Abstract
Aim: To report pathologic findings in the gastrointestinal (GI) tract of coronavirus disease 2019 (COVID-19) patients. Material and Methods: we evaluated clinical and GI tract histologic findings in six COVID-19 patients that presented with GI symptoms like diarrhea, and abdominal pain. This study includes surgical resection specimens from five patients and two sets of biopsy specimens from one patient. Results: Idiopathic inflammatory bowel disease was considered in three of six cases based on clinical, radiologic, and endoscopic presentation. Histologically, the enteric mucosa had a spectrum of histologic changes, including active enteritis, chronic active enteritis, and transmural necrosis. Extensive thrombi in vessels and/or vasculitis were identified in three out of the six cases. The presence of extensive vascular thrombi is associated with poor prognosis, and the three patients deceased in a short period of time (ranges from 7-67 days, median 14 days) after admission for GI symptoms. Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) RNA was detected in bowel tissue of one case. The other three patients recovered and were discharged and free of GI symptoms (follow-up period ranges from 235 days to 270 days, median 237 days). Conclusion: COVID-19 associated enteritis may mimic Crohn’s disease clinically, radiologically and endoscopically, and these two entities can be differentiated by pathologic findings. COVID-19 patients with GI symptoms may warrant a workup to evaluate for pathologic changes, as the presence of vasculitis and microthrombi may predict poor clinical outcome.
Keywords: Thrombosis, vasculitis, gastrointestinal tract, COVID-19
Introduction
The SARS-CoV-2 virus, the cause of coronavirus disease 19 (COVID-19), first appeared in the Wuhan Province of the People’s Republic of China in 2019 and has since spread rapidly, with the World Health Organization declaring COVID-19 a pandemic in March 2020. Although COVID-19 mainly manifests as a respiratory disease, digestive symptoms have been reported in 3% to 18.6% of patients in various studies [1-3] and intestinal ischemia has been reported in patients with COVID-19 [4-9]. In children, a study reports temporal association of SARS-CoV-2 infection with acute appendicitis [10]. To further the understanding of GI involvement in COVID-19, we report clinical and pathological findings in the GI tracts of six patients.
Materials and methods
Case selection and data collection
From May 2020 to January 2021, we collected GI tract tissue samples of patients with current or prior positive PCR results for COVID-19 in nasopharyngeal swabs. Clinical information and follow-up data were obtained from the electronic medical record. Data included age, gender, treatments, and clinical course (Table 1). Slides were reviewed by two practicing board-certified pathologists. This study was approved by the Institutional Review Board at our institute.
Table 1.
Clinical and pathologic features of six patients with COVID-19
| Case # | Sex | Age (y) | First Positive PCR nasal swab | Procedure | Main histologic findings | RT-PCR in bowel tissue SARS-CoV-2 | Follow-up period and outcome |
|---|---|---|---|---|---|---|---|
| 1 | F | 55 | One week after onset of diarrhea | Small bowel resection | Transmural necrosis, vessel thrombi | Negative | Deceased 14 days after admission |
| 2 | F | 20 | 2.5 months prior to GI symptoms onset | Transverse colectomy | Active colitis, vessel thrombi and vasculitis | Positive | Deceased 7 days after admission |
| 3 | F | 59 | 4 months prior to abdominal pain onset | Colonoscopies | Ischemic colitis, vessel thrombi, vasculitis, CMV | Negative | Deceased 67 days after ED visit |
| 4 | M | 21 | Two weeks prior to testicular pain | Appendectomy | Active inflammation, congestion, vessel thrombi | Not performed | 235 days, recovered and free of GI symptom |
| 5 | F | 65 | On the day of admission for abdominal pain | Sigmoid colectomy | Transmural necrosis | Not performed | 237 days, recovered and free of GI symptom |
| 6 | F | 29 | 3 months after abdominal pain onset | Ileum and cecum resection | Ulcer, transmural inflammation | Negative | 270 days, recovered and free of GI symptom |
PCR analysis for COVID-19 viral mRNA
Nasopharyngeal swab PCR tests were performed as previously described [11]. RNA was extracted using Maxwell RSC RNA FFPE Kit (Promega). The CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel assay was adapted using TaqPath 1-step RT-qPCR Mastermix (Thermo Fisher), 2019-nCoV N1 and N2 primer/probe sets, and control RNA primer/probe set (IDT Inc. Coralville, IA). Cycle threshold values less than 40 were considered positive. PCR tests on bowel tissue were performed at GoPath (IL, USA). Formalin-fixed paraffin-embedded (FFPE) tissue was deparaffinized in xylene and ethanol gradient. After proteinase K digestion, total RNA was extracted using AllPrep DNA/RNA FFPE kit from Qiagen Inc. (Hilden, Germany). Real Time PCR was performed with a One-Step RT-PCR kit from Meridian Bioscience Inc. (Cincinnati, OH). Primers and probes for the N1 and N2 genes of SARS-CoV-2 virus and for the reference gene RPP30 were purchased from IDT using the CDC recommended target sequences. Samples with amplification of N1 and N2 were interpreted as SARS-CoV-2 positive. Absence of N1 and N2 gene amplification with amplification of the reference gene (RPP30) indicated a sample was negative for SARS-CoV-2 virus. Cycle threshold values less than 40 were considered positive.
