Abstract
Partial liver resection is an established treatment for hepatic disorders. However, surgical bleeding, intra-abdominal adhesion and rapid liver regeneration are still major challenges after partial liver resection, associated with morbidity and mortality. Herein, a biomimetic hybrid hydrogel, composed of oxidized hyaluronic acid, glycol chitosan and MenSCs-derived conditioned medium (CM), is presented to address these issues. The hybrid hydrogel is formed through reversible Schiff base, and possesses injectability and self-healing capability. Moreover, hybrid hydrogel exhibits the capabilities of hemostasis, anti-infection, tissue adhesion and controllable release of cargoes. Based on in vivo studies of the multifunctional hybrid hydrogel, it is demonstrated that acute bleeding in partial liver resection can be ceased immediately by virtue of the hemostasis features of hybrid hydrogel. Also, a significant reduction of intra-abdominal adhesion is confirmed in hybrid hydrogel-treated resection surface. Furthermore, upon the treatment of hybrid hydrogel, hepatic cell proliferation and tissue regeneration can be significantly improved due to the controllably released cytokines from MenSCs-derived CM, exerting the effects of mitogenesis and anti-inflammation in vivo. Thus, the biomimetic hybrid hydrogel can be a promising candidate with great potential for application in partial liver resection.
Keywords: Hybrid hydrogel, Partial liver resection, Hemostasis, Adhesion prevention, Liver regeneration
Graphical abstract
A biomimetic hybrid hydrogel, comprising oxidized hyaluronic acid, glycol chitosan and MenSCs-derived conditioned medium (CM), is developed. The hybrid hydrogel possesses injectability and self-healing capacity, and exhibits the capabilities of blood clotting, anti-infection, tissue adhesion and controllable release of cargoes. This multifunctional hybrid hydrogel shows great potential for hemostasis, adhesion prevention and promoting regeneration after partial liver resection.
Highlights
-
•
A biomimetic hybrid hydrogel, consisting of O-HA, GC and MenSCs-derived CM, is developed.
-
•
Hybrid hydrogel can cease bleeding and reduce intra-abdominal adhesion after partial liver resection.
-
•
Hybrid hydrogel improves cell proliferation and tissue regeneration after partial liver resection.
1. Introduction
Partial liver resection is an established and potentially curative treatment for hepatic carcinoma, living donor liver transplantation, liver abscess and calculus of intrahepatic duct [1,2]. However, the risk of resection surface-related complications, such as intra-operative bleeding, wound infection and intra-abdominal adhesion, is substantially associated with morbidity and mortality after liver resection [3]. More importantly, post-operatively rapid liver regeneration is crucial to warrant sufficient liver function [4]. In order to control intra- and post-operative bleeding, some topical hemostatic agents have been developed to reduce blood loss in liver surgery. Fibrin glues are widely used topical hemostatic agents, and have been proved to have hemostatic effect, biocompatibility and biodegradability [5]. But, fibrin glues have the potential risk of viral or prion transmission, as well as higher price, due to their source from human plasma [6].
Hydrogels derived from polysaccharides have attracted much attention in biomedical applications due to their biomimetic nature, biodegradability and biocompatibility [7]. Injectable hydrogels constructed via Schiff base exhibit their abilities for hemorrhage control and bacterial inhibition to promote wound healing [8]. Rapid formation of in situ hydrogels is achieved by blending two precursor solutions without external stimulus under physiological conditions. In addition, bioactive factors or therapeutic agents can be easily incorporated into hydrogels by mixing with precursor solutions. For instance, a bioinspired multifunctional hydrogel has been developed to repair acute tissue injuries and enhance wound-healing process for infectious skin defect [9].
Stem cell-based therapies have aroused massive interest regarding their potential applications in regenerative medicine [10]. Menstrual blood-derived stem cells (MenSCs) were proved to have high proliferation, broad multipotency and low immunogenicity [11]. Previous studies revealed that paracrine function contributed significantly to the therapeutic potential of stem cells. Paracrine factors, containing a large number of growth factors and cytokines, can be practically accumulated in conditioned medium (CM) of MenSCs. In vitro or in vivo effects of anti-apoptosis, immunomodulation and anti-inflammation were proved with MenSCs-secreted bioactive factors [12]. Therefore, MenSCs-derived CM has been explored as a potential therapeutic strategy to promote liver tissue regeneration.
