Abstract
We report a rare case of double primary germ cell tumor: right ovarian yolk sac tumor and left ovarian dysgerminoma. A 45 year-old woman was admitted to our hospital due to irregular bleeding for 2 days and extended menstrual period. Right ovarian mass was discovered on transvaginal ultrasound. The pathology results revealed that right ovarian yolk sac tumor and left ovarian dysgerminoma. Total abdominal hysterectomy with bilateral salpingo-oophorectomy with debulking with pelvic lymphadenectomy was performed. The patient underwent adjuvant chemotherapy with BEP six courses in four months and AFP dropped from 8490 ng/ml to nearly 10 ng/ml. Conclusion: Total abdominal hysterectomy with bilateral salpingo-oophorectomy followed by combination chemotherapy must be the treatment of first choice of germ cell tumor.
Keywords: Malignant germ cell tumor, double primary tumor, dysgerminoma, yolk sac tumor
Introduction
Malignant germ cell tumors are less than 5% of all ovarian neoplasms. Dysgerminoma (80%), yolk sac tumor (70%), and immature teratoma (53%) are the most common forms of malignant germ cell tumor [1]. Tumor markers such as AFP (alpha fetoprotein), hCG, and LDH are of great use in the diagnosis, prognosis, and follow-up of the disease. We report a rare case of double primary germ cell tumor: right ovarian yolk sac tumor and left ovarian dysgerminoma. We discuss the histopathological features, therapy and prognosis.
Methods and materials
Patient and sample
Germ cell tumors occurred in both sides of ovary in this case, which was extracted from the Department of Gynecology and Pathology, Hebei General Hospital. Formalin-fixed paraffin-embedded tissues were reprocessed for hematoxylin-eosin (H&E) staining and immunohistochemistry (IHC) staining.
Immunohistochemistry
Immunohistochemical staining was performed on paraffin-embedded sections using a panel of antibodies: CD117 (SP26, Roche, China), OCT3/4 (ZM-0233, ZSGB-Bio, China), PLAP (ZA-0513, ZSGB-Bio, China), vsimentin (AR0234, Talent Biomedical, China), CKpan (ZM-0069, ZSGB-Bio, China), AFP (AP0008, Talent Biomedical, China), CD30 (ZM-0043, ZSGB-Bio, China), CK7 (AM0098, Talent Biomedical, China), Inhibin-α (ZM-0460, ZSGB-Bio, China), ER (1D5, Dako, CA) and HCG-β (ZM-0134, ZSGB-Bio, China). The sections were pretreated, following the manufacturer’s instructions. Positive and negative controls were run concurrently for the staining of all immunomarkers.
Case report
A 45 year old woman, gravida 1 para 1, was admitted to our hospital owing to irregular bleeding for 2 days. History of the patient revealed that her menstrual cycle was regular until 8 months ago. Meanwhile, her menstrual period extended from 6-7 days to more than 20 days. She had a blood transfusion because of anemia at a local clinic. Ovarian mass was discovered on transvaginal ultrasound in our hospital, which showed a 3.9 cm × 4.1 cm × 3.2 cm mass with low echogenicity, many liquid dark areas (internal) and short rod blood flow signal, in the right adnexal area. The left ovary was about 2.7 cm × 1.5 cm, in which there was many high echogenicity spots. The initial serum AFP level (8490 ng/ml) was much higher than normal.
Exploratory laparotomy was carried out on day 3 of her admission; an approximately 4.8 cm × 3.5 cm × 3.2 cm mass with a smooth surface was seen in the right ovary. The tumor was well-circumscribed with a gray-yellow brittle cut surface. Intraoperative examination of a frozen specimen revealed a malignant tumor. Considering where fertility preservation was not a concern, total abdominal hysterectomy with bilateral salpingo-oophorectomy with debulking with pelvic lymphadenectomy was performed. The removed uterus measured 7.0 cm × 6.0 cm × 3.0 cm. The thickness of the endometrium was 0.1 cm and the total myometrial thickness was 3.0 cm. The appearance of the cervix was normal, with length 4 cm and diameter of 4 cm. The uterine serosa, bilateral infundibulopevic ligament and fallopian tubes, left ovary, and omentum had no unusual gross features.