Results
Case 1
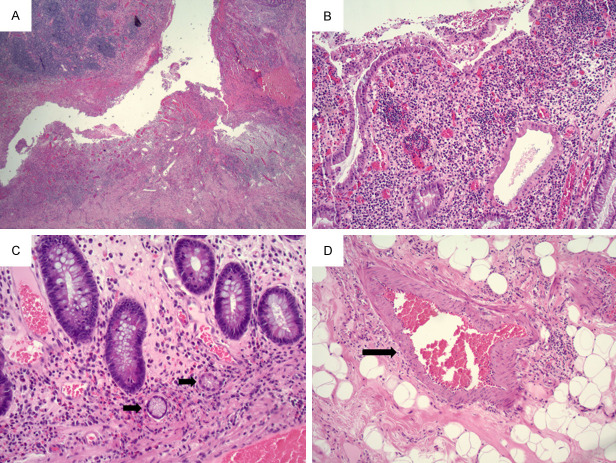
A 55-year-old female with morbid obesity, hypertension, heart failure, type 2 diabetes mellitus, and atrial fibrillation presented with a history of one week of fever, headache, diarrhea, and fatigue. The patient noted one episode of nausea and vomiting on the day of admission and did not report cough, chest pain, or difficulty breathing. CT showed multiple bilateral peripheral ground-glass and consolidative opacities, consistent with multifocal pneumonia. On admission to the Emergency Department, PCR from nasopharyngeal swab was negative for SARS-CoV-2 and fibrinogen was elevated. Two days later, the patient tested positive for SARS-CoV-2 by PCR from nasopharyngeal swab. Additionally, D-dimer was also elevated. The patient’s hospital course was complicated by renal failure and anemia. Thirteen days after admission, the patient became hypotensive and febrile and CT scan demonstrated pneumatosis and portal venous gas. The subsequent day, the patient underwent exploratory laparotomy. At this time, her D-dimer was significantly elevated. More than 3 meters of the small bowel was necrotic, resulting in resection of most of the jejunum and ileum. As shown in Figure 1, most of the sections showed ischemic mucosal changes with extensive thrombi in underlying vessels (Figure 1A, 1B). Rare focal viable mucosa can be identified and the vessels in the submucosa show congestion and rare thrombus (Figure 1C, 1D). RT-PCR from small bowel tissue was negative for COVID-19 viral mRNA with proper positive control. The patient expired after returning from the operating room, 3 weeks after onset of her symptoms and 14 days after the admission.
Figure 1.
(A) 10X objective, (B) 20X objective. Ischemic colitis with necrosis, congestion, and hemorrhage in mucosa and vascular thrombi in submucosa. (C) 10X objective, (D) 20X objective. Rare focal viable mucosa with congestion and focal thrombus (black arrow) in blood vessels in submucosa.
Case 2
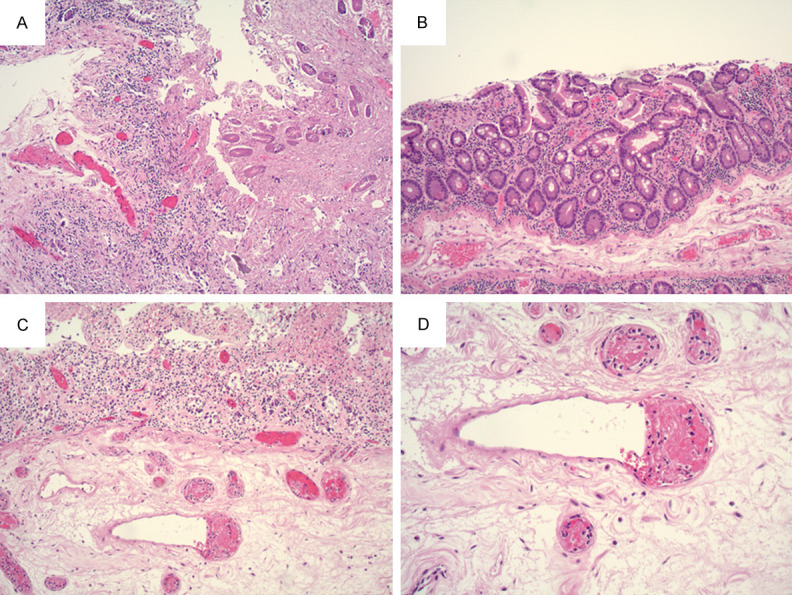
A 20-year-old female with a history of asthma and obesity presented with four days of nausea associated with abdominal pain, vomiting, bloating, non-bloody diarrhea, dizziness, and headaches. The patient tested positive for SARS-CoV-2 PCR 2.5 months prior and had been tested routinely due to working in an assisted living facility. After initial positive PCR test, the patient developed diarrhea, malaise, and fatigue that resolved in less than one week. She tested positive by PCR three days before admission and positive by IgG for SARS-CoV-2 after admission. The patient presented with hypotension and CT showed colitis of unknown etiology. On admission, the patient underwent exploratory laparotomy and transverse colectomy. Both fibrinogen and D-dimer were elevated. An intraoperative colonoscopy showed segmental thickened colonic mucosa and segmental inflammation, concerning for acute onset inflammatory bowel disease clinically. Microscopically, active colitis and thrombi in the blood vessels of the lamina propria was present in colonic mucosa (Figure 2A, 2B). Additionally, vasculitis and extensive thrombi were present in the blood vessels of the submucosa (Figure 2C, 2D). No granulomas or morphologic evidence of chronic colitis were identified to support the diagnosis of Crohn’s disease. RT-PCR from colonic tissue was positive for SARS-CoV-2 viral mRNA (Supplementary Figure 1). The patient was treated with broad-spectrum antibiotics, steroids, and IVIG. Three days after surgery, the patient exhibited acute neurological status change and CT/MRI demonstrated diffuse cerebral edema. Craniotomy was offered but was declined by her family due to poor prognosis. Seven days after surgery, the patient died, 2-3 months after her initial GI symptoms and 7 days after admission. An autopsy was performed. The remaining colon was grossly unremarkable. Microscopically, there was no colitis in the mucosa and no thrombus was identified in the vessels of the colonic sections with extensive sampling. Microthrombi were identified only in the lungs and thrombosis was not identified in any other organs.
Figure 2.
(A) 10X objective, (B) 20X objective. Colonic mucosa with cryptitis and vessel thrombus (black arrow) in lamina propria. (C) 10X objective, (D) 20X objective. Extensive vessel thrombi and vasculitis (black arrow) in blood vessels in submucosa.
Case 3
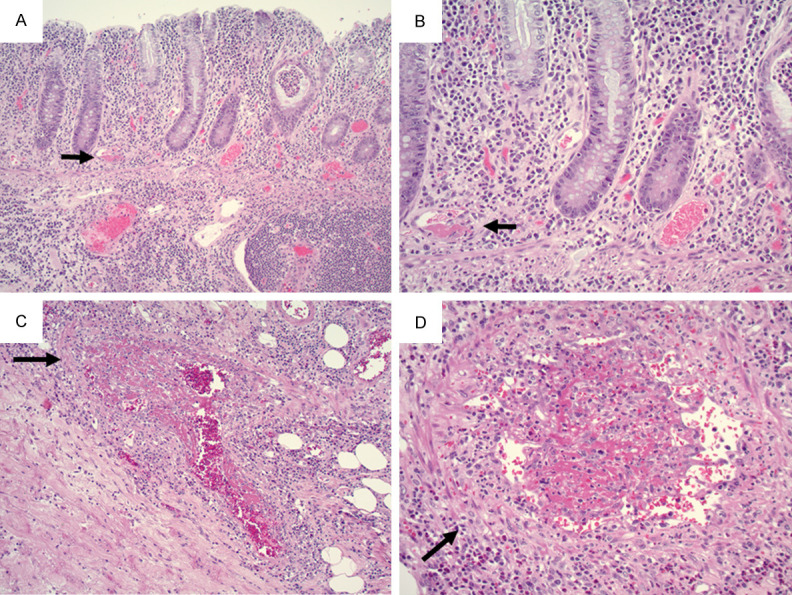
A 59-year-old female with a medical history of asthma and breast cancer presented with GI tract bleeding and diarrhea. Though she tested positive for SARS-Cov-2 with PCR by nasopharyngeal swab four months earlier, four follow-up PCR tests were negative. She had minimal symptoms at the time of her positive PCR test, including intermittent cough with congestion, runny nose, and postnasal drip with no fever, chills, body aches, and fatigue. These respiratory systems may have been confounded by the patient’s history of asthma. Colonoscopy showed a diffuse area of severely erythematous mucosa in the sigmoid colon. Histology showed ischemic-type changes, including “withered” crypts, regenerative changes, and mildly active colitis with cryptitis (Figure 3A). Idiopathic inflammatory bowel disease was suspected clinically and the patient was started on PO prednisone for inflammatory colitis. Three weeks later, a follow-up colonoscopy showed normal ileum alongside diffuse and patchy severe inflammation in the cecum, hepatic flexure, splenic flexure, and descending colon. Endoscopic impression was consistent with Crohn’s disease. Biopsy from the cecum showed mildly active colitis and submucosal vasculitis (Figure 3B). Biopsy from the ascending colon showed chronic active colitis with cryptitis, lymphoplasmacytosis, vasculitis and prominent vessel thrombi (Figure 3C). The transverse colon and descending colon showed chronic active colitis, ulcers, granulation tissue, and cytomegalovirus (CMV) inclusions (Figure 3D), which was confirmed by immunostain (not shown). RT-PCR performed on the biopsy of the ascending colon was negative for SARS-Cov-2 mRNA. Plasma CMV PCR was positive. No measurement of D-dimer or fibrinogen was performed during either colonoscopy. The patient was treated with Valganciclovir for CMV infection. Although CMV levels dropped below detection after treatment, the patient continued to have abdominal pain and fatigue and she was found to have deep vein thrombosis of the bilateral lower extremities. She was discharged with Apixaban. One month after the second colonoscopy, the patient had bowel perforation and underwent bowel resection, which revealed ischemic necrosis of the bowel. Five days after the surgery, the patient passed away due to sepsis secondary to perforated bowel, approximately 2 months after initial onset of GI symptoms.