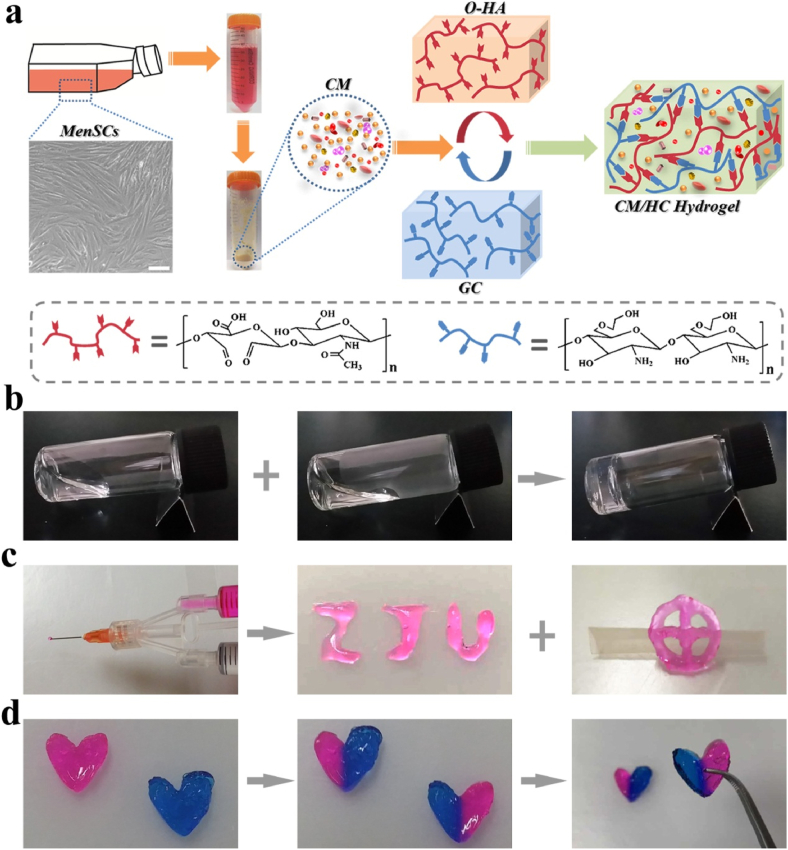
In this work, to address the issues in partial liver resection, including bleeding, intra-abdominal adhesions and post-operative regeneration, we proposed a hybrid hydrogel consisting of oxidized hyaluronic acid (O-HA), glycol chitosan (GC) and MenSCs-derived CM to meet the desired multifunctional features for partial liver resection, as depicted in Fig. 1a. This injectable hydrogel achieved rapid in situ formation with self-healing capability through dynamic Schiff-base linkages, simply by blending two precursor solutions preloaded into a medicine mixer. The capabilities of hemostasis, tissue adhesion, and anti-bacterial activities were also achieved due to dense positive charges, Schiff base, swelling property or inherent viscosity. Moreover, the loading and controllable release of biological factors of CM in hybrid hydrogel rendered great potential for hepatocyte proliferation and tissue regeneration. Upon the hybrid hydrogel, acute bleeding of partial liver resection in rats could be quickly stopped as a result of tissue adhesion and hemostasis properties of hybrid hydrogel. Further, the remarkable reduction of post-operatively intra-abdominal adhesion formation was found in hybrid hydrogel-treated resection surface. Eventually, hybrid hydrogel significantly enhanced hepatic cell proliferation and tissue regeneration after partial liver resection.
Fig. 1.
a) Schematic illustration of the fabrication of biomimetic hybrid hydrogel comprising O-HA, GC and MenSCs-derived CM. Scale bar: 10 μm. b) The formation of hybrid hydrogel through mixing two precursor solutions. c) Two precursor solutions loaded in medicine mixer was injected to form the characters of “ZJU” and “cross-ring” shape. d) Self-healing process of hybrid hydrogel. Precast heart-shaped hybrid hydrogels with pink and blue color were cut into two pieces. Two pieces with different colors could be combined into new heart-shaped hydrogel that can maintain integrity under gravity. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Materials and methods
2.1. Materials
Glycol chitosan, ethylene glycol and sodium periodate were purchased from Sigma-Aldrich (USA). Sodium hyaluronate, methylene blue and rhodamine B were bought from Aladdin (China). Medicine mixer was bought from Shanghai mishawa Medical Industry (China). Cell Counting Kit-8 was purchased from Dojindo laboratories (Janpan). FITC-BSA was bought from Solarbio (China). Fibrin glue was bought from Shanghai RAAS blood products Co Ltd (China). DMEM and fetal bovine serum were bought from Gibco Life Technologies (USA). TRIzol reagent was purchased from Invitrogen (USA). Prime Script™ RT Reagent Kit and TB Green Premix Ex Taq ΙΙ Kit were purchased from Takara Bio Inc (Janpan). Antibodies of PE-conjugated CD29, CD34, CD73, CD90, and HLA-DR were bought from BD Biosciences (USA). RIPA lysis buffer was purchased from Beyotime biotechnology (China). BCA Kit was bought from Thermo-Fisher Scientific (USA). Antibodies of Ki67, PCNA and Cyclin D1 were bought from Cell Signaling Technology (USA). Mini-PROTEAN TGX Precast Protein Gels was from Bio-Rad (USA).
2.2. Culture of menstrual blood-derived stem cell and preparation of conditioned medium
MenSCs were cultured following methods previously described [12]. To identify the expression of surface markers, MenSCs were evaluated using flow cytometry. Primary antibody included PE-conjugated CD29, CD34, CD73, CD90, and HLA-DR. IgG1 and IgG2a were used as isotype control for above primary antibody. When cells reached 70%–80% confluence, the MenSCs were cultured in serum-free low glucose DMEM for another 24 h. Conditioned medium was collected and centrifuged to remove cell debris. Then, the obtained medium was lyophilized into powder and kept under −80 °C.