The pathology result revealed a right ovarian yolk sac tumor (Figure 1). It showed microcystic and reticular structures, which consisted of primitive cells, with loose and myxoid stroma. The tumor cells expressed CK-pan, AFP, CD117 and PLAP, but were negative for vimentin, CD30, OCT3/4, inhibin-α, HCG-β, CK7, ER, and PR (Figure 2). Surgical staging was Ib.
Figure 1.
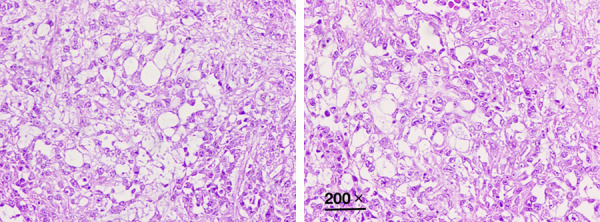
Photomicrograph showing right ovarian yolk sac tumor. H&E 200 ×.
Figure 2.
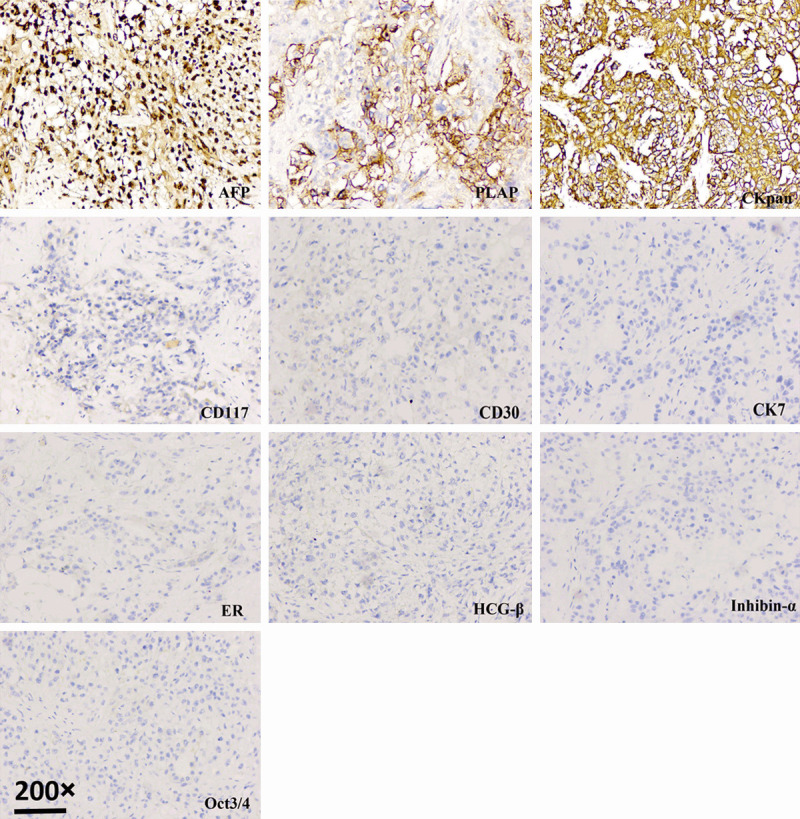
Photomicrograph showing right ovarian yolk sac tumor. Immunohistochemical staining 200 ×.
However, microscopic examination showed left ovarian dysgerminoma (Figure 3). It consisted of sheets of polygonal cells with abundant granular eosinophilic or clear cytoplasm and distinct cell membranes. The tumor cells had middle-sized nuclei with vesicular chromatin and prominent nucleoli. There were fibrous septa, which consisted of Iymphocytes (mostly T-cells) and epithelioid histiocytes, intersecting the tumor. Lymphocytes and epithelioid histiocytes were also sprinkled among the tumor cells. The tumor cells were positive for CK pan, vimentin, PLAP, CD117 and OCT3/4, while not expressed AFP, CD30, inhibin-α, HCG-β, CK7, ER and PR (Figure 4). Surgical stage was Ib.
Figure 3.
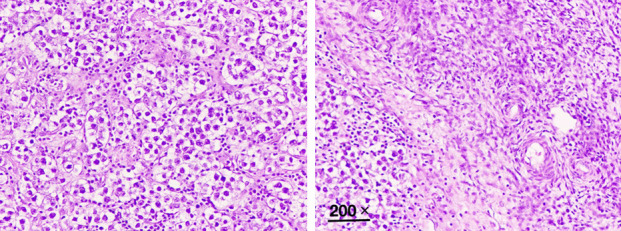
Photomicrograph showing left ovarian dysgerminoma. H&E 200 ×.