Figure 3.
A. From the first colonoscopy, sigmoid colon biopsy showed ischemic type changes with crypt atrophy (black arrow) and regenerative changes (10X objective). B. Three weeks later, cecal biopsy showed vasculitis (black arrow) with mildly active colitis in overlying colonic mucosa (20X objective). C. Ascending colon biopsy showed marked vascular thrombi (black arrow, 20X objective). D. Granulation tissue from transverse colon biopsy showed CMV viral inclusions (black arrow, 40X objective).
Case 4
A 21-year-old male with no significant past medical history presented to the Emergency Department with testicular pain. Two weeks prior to the visit, he was diagnosed with COVID-19 with a positive SARS-Cov-2 PCR and symptoms of cough, congestion, rhinorrhea, and fevers. He was also diagnosed with epididymitis and started on doxycycline three days prior to the visit. Ultrasound of the testicles showed no torsion or other acute abnormality. Six days later, the patient presented to the Emergency Department for abdominal pain. Imaging showed a ruptured appendix. PCR for SARS-Cov-2 from nasal swab was negative. Intraoperative findings include omentum surrounding the appendix and a perforation within the mid appendix. Resection specimens showed acute appendicitis and periappendicitis with acute inflammatory exudates in the appendiceal lumen (Figure 4A) and marked vascular congestion with abundant neutrophils and histiocytes in periappendiceal tissue (Figure 4B). Fibrin thrombi were present in blood vessels in periappendiceal tissue away from perforation site (Figure 4C, 4D). No measurement of D-dimer or fibrinogen was performed. He recovered and was discharged five days after surgery.
Figure 4.
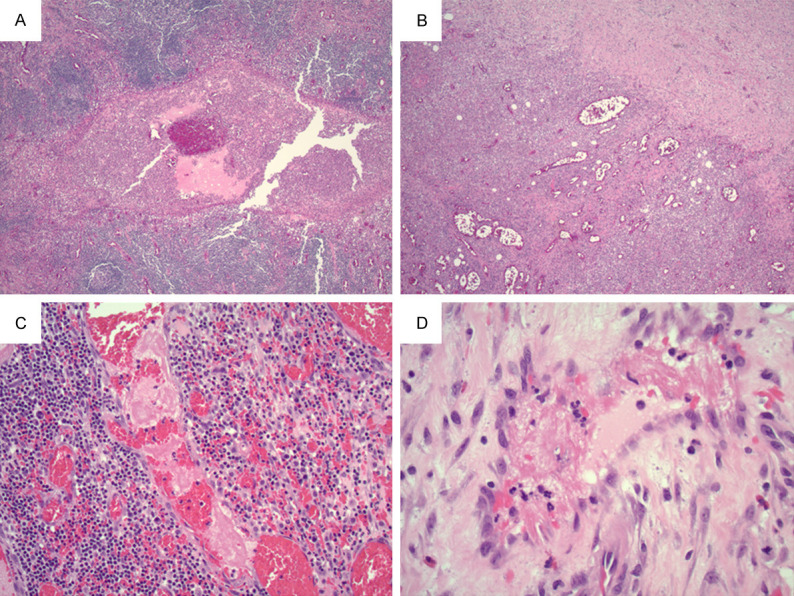
(A) Acute appendicitis with acute inflammatory exudates in the appendiceal lumen (10X objective). (B) Marked congestion with abundant inflammatory cells in the periappendiceal tissue. (10X objective). (C) 20X objective, (D) 40X objective. Vessel fibrin thrombi in the periappendiceal tissue.