2.3. Synthesis of oxidized hyaluronic acid
O-HA was synthesized using periodate oxidation method according to previous report [13]. Briefly, sodium hyaluroniate was dissolved in deionized water at a concentration of 5 mg/mL, and NaIO4 in deionized water was added dropwise with 1:4, 1:2.6 and 1:2 M ratio of NaIO4 to sodium hyaluroniate (disaccharide unite) to obtain 25%, 37.5% and 50% O-HA. Then, the mixed solution was stirred for 2 h in darkness at room temperature, followed by addition of ethylene glycol to react with excess NaIO4 for another 1 h. O-HA in solution was purified by extensive dialysis against deionized water for 48 h, followed with freeze-drying to obtain dry product. 1H NMR was performed to confirm the formation of aldehyde groups in O-HA.
2.4. Fabrication and characterization of hybrid hydrogel
2.4.1. Fabrication and of hybrid hydrogel
Hybrid hydrogel, also assigned as CM/HC hydrogel, was obtained by mixing equal volume of GC-CM solution and O-HA solution. Briefly, 2 wt%, 2.5 wt% and 3 wt% GC solution were prepared by dissolving certain amounts of GC in 1 mL PBS (pH 7.4), respectively. 2 mg CM powder was directly dissolved in 1 mL of 2 wt%, 2.5 wt% or 3 wt% GC solution to form GC-CM solution. 2 wt% with 25% O-HA, 2.5 wt% with 37.5% O-HA and 3 wt% with 50% O-HA solutions were prepared by dissolving certain amounts of O-HA in 1 mL PBS (pH 7.4), respectively. Hybrid hydrogels were fabricated with different mass ratio of O-HA and GC, as well as different total solid contents: (Ι) 2 wt% with 25% O-HA + 2 wt% GC-CM, (ΙΙ) 2.5 wt% with 37.5% O-HA + 2.5 wt% GC-CM, (ΙΙΙ) 3 wt% with 50% O-HA + 3 wt% GC-CM. HC hydrogels were made by mixing equal volume of GC and O-HA solutions.
2.4.2. Rheological analysis of hybrid hydrogel
Rheological measurement was conducted on a TA DHR-2 rheometer with 25 mm parallel plate attached to a transducer. Storage modulus G′ and loss modulus G″ were analyzed in these studies. G′ and G″ of CM/HC hydrogels, (Ι) 2 wt% with 25% O-HA + 2 wt% GC-CM, (ΙΙ) 2.5 wt% with 37.5% O-HA + 2.5 wt% GC-CM, (ΙΙΙ) 3 wt% with 50% O-HA + 3 wt% GC-CM, were tested under a 1% strain with the angular frequency from 0.1 to 100 rad/s. G′ and G″ of CM/HC hydrogel (3 wt% with 50% O-HA + 3 wt% GC-CM) were measured under strain sweep at 1 Hz frequency. Continuous step-strain sweep of CM/HC hydrogel (3 wt% with 50% O-HA + 3 wt% GC-CM) was performed at 1 Hz frequency and strain changing from 1% to 400% with 60 s interval.
2.4.3. Swelling ratio of hybrid hydrogel
Swelling ratio of CM/HC hydrogel was calculated through gravimetric method. The weighed freeze-dried CM/HC hydrogel discs were immersed in PBS solution (pH 7.4) at 37 °C. At specific time intervals, the swollen discs were taken out and weighed. All experiments were performed in triplicate. The swelling ration was calculated using the following equation: Swelling ratio (%) = (Ws – Wd)/Wd × 100%, where Ws and Wd were the weight of hydrogels at swelling state and freeze-dried state.
2.4.4. Injectability and self-healing of hybrid hydrogel
The injectable property of CM/HC hydrogel was evaluated as follows. GC-CM and O-HA solutions stained by rhodamine B were separately loaded into the two syringes of Medicine Mixer, and injected onto board to make the characters of “ZJU” and “cross-ring” shape. Self-healing performance of CM/HC hydrogel was exhibited as follows. Two pieces of heart-shaped hybrid hydrogel stained by methylene blue and rhodamine B were equally cut into two parts, and separate pieces with different colors were combined into new heart-shaped pieces for 10 min at room temperature. Self-healing property of mixed heart-shaped hydrogel was observed by holding its structure under gravity. Two disc-shaped hybrid hydrogels were conducted following above procedure, but immersed in PBS for 2 h before lift under gravity.
2.4.5. Cytotoxicity of HC hydrogel
HC hydrogel extracts were utilized to test their cytotoxicity as follows. HC hydrogel was extracted using DMEM with 10% FBS for 48 h to obtain 100% stock solution at 37 °C. Then, 12.5%, 25% and 50% dilutions of 100% stock solution were prepared to obtain different concentration of extracted solution. C3A, HUVEC and LX2 cells were seeded into 96-well plates and cultured for 12 h, respectively. After that, culture medium were replaced by 12.5%, 25%, 50% and 100% extracted solutions, and incubated for another 24 h respectively. Cell viability of each group was measured by Cell Counting Kit-8, and expressed by the percentage of living cells versus control cells.
