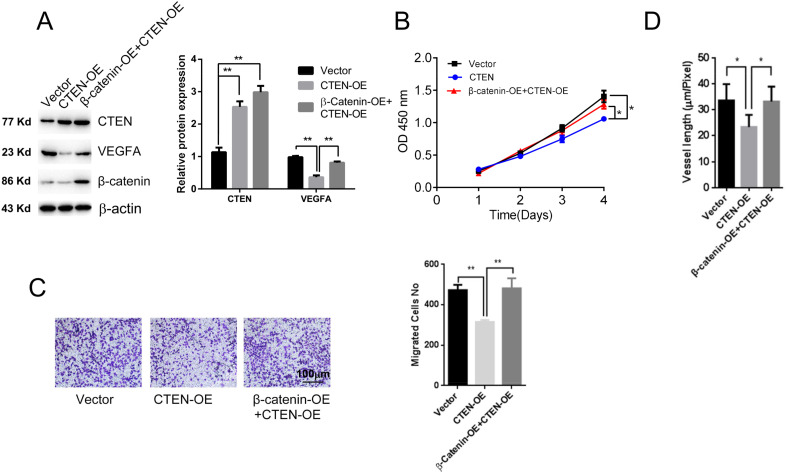
To verify our hypothesis, we examined whether rescuing β-catenin expression can attenuate the effects of CTEN overexpression in cell proliferation, migration, and tube formation of HUVECs. First, β-catenin was overexpressed in HUVECs that stably overexpresses CTEN. Western blot revealed that β-catenin overexpression effectively eliminated CTEN-overexpression-induced VEGFA downregulation, while no changes were observed in the expression and Tyr-731 phosphorylation of c-Cbl (Figure 5A). Moreover, β-catenin overexpression greatly elevated the ability of cell proliferation and migration, which were inhibited by CTEN overexpression (Figure 5B and C). Tube formation assay results demonstrated that the impairment of tube formation by CTEN overexpression was also greatly attenuated by β-catenin overexpression (Figure 5D).
Figure 5.
Rescuing β-catenin expression effectively ameliorated the inhibitory effects of CTEN overexpression in cell proliferation, migration, and tube formation of HUVECs. (A) Western blot results showing the expression level of VEGFA and β-catenin. n = 4. (B) The CCK-8 kit assay showing cell proliferation after CTEN overexpression or co-expression of CTEN and β-catenin. n = 5. (C) Transwell assay showing the cell migration. (D) Blood vessel formation assay. n = 5. **P < .01.
Abbreviations: CCK-8, cell counting kit-8; CTEN, C-terminal tensin-like; HUVEC, human umbilical vein endothelial cell; VEGFA, vascular endothelial growth factor A.
In addition, we also checked the effect of the c-Cbl knockdown. As shown in Supplemental Figure 3, knockdown of c-Cbl greatly attenuated CTEN-overexpression-induced inhibition of cell proliferation and tube formation in MCF7 cells. Thus, our results demonstrated a new function of CTEN in tumor angiogenesis by regulating VEGFA expression through c-Cbl-mediating β-catenin down-regulation.
Discussion
In this study, we evaluated the mRNA and protein expression levels of CTEN in the human BRCA samples from the TCGA and CPTAC database and found that CTEN was consistently downregulated in different types of BRCA, including luminal, HER2 positive, and triple-negative BRCA. These observations are in sharp contrast with a previous study, 22 where increased CTEN expression was found in BRCA using immunohistochemistry and tissue microarray and significantly associated with poor prognostic variables. This contradiction may result from different stages of BRCA or different assays for the expression detection. In fact, CTEN overexpression in HUVECs and MCF7 cells effectively suppressed cell proliferation, migration, and tube formation. We further uncovered that the role of CTEN was mediated by the downregulation of VEGFA through c-Cbl-mediated down-regulation of β-catenin. Based on our findings, CTEN may serve as a tumor suppressor rather than the oncogenic function in BRCA.