Case 5
A 65-year-old female with a history of diverticulosis (diagnosed by colonoscopy eight years prior), hypertension, and hypothyroidism presented with one day of abdominal pain, rectal bleeding, and nausea associated with fever and chills. CT showed pneumoperitoneum with concern for sigmoid colon perforation. PCR from a nasopharyngeal swab was positive for SARS-Cov-2. On the day of admission, the patient underwent exploratory laparotomy and resection of the perforated sigmoid colon. Fibrinogen and D-dimer measurements were not performed before surgery, and measurements taken four days after were both reported to be elevated. Transmural necrosis was present at the perforation site (Figure 5A) and adjacent colonic mucosa was unremarkable (Figure 5B). Rare fibrin thrombi were identified only at the perforation site (Figure 5C). Most of the blood vessels showed congestion with edema with mild active inflammation in the submucosa (Figure 5D). The patient recovered and was discharged one month after surgery.
Figure 5.
A. Transmural necrosis at the perforation site (10X objective). B. Colonic mucosa adjacent to perforation site shows no abnormalities (120X objective). C. Very rare fibrin thrombi identified at the perforation site (10X objective). D. Congestion and edema in the submucosa.
Case 6
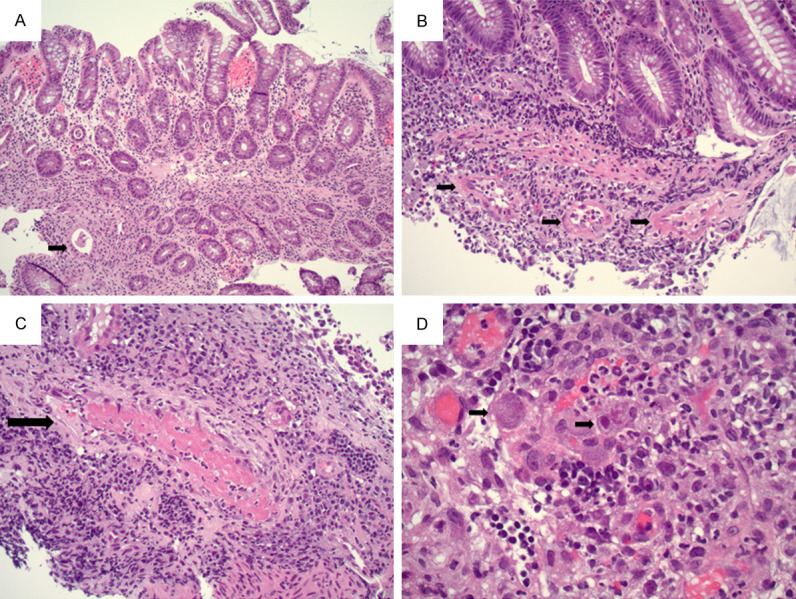
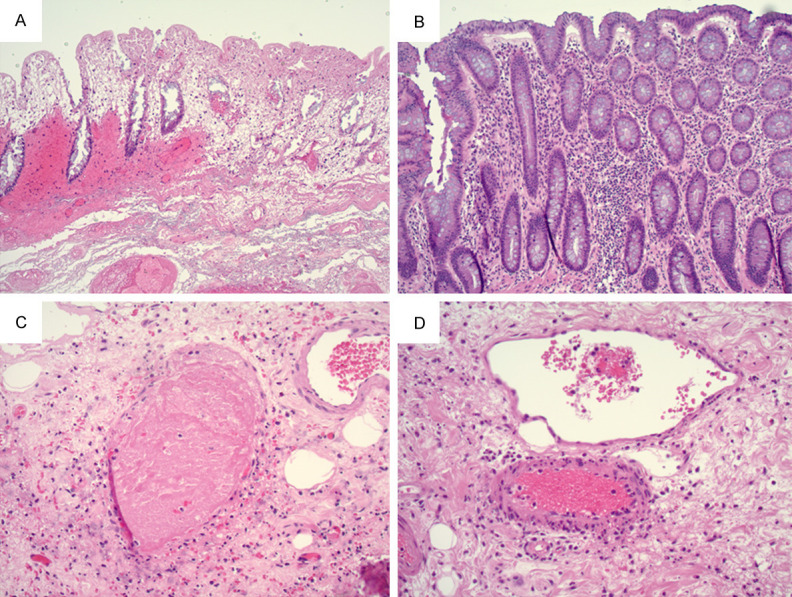
A 29-year-old female with no significant prior medical history presented to the Emergency Department with two months of intermittent abdominal pain. CT revealed bowel wall thickening and inflammatory changes in the terminal ileum with secondary luminal narrowing and stranding, suggesting Crohn’s disease. Imaging identified a focal phlegmon/abscess in the left hemi-abdomen. PCR from a nasopharyngeal swab was negative for SARS-Cov-2. Colonoscopy revealed stenosis at the ileocecal valve and diffuse inflammation (including altered vascularity, congestion, erythema, friability and shallow ulcer) in the terminal ileum and the ileocecal valve. Antibiotics were prescribed to manage the phlegmon/abscess. One month after the colonoscopy, the patient presented with runny nose, congestion, and headache. PCR from a nasopharyngeal swab was positive for SARS-Cov-2. After self-quarantining, the patient recovered. She underwent resection of terminal ileum and cecum two months after the colonoscopy. At the time of surgery, PCR from a nasopharyngeal swab was negative for SARS-Cov-2. Microscopically, the specimen showed a fissuring ulcer (Figure 6A), chronic active ileitis (Figure 6B), and pyloric gland metaplasia (Figure 6C). Submucosal blood vessels were congested and no vascular thrombi was identified (Figure 6D). PCR on bowel tissue for SARS-Cov-2 RNA was negative. The patient recovered after surgery, 4-5 months after the onset of GI symptoms, and was managed with Inflectra.