2.4.6. Antibacterial activity of hybrid hydrogel
Gram-negative bacteria P.aeruginosa and Gram-positive bacteria S.aureus were used to investigate surface antibacterial activity. Precursor solution was sterilized though 0.22 μm syringe filters, and 200 μL CM/HC hydrogels were fabricated in 48-well plate with 1 h incubation at room temperature. After hybrid hydrogels were rinsed with sterilized PBS, 10 μL of 106 CFU/mL bacterial suspension was evenly spread onto each surface of hybrid hydrogel and incubated at 37 °C for 4 h. Tissue culture treated polystyrene surface was utilized as a control. Subsequently, 1 mL sterilized PBS was added into each well to re-suspend survived bacteria, and 50 μL suspension was spread onto the surface of LB agar gel. After incubation for 12 h at 37 °C, the CFUs on LB agar gel plates were counted.
2.4.7. In vitro hemostatic ability of hybrid hydrogel
Heparinized whole blood from mouse was used to evaluate the hemostatic ability of GC solution, O-HA solution, HC hydrogel and CM/HC hydrogel. 200 μL heparinized whole blood was blended with 200 μL of GC solution, O-HA solution, HC hydrogel or CM/HC hydrogel in a tube, respectively. Self-supporting gel formation following tube inversion was recorded.
2.4.8. Tissue adhesive properties of hybrid hydrogel
To investigate tissue adhesion of CM/HC hydrogel, torsion experiment was firstly performed. CM/HC hydrogel stained by rhodamine B in situ formed on the surface of fresh porcine skin. After standing by at room temperature for 20 min, torsion stress with diverse directions and distribution was exerted on porcine skin to examine the adhesive flexibility.
2.4.9. In vitro release of protein loaded in hydrogel
1 mL HC hydrogel containing 1 mg FITC-BSA was immersed in 3 mL PBS solution (pH 7.4) at 37 °C. At specific time interval, 1 mL PBS was taken out and replaced by fresh PBS to continue incubation. The concentration of FITC-BSA was obtained according to standard curve, and then cumulative release was calculated. All experiments were conducted in triplicates.
2.5. In vivo degradation behavior of hybrid hydrogel
Sprague-Dawley rats (SD rats) were provided by Laboratory Animal Center of Zhejiang University. All experimental protocols were approved by the Animal Experimental Ethical Inspection of the First Affiliated Hospital, Zhejiang University School of Medicine, and all animals received humane care according to the criteria of Guide for the Care and Use of Laboratory Animals. Six-week-old male SD rats (180–200 g) were employed to evaluate the in vivo degradation behavior. The rats were randomly divided into different groups with three rats per group. 400 μL of CM/HC hydrogel was administrated by dorsal subcutaneous injection. The rats were sacrificed at 0h, 12h, 1d, 3d, 5d and 7d, respectively. Then, the skins of rats were incised to expose injection site to observe hydrogel degradation. Tissues around injection site was removed and fixed with 10% formalin, followed by dehydration and embedded in paraffin. For histopathological analysis, tissue sections were stained with hematoxylin-eosin (HE) and masson-trichrome (MT).
2.6. Hemostatic effect of hybrid hydrogel after partial liver resection
To evaluate the hemostatic effect of hybrid hydrogel, six-week-old male SD rats (180–200 g) were used to generate bleeding model. The rats were randomly divided into different groups with four rats per group according to the materials administered: control group, Fibrin glue group, HC hydrogel group and CM/HC hydrogel group. The rats were anesthetized with isoflurane. After abdominal incision, serous fluid around liver was gently removed, and a pre-weighed filter paper on a parafilm was carefully placed beneath the exposed liver. To generate bleeding model, 10% volume of the left lateral lobe was quickly resected to bleed with a surgical scissors. There is no treatment at the initial bleeding in control group, while Fibrin glue, HC hydrogel and CM/HC hydrogel were immediately injected onto the surface of resection in each experimental group. When the liver bleeding was stopped, the mass of blood absorbed on filter paper was measured, and the bleeding time after partial liver resection was recorded.
2.7. Anti-adhesion effect of hybrid hydrogel after partial liver resection
Six-week-old male SD rats (180–200 g) were divided into four groups with six rats per group: control group, Fibrin glue group, HC hydrogel group and CM/HC hydrogel group. After anesthetized with isoflurane, the liver of rat was exposed through abdominal incision. To assess anti-adhesion effect, 10% volume of the left lateral lobe was quickly resected with a surgical scissors to form a cutting surface. Then, Fibrin glue, HC hydrogel and CM/HC hydrogel were spread around cutting site and adjacent region to form a thick gel layer, respectively. Four days after surgery, second abdominal incisions were performed on treated rats of each group, and the intra-abdominal adhesion between liver and surrounding tissue were evaluated macroscopically. The severity of adhesion was graded semiquantitatively from 1 (none), 2 (mild), and 3 (moderate) to 4 (severe) by a modified method [14]. Liver tissue samples of each group were collected and fixed in 10% formalin solution to conduct HE and MT staining.