Figure 4.
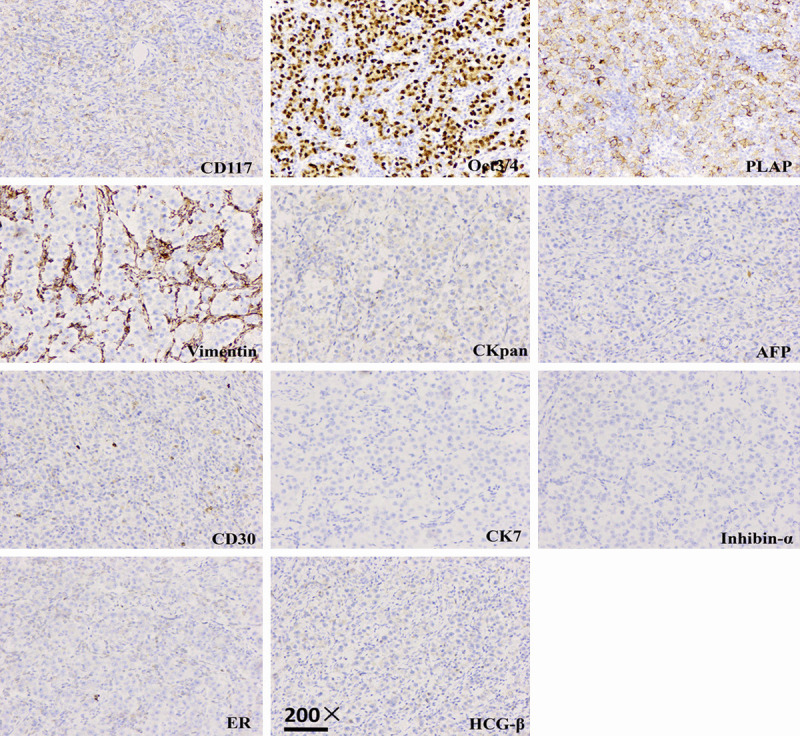
Photomicrograph showing left ovarian dysgerminoma. Immunohistochemical staining 200 ×.
The patient underwent adjuvant chemotherapy with BEP (BLM; Etoposide VP-16; DDP) six cycles in four months. Her tumor marker of AFP was dropped from 8490 ng/ml to nearly 10 ng/ml (Figure 5). During the sixth admission, she was critically ill for myelosuppression. After the treatment of leukopenia, she was better and discharged from hospital. She had survived with no evidence of disease on one year follow up.
Figure 5.
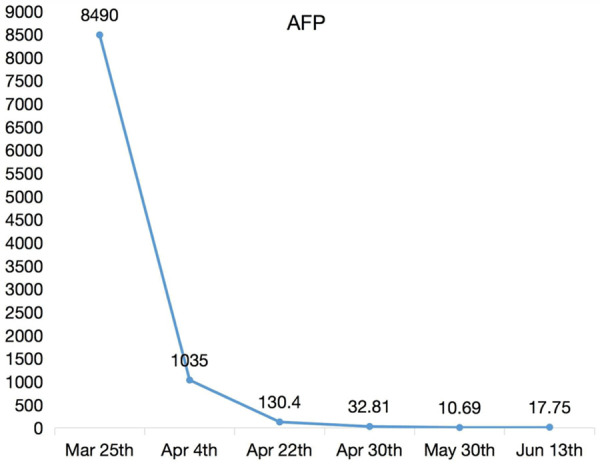
Changes in AFP during six periods chemotherapy.
Discussion
Malignant germ cell tumor accounts for less than 5% of all ovarian neoplasms. The incidence is 8-19% in Asia [2]. World Health Organization classified malignant germ cell tumors such as dysgerminoma, yolk sac tumor, and immature teratoma, non-gestational choriocarcinoma, embryonal carcinoma, and mixed germ cell tumor [3]. Malignant mixed germ cell tumor consists of two or more malignant germ cell components, which is quite rare but much more aggressive [4]. The most common combination reported is dysgerminoma and yolk sac tumor [5]. However, we report this case of double primary germ cell tumor. We could not find any evidence of mixed germ cell component on histopathology in either ovary.