Does CTEN play a role in tumorigenesis? Recently, increasing evidence has demonstrated that CTEN has both oncogenic and tumor suppressor function depending on the cancer tissue type. 8 CTEN was originally found to be abundantly expressed in the normal placenta and prostate. 11 Upregulation of CTEN was detected in lung, colon, stomach, pancreas, and gastric cancer and was highly associated with poor prognosis.12-19 The upregulation of CTEN was induced by a variety of cytokines and cancer-associated growth factors, and then promoted tumorigenicity. 28 However, compared to normal prostate, the CTEN expression was downregulated in prostate and kidney cancers,11,20,21 indicating an inhibitory role of CTEN in prostate cancer, although the underlying mechanisms are still unknown. Here, we also observed the downregulation and a tumor suppressor function of CTEN in BRCA, raising the possibility that CTEN may function similarly in prostate cancer and BRCA.
The mechanism of how CTEN suppresses tumor growth was also investigated in this study. CTEN was found to bind with c-Cbl, a unique E3 ubiquitin ligase that promotes degradation of active β-catenin and negatively regulates angiogenesis by its tyrosine phosphorylation through the Wnt signal pathway. 26 Recent work has demonstrated that Wnt/β-catenin signaling can regulate vessel development in normal and pathophysiological conditions. 29 Accumulation of β-catenin in the cytosol and nucleus have been found in the vascular cells during vessel proliferation and pathological angiogenesis. 30 Interestingly, VEGFA, a potent angiogenic factor functions as an essential growth factor for vascular endothelial cells was described as a target of β-catenin in HeLa and colon cancer cells. 27 β-Catenin indeed regulated the expression of VEGFA in colon cancer, 27 suggesting an important role of β-catenin in the colon cancer angiogenesis. In the present study, the mRNA and protein expression levels of VEGFA were found to be upregulated in different subtypes of BRCA, inversely correlating with CTEN expression. Based on these observations, we hypothesized that CTEN suppressed tumor growth and angiogenesis by downregulating VEGFA through binding with c-Cbl and down-regulation of β-catenin. Indeed, rescuing β-catenin expression effectively ameliorated the inhibitory effect of CTEN in tumor growth and angiogenesis in HUVECs, supporting our hypothesis. However, the mechanism underlying the interaction between CTEN and c-Cbl still needs to be further investigated.
In summary, we found that the expression level of CTEN was significantly downregulated in different subtypes of human BRCA. CTEN exerts an inhibitory effect in tumor growth and angiogenesis by c-Cbl/β-catenin/VEGFA pathway axis in BRCA. Our findings suggested that CTEN may serve as a tumor suppressor in BRCA.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-2-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-pdf-4-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Abbreviations
- BRCA
breast cancer
- c-Cbl
casitas B-lineage lymphoma
- CCK-8
cell counting kit-8
- CPTAC
Proteomic Tumor Analysis Consortium
- Ct
threshold cycle
- CTEN
C-terminal tensin-like
- HER2
human epidermal growth factor receptor 2
- HUVEC
human umbilical vein endothelial cell
- RT-PCR
reverse transcriptase-polymerase chain reaction
- VEGFA
vascular endothelial growth factor A.
Footnotes
Authors Note: The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was approved by The Research Ethics Committee of Jiangyin People's Hospital (Approval No. 2018004).
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Clinical Science and Technology Development Fund of Jiangsu University (grant number JLY20180185).