2.8. Evaluation of enhanced regeneration after partial liver resection
Six-week-old male SD rats (180–200 g) were divided into five groups with six rats per group: Sham group, Control group, Fibrin glue group, HC hydrogel group and CM/HC hydrogel group. To generate liver regeneration model, the partial liver resection was conducted as followed: the left lateral lobe, caudate lobe and right inferior lobe were resected, and 5% right superior lobe was resected to create a cutting surface. In Control group, medical absorbent cotton was used to stop bleeding in cutting surface of right superior lobe. Fibrin glue, HC hydrogel and CM/HC hydrogel were immediately injected around wound in right superior lobe to form thick gel layer, respectively. Sham group only underwent abdominal incision and suture without partial liver resection. All rats were sacrificed 24 h after partial liver resection, and their blood and liver tissue samples were collected for further investigation. The liver weight and body weight were recorded at the time of sacrifice to calculate the ratio of liver to body weight. Blood samples were centrifuged to separate serum. Serum levels of bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using an automatic biochemistry analyzer.
2.9. Quantitative real-time PCR (qRT-PCR) and Western blot analysis
Total RNA of liver tissue was extracted using TRIzol reagent according to manufacturer's instructions. Then, 1 μg of total RNA was utilized to produce cDNA with Prime Script™ RT Reagent Kit. qRT-PCR was performed with TB Green Premix Ex Taq ΙΙ Kit in Applied Biosystems 7500 Fast Real-Time PCR System. The primers used for qRT-PCR were listed in Table S2. Expression levels of target genes were normalized to GAPDH gene, and calculated using comparative threshold cycle (CT) method. Liver tissue samples were lysed in RIPA lysis buffer to obtain total proteins. The protein concentration was determined by BCA assay. Targeted proteins in lysates were separated by SDS-PAGE electrophoresis, and were immune-blotted with their specific antibodies. The bands of proteins were visualized by using chemi-luminescence substrate. Intensity of protein bands was normalized against β-actin and was quantified by Image J software.
2.10. Histopathology and immunohistochemistry
Liver tissue samples were fixed in 10% formalin, followed by dehydration and embedded in paraffin. Then, 5-μm thickness tissue sections were prepared by a microtome according to standard protocols. For histopathological analysis, tissue sections were stained with HE and MT. To conduct immunohistochemical analysis, tissue sections were stained with primary antibodies of Ki67, PCNA, Cyclin D1, and subsequently incubated with second antibodies, followed by treatment with horseradish peroxidase and 3,3′-diaminobenzidine solution. At last, tissue sections were counterstained with hematoxylin.
2.11. Statistical analysis
All experiments were conducted at least three independent repeats. All data were showed as mean ± standard deviation. One-way ANOVA was used to determine differences between groups. P-values < 0.05 (*) or < 0.01 (**) were considered to indicate statistical significance.
3. Results and discussion
3.1. Culture of MenSCs and preparation of CM
To obtain MenSCs-derived CM, MenSCs were isolated and cultured. Morphology and immunophenotype of MenSCs are similar to mensenchymal stem cells. MenSCs showed a spindle-shaped, fibroblast-like morphology (Fig. 1a). They expressed high levels of CD29, CD73, CD90, and did not express CD34 or HLA-DR (Fig. S1). Collected conditioned medium from MenSCs culture was lyophilized into powder for fabrication of hybrid hydrogel (Fig. 1a). Due to the different source of MenSCs, it seems like that the variability of biological activities in CM from batch to batch can't be avoided, but the preparation standardization could remarkably reduce the variability. The preparation standardization includes (1) screening criteria for MenSCs donors, such as age, marriage and childbirth, disease status, etc. (2) standard operations and conditions for collection, isolation and culture of MenSCs, (3) CM preparation utilizing same passage of MenSCs, (4) standard operations and conditions for lyophilization and storage of CM.
3.2. Preparation and characterization of hybrid hydrogel
In typical synthetic route, O-HA was prepared by reacting sodium hyaluronate with sodium periodate, where the vicinal hydroxyl groups of sugar ring were oxidized into dialdehydes (Fig. S2). Hybrid hydrogel, also assigned as CM/HC hydrogel, was formed by blending precursor solutions containing O-HA, GC and CM at room temperature. The mixture transformed into a transparent hydrogel based on the Schiff-base reaction between aldehyde and amino groups in polymers (Fig. 1b). Gelation time was determined via vial inverting method and listed in Table S1. Mechanical strength of the CM/HC hydrogel was measured by rheological analysis with different mass ratios of O-HA and GC, as well as different total solid contents. The results indicated that the group of CM/HC hydrogel (3 wt% with 50% O-HA + 3 wt% GC-CM) possessed stronger mechanical property than the other two groups (Fig. S3a). Viscoelastic property of hybrid hydrogel was presented by determination of its linear range. It was indicated that G′ and G″ were independent of the strain up to about 200%. When the strain was larger than critical point, G′ would be smaller than G″, suggesting the transformation from gel state into quasi-liquid state (Fig. S3b). To investigate the injectability of CM/HC hydrogel, two precursor solutions were separately loaded into the two syringes of medicine mixer for injection. As shown in Fig. 1c, the injected solution quickly gelled and formed the characters of “ZJU” and “cross-ring” shape, demonstrating the availability and convenience for biomedical applications.