Figure 6.
A. Deep fissuring ulcer in the terminal ileum (2X objective). B. Chronic active ileitis (10X objective). C. Ileal mucosa with pyloric gland metaplasia (black arrow, 20X objective). D. Congested submucosal blood vessel with no thrombi (black arrow, 10X objective).
Discussion
Although pneumonia is the most common symptom of COVID-19 [12], GI symptoms are also prominent in some patients. A systemic review and meta-analysis of 29 studies reported a pooled prevalence of digestive symptoms of 15%. Nausea or vomiting, diarrhea, and loss of appetite were the most common GI symptoms [13]. Additionally, one study reported that 45% of patients with severe COVID-19 had GI symptoms (abdominal pain, diarrhea, and vomiting) at hospital presentation [14]. A case series from Italy reported 1.7-fold higher mortality in patients with COVID-19-related intestinal ischemia compared to patients with intestinal ischemia pre-COVID-19 [15]. Additionally, an autopsy series reported ischemic enteritis in 3 out of 12 consecutive COVID-19 positive deaths [16].
Some literature has suggested association between intestinal thrombosis and COVID-19. Singh et al reported that an 82-year-old woman with a history of hypertension and diabetes had gangrenous ascending colon after diagnosis of COVID-19. The resection specimen showed mucosal, submucosal necrosis and microthrombi [8]. Additionally, Norsa et al reported a case of fatal intestinal infarction in a 62-year-old man [9]. The resected small bowel showed complete ischemic necrosis of the mucosal layer and acute perivisceral inflammation. The authors also reported that the mesenteric vessels showed complete recent thrombosis and inflammatory infiltration of the endothelium.
Although organ damage may result from viral immune response (cytokine storm) and systemic coagulopathy [18], there is also evidence suggesting that SARS-CoV-2 may directly target epithelial cells in the GI system and cause damage. ACE2, a cell receptor for the virus, is expressed in the GI system, especially the small bowel and colon [17]. Viral RNA was detected in 48.1% of stool specimens from a meta-analysis of 60 studies, even after respiratory samples were negative in some of the cases [19]. Current literature shows variable results regarding the presence of virus in the bowel tissue. Although coronavirus-like particles were reported to be found in GI tract by Bradley and colleagues [20], other authors noted that some of the paper’s figures misinterpreted cellular structures as viral particles due to the declining usage and loss of expertise regarding electron microscopy [21]. Using RT-PCR, SARS-CoV-2 was not detected in the small intestine and large intestine in an autopsy study [22]. Another autopsy study reported negative findings in the small intestine by RNA-ISH, immunohistochemistry (IHC), and qPCR [23]. A recent study by Zhang et al reported negative IHC for SARS-CoV-2 in two cases and chromogenic in situ hybridization in seven cases [24]. In contrast, Carnevale et al reported direct endothelial damage and vasculitis in the submucosa of the ileocecal valve, with viral particles detected in endothelial cells by IHC [25]. In a case report by Norsa et al [9], a patient who tested negative in a nasopharyngeal swab and bronco-alveolar lavage also tested positive for SARS-CoV-2 in intestinal mucosa by RNA in situ hybridization.
COVID-19-associated coagulopathy is well-documented in the literature [26]. Pulmonary endotheliitis, thrombosis, and angiogenesis have been reported [27]. Endotheliitis was also reported in a small bowel resection specimen [28]. However, there have been limited reports on pathologic findings of the GI tract in patients with COVID-19. Additionally, the majority of the cases in the literature are autopsy studies and are unsuitable for evaluating histologic findings in the GI tract due to autolysis. Our study provides unique insights into pathologic findings in the GI tract of COVID-19 infected patients.