ORCID iD: Tao Zhao https://orcid.org/0000-0003-2868-0864
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730-756. [DOI] [PubMed] [Google Scholar]
- 4.Rivera-Franco MM, Leon-Rodriguez E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer. 2018;8:12. 10.1177/1178223417752677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francies FZ, Hull R, Khanyile R, Dlamini Z. Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options. Am J Cancer Res. 2020;10(5):1568-1591. [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsburg O, Yip CH, Brooks A, et al. Breast cancer early detection: a phased approach to implementation. Cancer. 2020;126(Suppl 10):2379-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaum JK, Duggan C, Anderson BO, Etzioni R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Glob Health. 2018;6(8):e885-e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo SH. C-terminal tensin-like (CTEN): a promising biomarker and target for cancer. Int J Biochem Cell Biol. 2014;51:150-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo SH. Tensin. Int J Biochem Cell Biol. 2004;36(1):31-34. [DOI] [PubMed] [Google Scholar]
- 10.Lo SH, Weisberg E, Chen LB. Tensin: a potential link between the cytoskeleton and signal transduction. Bioessays. 1994;16(11):817-823. [DOI] [PubMed] [Google Scholar]
- 11.Lo SH, Lo TB. CTEN, a COOH-terminal tensin-like protein with prostate restricted expression, is down-regulated in prostate cancer. Cancer Res. 2002;62(15):4217-4221. [PubMed] [Google Scholar]
- 12.Liao YC, Chen NT, Shih YP, Dong Y, Lo SH. Up-regulation of C-terminal tensin-like molecule promotes the tumorigenicity of colon cancer through beta-catenin. Cancer Res. 2009;69(11):4563-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki H, Moriyama S, Mizuno K, et al. CTEN mRNA expression was correlated with tumor progression in lung cancers. Lung Cancer. 2003;40(2):151-155. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, Yukiue H, Kobayashi Y, Fukai I, Fujii Y. CTEN mRNA expression is correlated with tumor progression in thymoma. Tumour Biol. 2003;24(5):271-274. [DOI] [PubMed] [Google Scholar]
- 15.Katz M, Amit I, Citri A, et al. A reciprocal tensin-3-CTEN switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007;9(8):961-969. [DOI] [PubMed] [Google Scholar]
- 16.Albasri A, Al-Ghamdi S, Fadhil W, et al. CTEN signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011;30(26):2997-3002. [DOI] [PubMed] [Google Scholar]
- 17.Asiri A, Toss MS, Raposo TP, et al. Cten promotes epithelial-mesenchymal transition (EMT) in colorectal cancer through stabilisation of Src. Pathol Int. 2019;69(7):381-391. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Mizokami A, Izumi K, et al. CTEN/tensin 4 expression induces sensitivity to paclitaxel in prostate cancer. Prostate. 2010;70(1):48-60. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ghamdi S, Albasri A, Cachat J, et al. CTEN is targeted by KRAS signalling to regulate cell motility in the colon and pancreas. PLoS One. 2011;6(6):e20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu WM, Liao YC. Downregulation of C-terminal tensin-like protein (CTEN) suppresses prostate cell proliferation and contributes to acinar morphogenesis. Int J Mol Sci. 2018;19(10):3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martuszewska D, Ljungberg B, Johansson M, et al. Tensin3 is a negative regulator of cell migration and all four tensin family members are downregulated in human kidney cancer. PLoS One. 2009;4(2):e4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albasri A, Aleskandarany M, Benhasouna A, et al. CTEN (C-terminal tensin-like), a novel oncogene overexpressed in invasive breast carcinoma of poor prognosis. Breast Cancer Res Treat. 2011;126(1):47-54. [DOI] [PubMed] [Google Scholar]
- 23.Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56(3):794-814. [DOI] [PubMed] [Google Scholar]
- 25.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shivanna S, Harrold I, Shashar M, et al. The c-Cbl ubiquitin ligase regulates nuclear beta-catenin and angiogenesis by its tyrosine phosphorylation mediated through the Wnt signaling pathway. J Biol Chem. 2015;290(20):12537-12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Easwaran V, Lee SH, Inge L, et al. Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63(12):3145-3153. [PubMed] [Google Scholar]
- 28.Hung SY, Shih YP, Chen M, Lo SH. Up-regulated CTEN by FGF2 contributes to FGF2-mediated cell migration. Mol Carcinog. 2014;53(10):787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen JJ, Pohl SO, Deshmukh A, et al. The role of Wnt signalling in angiogenesis. Clin Biochem Rev. 2017;38(3):131-142. [PMC free article] [PubMed] [Google Scholar]
- 30.Kiewisz J, Wasniewski T, Kmiec Z. Participation of WNT and beta-catenin in physiological and pathological endometrial changes: association with angiogenesis. Biomed Res Int. 2015;2015:854056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-2-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment
Supplemental material, sj-pdf-4-tct-10.1177_15330338211045506 for CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer by Xiangdong Lu, Bin Zhou, Minmin Cao, Qin Shao, Yukai Pan and Tao Zhao in Technology in Cancer Research & Treatment