As the hybrid hydrogel is applied in vivo, it may suffer the damage from external mechanical force. In that case, self-healing property of hydrogel could retain the structural integrity for prolonged lifetime. Macroscopic experiments were performed to visually evaluate the self-healing capacity on two precast heart-shaped CM/HC hydrogels with pink (stained with rhodamine B) and blue color (stained with methylene blue). It was shown that two pieces of hydrogels were able to integrate into one heart-shaped hydrogel without any external stimulus, and the self-healed hydrogel could maintain integrity under gravity (Fig. 1d). In addition, the self-healing property of CM/HC hydrogel was merely influenced after immersion in PBS solution (Fig. S4). Self-healing capacity of hybrid hydrogel mainly came from the dynamic equilibrium of uncoupling and recoupling of Schiff-base linkages in hydrogel networks. To further investigate self-healing property, continuous step alterations of strain between 1% and 400% were applied to the CM/HC hydrogel. It was found that 400% strain disrupted hydrogel networks and promoted gel-to-sol transition, whereas the G′ quickly restored to the initial value under 1% strain, indicating the recovery of hydrogel structure via sol-to-gel transition (Fig. S3c). Hence, under the cycles of 1% and 400% strain, CM/HC hydrogel still kept the capability of rapid recovery, and could restore to original structure.
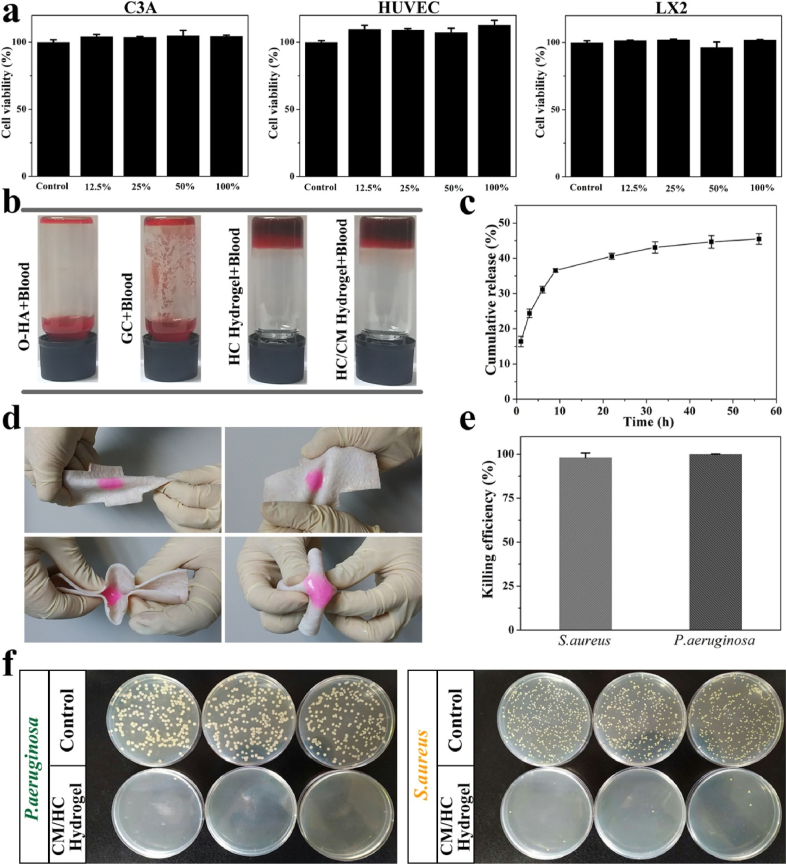
To meet the demand of potential applications in liver, the cytotoxicity of hydrogel was investigated. To this end, C3A, HUVEC and LX2 cells were cultured to assess the biocompatibility of hydrogel extract solution. The cell viabilities kept almost 100% at different concentrations after 24 h incubation for all three cell lines compared with control group (Fig. 2a). This is attributed to the fact that the compositions of hybrid hydrogel were hyaluronic acid and chitosan, both of which are well-known natural biomaterials.
Fig. 2.
a) Cytotoxicity of hydrogel extract solution in C3A, HUVEC and LX2 cells. b) Hemostatic effects of O-HA, GC, HC hydrogel and CM/HC hydrogel on heparinized rat whole blood. c) In vitro cumulative release of FITC-BSA loaded in hybrid hydrogel. d) Torsion tests of CM/HC hydrogel on fresh porcine skin. e) Killing efficiency of hybrid hydrogel against Gram-negative bacteria P.aeruginosa and Gram-positive bacteria S.aureus. Data represent mean ± SD, n = 3. f) CFUs of P.aeruginosa and S.aureus after surface antibacterial assay with CM/HC hydrogel.
Would healing can be impaired due to bacterial infection. Antibacterial capability was desired for hybrid hydrogel in its application. To investigate the antibacterial efficacy, the antibacterial activities of CM/HC hydrogel against Gram-negative bacteria P.aeruginosa and Gram-positive bacteria S.aureus were evaluated by surface antibacterial assay (Fig. 2f). At the initial bacterial concentration of 106 CFU/mL, new colony of these two bacterial were barely found on agar gels after incubation with CM/HC hydrogel, exhibiting almost 100% killing efficiency under this bacterial concentration (Fig. 2e). The valid antibacterial activities probably came from the reaction of aldehyde groups with bacterial outer membranes, and protonated amine groups of glycol chitosan that disrupt bacterial membranes by strong electrostatic interaction [15].