Cases 1, 2, and 3 showed vessel microthrombi, and presented at various stages of the process. Case 1 was end stage with transmural necrosis. Case 2 was at a relatively early stage with well-formed vessel thrombi and intact colonic mucosa with active colitis. Case 3 demonstrated chronic ischemic changes in colonic mucosa with well-formed vessel thrombi and coexisting CMV infection in the second set of biopsies. Cases 2 mimicked Crohn’s disease clinically. In Case 2, given that microthrombi were only identified in the lungs at the autopsy and the remaining colon was grossly and microscopically unremarkable, vasculitis and thrombosis in the GI tract were likely segmental. Based on the current understanding of the process, the formation of microthrombi in the GI tract and lungs are most likely secondary to endothelial damage induced vasculitis and thrombosis. Case 3 mimicked Crohn’s disease endoscopically. In Case 3, the patient tested negative for SARS-CoV-2 by nasopharyngeal swab multiple times and RT-PCR for SARS-CoV-2 mRNA was negative in bowel tissue. CMV treatment in this patient did not improve GI symptoms, suggesting that CMV infection may have been a concurrent condition instead of the driver of the disease process.
In these three cases, only case 2 demonstrated presence of SARS-CoV-2 mRNA in the bowel tissue. Our findings, combined with previous literature of variable findings of viral detection in the bowel tissue, suggest that patients can have bowel ischemia secondary to viral effect without evidence of virus in the bowel tissue.
The role of SARS-CoV-2 in Cases 4 and 5 is less clear. In Case 4, clinical and pathological findings appear to be limited to the appendix. At the time of appendectomy, the patient’s nasopharyngeal swab was negative, although he tested positive 16 days prior to the appendectomy. Histologically, vascular thrombi can be seen in some cases of acute appendicitis; however, the prominent vascular congestion in periappendiceal tissue is not commonly seen in acute appendectomy specimens. Interestingly, Ahmed et al reported a 28-year-old man with acute appendicitis with positive PCR finding for SARS-CoV-2 from appendiceal tissue and three negative PCR results from nasopharyngeal and throat swabs [29]. The pathology report described acute appendicitis with a severe unusual mesenteric lymphadenitis and suppurative necrotizing reaction, suggesting Yersinia enterocolitis or Crohn’s disease. Case 5 had a well-documented history of diverticulosis, which may have caused bowel perforation. The vessel thrombi present at the perforation site may be attributed to development of a localized transient hypercoagulative state rather than etiology [30]. Moreover, bowel perforation and SARS-CoV-2 may have been coincidental; on the day of admission, the positive rate of COVID-19 in the patient’s state of residence was 13.3%.
Case 6 is most likely coincidental SARS-CoV-2 infection in a patient with Crohn’s disease. GI symptoms manifested three months before a positive PCR test and COVID-19 respiratory symptoms. The negative RT-PCR from resection bowel tissue and lack of vessel thrombi or vasculitis indicates GI symptoms were not the result of viral infection.
Our study has some limitations. First, the number of the cases is limited due to a single institution study. Second, although RT-PCR for SARS-CoV-2 mRNA was positive in one of the cases, it’s unclear which cell type, endothelial cells, epithelial cells or both, account for the positivity. Based on a recent study, viral protein and RNA were detected in superficial enterocytes [31]. Another study showed viral RNA was detected by in situ hybridization in bowel epithelium in one of 25 CVOID-19 cases tested [32]. Further studies with more cases and extended follow-up period would help understand the role of enteric viral infection in GI symptoms of COVID-19 patients.
In summary, our cases show a spectrum of GI tract findings in COVID-19 patients. Patients with COVID-19 can present with GI symptoms before a positive nasal swab or after the viral level drops below detection limit. COVID-19 associated colitis may mimic Crohn’s disease clinically, radiologically and endoscopically, and these two entities can be differentiated by pathological findings. COVID-19 patients with GI symptoms may warrant a workup to evaluate for pathologic changes, as thepresence of vasculitis and microthrombi may predict poor clinical outcome. Whether COVID-19 can contribute to other GI complications such as appendicitis, diverticulitis, and pre-existing Crohn’s disease warrants further investigation.