Ovarian germ cell tumors typically occur in adolescent girls and young women [6]. Most common presenting symptoms in such patients are painful abdomen, abdominal distention, or a mass in abdomen with or without irregular bleeding. Conservation of reproductive potential is of great importance as most of the patients are very young. Since these tumors are mostly unilateral and highly sensitive to chemotherapy, conservative, fertility-sparing surgery is appropriate as long as chemotherapeutic agents are given, with the addition of careful staging [7,8]. Since fertility preservation was not required in this case, total abdominal hysterectomy with bilateral salpingo-oophorectomy with debulking with pelvic lymphadenectomy was performed, followed by postoperative chemotherapy. Combination chemotherapy using BEP was given for 6 cycles.
Combination chemotherapy after surgery has greatly improved the survival rate. BEP regimen is the first-line chemotherapy for malignant germ cell tumors of ovary [9]. The tumor markers that contribute to diagnosis, prognosis, and assessing therapeutic response, are β-hCG, AFP and LDH. AFP>10,000 ng/ml or β-hCG levels >50,000 mIU/ml at initial diagnosis indicate poor prognosis, with a survival rate of 50% in 5-year [10]. After 6 cycles of chemotherapy, our patient’s AFP was basically stable (nearly 10 ng/ml) with no evidence of recurrence.
In conclusion, we report one case of double primary germ cell tumor. Germ cell tumor is highly aggressive and often results in poor prognosis. Total abdominal hysterectomy with bilateral salpingo-oophorectomy followed by combination chemotherapy must be the treatment of first choice.
Acknowledgements
The present study was supported by the Research Fund of Hebei Science Foundation for young scholars (Grant No. H2017307005).
Disclosure of conflict of interest
None.
References
- 1.Gershenson DM, Del Junco G, Copeland LJ, Rutledge FN. Mixed germ cell tumors of the ovary. Obstet Gynecol. 1984;64:200–6. [PubMed] [Google Scholar]
- 2.Lim FK, Chanrachakul B, Chong SM, Ratnam SS. Malignant ovarian germ cell tumors: experience in the national university hospital of Singapore. Ann Acad Med Singap. 1998;27:657–61. [PubMed] [Google Scholar]
- 3.Koshy M, Vijayananthan A, Vadiveloo V. Malignant ovarian mixed germ cell tumor: a rare combination. Biomed Imaging Interv J. 2005;1:e10. doi: 10.2349/biij.1.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munemane A, Munemane M. Malignant mixed germ cell tumors of the ovary. A report of 2 cases. Int J Med Sci Public Health. 2014;3:105–7. [Google Scholar]
- 5.Tiwary B, Sinha HH, Pandey VK. Malignant mixed germ cell tumor of ovary: a rare case report. Int J Reprod Contracept Obstet Gynecol. 2015;4:511–3. [Google Scholar]
- 6.Bhurgri Y, Shaheen Y, Kayani N, Nazir K, Ahmed R, Usman A, Bashir I, Setna F, Bhurgri A, Hasan SH, Zaidi SM. Incidence, trends and morphology of ovarian cancer in Karachi (1995-2002) Asian Pac J Cancer Prev. 2011;12:1567–71. [PubMed] [Google Scholar]
- 7.Gershensom DM. Treatment of ovarian cancer in young women. Clin Obstet Gynecol. 2012;55:65–74. doi: 10.1097/GRF.0b013e318248045b. [DOI] [PubMed] [Google Scholar]
- 8.Nishio S, Ushijima K, Fukui A, Fujiyoshi N, Kawano K, Komai K, Ota S, Fujiyoshi K, Kamura T. Fertility-preserving treatment for patients with malignant germ cell tumors of the ovary. J Obstet Gynaecol Res. 2006;32:416–21. doi: 10.1111/j.1447-0756.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao T, Liu Y, Wang X, Zhang H, Lu Y. The role of staging surgery in the treatment of apparent early-stage malignant ovarian germ cell tumors. Aust N Z J Obstet Gynaecol. 2016;56:398–402. doi: 10.1111/ajo.12468. [DOI] [PubMed] [Google Scholar]
- 10.Mazumdar M, Bajorin DF, Bacik J, Higgins G, Motzer RJ, Bosl GJ. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J. Clin. Oncol. 2001;19:2534–41. doi: 10.1200/JCO.2001.19.9.2534. [DOI] [PubMed] [Google Scholar]