In vitro hemostatic effect of hybrid hydrogel was tested using heparinized rat whole blood. It was found that the mixture of liquid blood and O-HA or GC remained free-flowing liquid in a short time. However, the liquid blood was quickly transformed into a blood gel when HC or CM/HC hydrogel was injected, which could hold its own weight upon tube inversion (Fig. 2b). During this process, it was likely that Schiff-base formation between aldehyde and amino groups of blood cells or plasma, swelling effects of hydrogel (Fig. S5), as well as inherent viscosity of biomaterials all contributed to the blood gelation [16].
For potential applications in hemostasis, hydrogel should have strong tissue adhesion property to bond themself around wound surface to control bleeding. To confirm this property, torsion tests on porcine skin were performed. CM/HC hydrogel was prepared in situ on surface of fresh porcine skin. When applied torsion stress with different directions, CM/HC hydrogel still adhered tightly to porcine skin and kept intact, indicating the tissue-adhesive ability and deformability (Fig. 2d). This strong tissue adhesion ability could be attributed to synergistic effects of Schiff-base linkages between aldehyde and amino groups in tissues, as well as electrostatic interaction between protonated amino groups and phospholipids of cell membrane [17].
MenSCs-secreted CM comprises a variety of soluble protein factors targeting multiple mitogenic pathways. The loading and controllable release of CM in hybrid hydrogel is important to exert the effects of cytokines for promoting liver regeneration. To evaluate the profile of in vitro release in hybrid hydrogel, FITC-BSA was selected as model protein to assess the cumulative release. As shown in Fig. 2c, the high release rate was occurred during first 10 h, followed with sustained release of loaded proteins until final time point. Controllable release of 40% loaded proteins was obtained at 22 h. This time interval covered the period from priming phase to proliferative phase during liver regeneration [18].
3.3. In vivo degradation of hybrid hydrogel
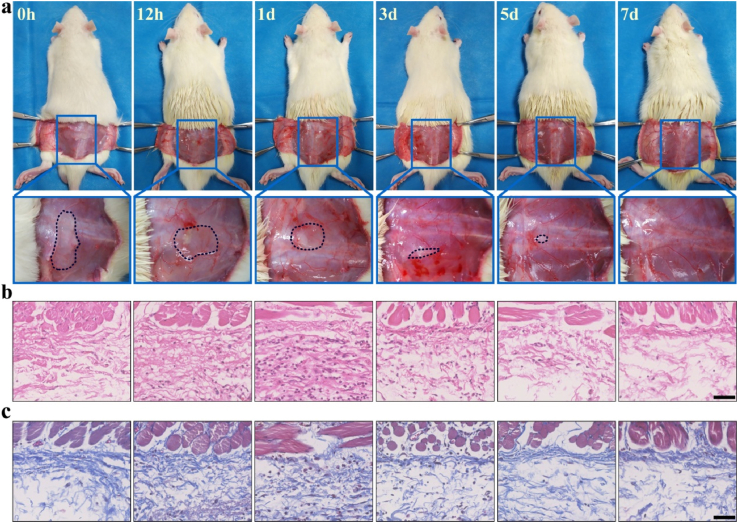
In vivo degradation of hybrid hydrogel was observed to evaluate the biodegradation and biocompatibility. The amount of hydrogel decreased gradually over time, and the hydrogel disappeared around 7 days after injection. There was no redness and swelling around the injection site in the process of degradation, suggesting good biocompatibility for hybrid hydrogel (Fig. 3a). Furthermore, HE and MT staining were employed to assess the histopathological changes. The results exhibited that the number of neutrophils and macrophages increased following hybrid hydrogel injection with significant increment at 24 h. Then, these inflammatory cells faded away along with degradation of hybrid hydrogel (Fig. 3b). The amount of collagen kept the same level from the presence of hybrid hydrogel to their complete degradation at injection site. In addition, only small quantities of fibroblasts were found around injection site without obvious proliferation in the process of degradation (Fig. 3c). These results indicated that hybrid hydrogel just caused slight invasion of fibroblasts and deposition of collagen, which was beneficial for adhesion prevention.
Fig. 3.
a) Gross observation of in vivo degradation for hybrid hydrogel at different time points. b) HE staining of the tissues around the injection site. c) MT staining of the tissues around the injection site. Scale bar: 50 μm.