Acknowledgements
The study was partially supported by faculty research startup fund from School of Medicine, Case Western Reserve University (Wei Xin and Lan Zhou).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-Sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida Vargas A, Valentí V, Sánchez Justicia C, Martínez Regueira F, Martí Cruchaga P, Luján Colás J, Aliseda Jover D, Esteban Gordillo S, Cienfuegos JA, Rotellar Sastre F. Severe colon ischemia in patients with severe coronavirus-19 (COVID-19) Rev EspEnferm Dig. 2020;112:784–787. doi: 10.17235/reed.2020.7329/2020. [DOI] [PubMed] [Google Scholar]
- 5.Paul T, Joy AR, Alsoub HARS, Parambil JV. Case report: ischemic colitis in severe COVID-19 pneumonia: an unforeseen gastrointestinal complication. Am J Trop Med Hyg. 2021;104:63–65. doi: 10.4269/ajtmh.20-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González Lázaro P, Lomas Meneses A, Del Val Zaballos F, Morandeira Rivas A. Ischemic colitis and short bowel disease due to coronavirus disease 2019 (COVID 19) Clin Nutr ESPEN. 2020;40:406–407. doi: 10.1016/j.clnesp.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KH, Lim SL, Damati A, Maruboyina SP, Bondili L, Abu Hanoud A, Slim J. Coronavirus disease 2019 (COVID-19) and ischemic colitis: an under-recognized complication. Am J Emerg Med. 2020;38:2758.e1–2758.e4. doi: 10.1016/j.ajem.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B, Mechineni A, Kaur P, Ajdir N, Maroules M, Shamoon F, Bikkina M. Acute intestinal ischemia in a patient with COVID-19 infection. Korean J Gastroenterol. 2020;76:164–166. doi: 10.4166/kjg.2020.76.3.164. [DOI] [PubMed] [Google Scholar]
- 9.Norsa L, Valle C, Morotti D, Bonaffini PA, Indriolo A, Sonzogni A. Intestinal ischemia in the COVID-19 era. Dig Liver Dis. 2020;52:1090–1091. doi: 10.1016/j.dld.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra A, Sturgill M, Whitley-Williams P, Lee YH, Esochaghi C, Rajasekhar H, Olson B, Gaur S. Pediatric COVID-19 and appendicitis: a gut reaction to SARS-CoV-2? Pediatr Infect Dis J. 2021;40:e49–e55. doi: 10.1097/INF.0000000000002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhoads DD, Cherian SS, Roman K, Stempak LM, Schmotzer CL, Sadri N. Comparison of Abbott ID now, diasorinsimplexa, and CDC FDA emergency use authorization methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from individuals diagnosed with COVID-19. J Clin Microbiol. 2020;58:e00760-20. doi: 10.1128/JCM.00760-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaafarani HMA, El Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, Gaitanidis A, Alser O, Mashbari H, Bankhead-Kendall B, Mokhtari A, Maurer L, Kapoen C, Langeveld K, El Hechi MW, Lee J, Mendoza AE, Saillant NN, Parks J, Fawley J, King DR, Fagenholz PJ, Velmahos GC. Gastrointestinal complications in critically Ill patients with COVID-19. Ann Surg. 2020;272:e61–e62. doi: 10.1097/SLA.0000000000004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor outcome of intestinal ischemic manifestations of COVID-19. Gastroenterology. 2020;159:1595–1597. doi: 10.1053/j.gastro.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang Y, Liu B, Wang W, Wei C, Yang J, Ye G, Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 20.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dittmayer C, Meinhardt J, Radbruch H, Radke J, Heppner BI, Heppner FL, Stenzel W, Holland G, Laue M. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet. 2020;396:e64–e65. doi: 10.1016/S0140-6736(20)32079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020;154:190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massoth LR, Desai N, Szabolcs A, Harris CK, Neyaz A, Crotty R, Chebib I, Rivera MN, Sholl LM, Stone JR, Ting DT, Deshpande V. Comparison of RNA in Situ hybridization and immunohistochemistry techniques for the detection and localization of SARS-CoV-2 in human tissues. Am J Surg Pathol. 2021;45:14–24. doi: 10.1097/PAS.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ML, Jacobsen F, Pepe-Mooney BJ, Mino-Kenudson M, Deshpande V, Shih AR, Mattia AR, Goessling W, Hwabejire JO, Velmahos GC, Misdraji J. Clinicopathologic findings in COVID-19-associated ischemic enterocolitis. Histopathology. 2021 doi: 10.1111/his.14457. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnevale S, Beretta P, Morbini P. Direct endothelial damage and vasculitis due to SARS-CoV-2 in small bowel submucosa of COVID-19 patient with diarrhea. J Med Virol. 2021;93:61–63. doi: 10.1002/jmv.26119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S, Ahmed RN, Jani P, Ullah M, Aboulgheit H. SARS-CoV-2 isolation from an appendix. J Surg Case Rep. 2020;2020:rjaa245. doi: 10.1093/jscr/rjaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odze Robert D, Goldblum John R. Surgical pathology of the GI tract, liver, biliary tract and pancreas (3nd edition) ISBN. 2015 978-1-4557-0747-8. [Google Scholar]
- 31.Yantiss RK, Qin L, He B, Crawford CV, Seshan S, Patel S, Wahid N, Jessurun J. Intestinal abnormalities in patients with SARS-CoV-2 Infection: histopathologic changes reflect mechanisms of disease. Am J Surg Pathol. 2021 doi: 10.1097/PAS.0000000000001755. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Westerhoff M, Jones D, Hrycaj SM, Chan MP, Pantanowitz L, Tu H, Choi K, Greenson J, Lamps L. Gastrointestinal pathology in samples from coronavirus disease 2019 (COVID-19)-positive patients. Arch Pathol Lab Med. 2021;145:1062–1068. doi: 10.5858/arpa.2021-0137-SA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.