3.4. Hemostatic effect after partial liver resection
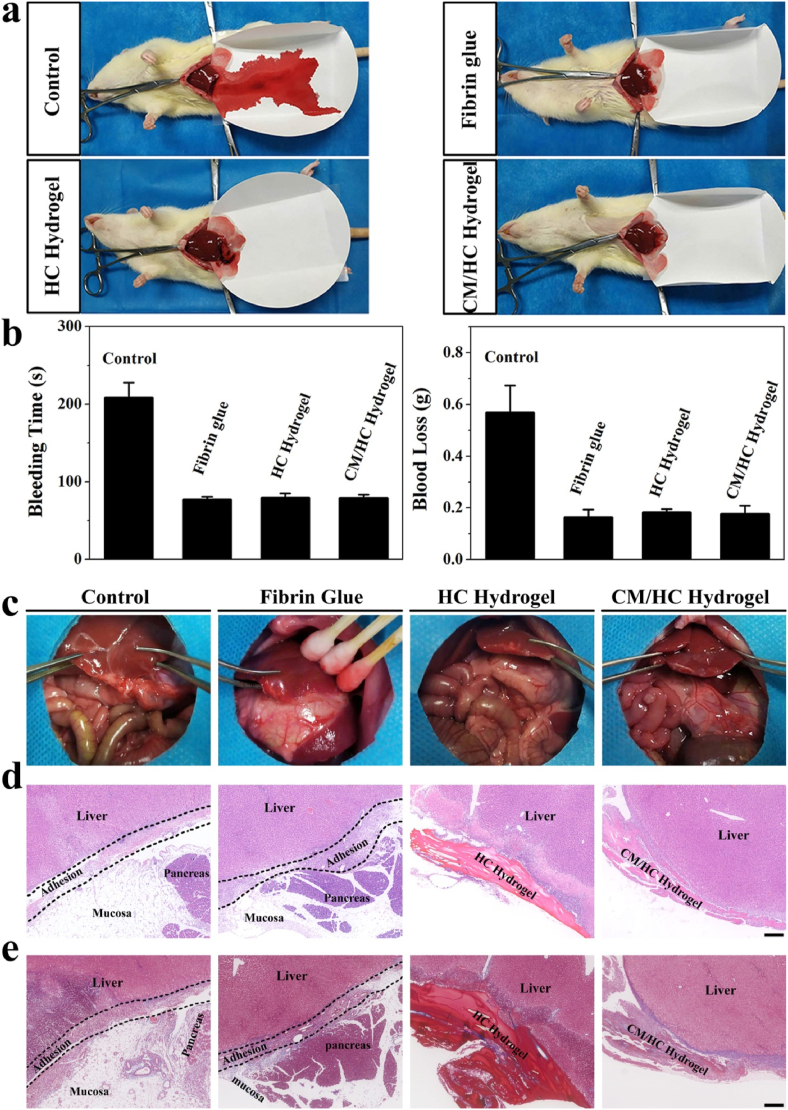
Liver tissue is a fragile structure with rich blood supply and specialized microvascular networks, which is incapable of smooth-muscle contraction to reduce blood flow. Liver bleeding is often difficult to control by conventional hemostatic methods such as suturing or cauterization. As an alternative option, fibrin glue is currently the most used hemostatic agents in liver surgery. To demonstrate the hemostatic capability of hybrid hydrogel, it was utilized to stanch bleeding after partial liver resection in rat (Fig. 4a). It was observed that a large amount of blood was lost from cutting surface under the condition of blood coagulation in control group without any treatment. In contrast, fibrin glue-treatment achieved quick stoppage of bleeding with a small quantity of blood loss, mimicking the final stage of coagulation cascade. Similarly, HC and CM/HC hydrogels rapidly finished hemostasis with limited blood loss. Quantitative results of bleeding time and total blood loss were consistent with the experimental observation (Fig. 4b). With the tissue adhesion and rapid hydrogel formation, hybrid hydrogel was able to fix itself to the tissues around cutting surface, forming a sticky sealant to prevent further hemorrhage. Hence, the hemostatic capability of hybrid hydrogel was comparable to that of fibrin glue.
Fig. 4.
a) Hemostatic capabilities of fibrin glue, HC hydrogel and CM/HC hydrogel after partial liver resection on rats. b) Bleeding time and blood loss of four groups under different treatments. Data represent mean ± SD, n = 4. c) Gross observation of intra-abdominal conditions for different treatments at 4 day after partial liver resection. d) HE staining of damaged liver tissues under different treatments to evaluate intra-abdominal adhesion. e) MT staining of damaged liver tissues under different treatments to assess intra-abdominal adhesion. Scale bar: 200 μm.
3.5. Anti-adhesion effect after partial liver resection
Intra-abdominal adhesion occurs as the consequence of mechanical or other damage after surgery, causing complications such as ileus or chronic abdominal pain. In order to assess the efficacy of adhesion prevention, the intra-abdominal conditions after each treatment were evaluated. With regard to adhesion scoring on different treatments, HC and CM/HC hydrogel-treated groups got lower semi-quantitative scores than that in control and fibrin glue-treated groups (Fig. S6). Severe adhesions could be observed between liver tissue and greater omentum in control and fibrin glue-treated groups. On the contrary, HC and CM/HC hydrogel treatments remarkably prevented the occurrence of intra-abdominal adhesion (Fig. 4c). HE and MT staining were performed further to conduct histological analysis. Adhesion zone emerged along the edge of resection, consisting of hepatic tissue, mucosa, inflammatory cells and deposited collagen (Fig. 4d and e). Previous studies reported contradictory results regarding the effects of fibrin glue on adhesion formation [19]. Our study indicated that fibrin glue was similar to control group in terms of adhesion formation without significant difference. Benefiting from physico-chemical properties, hybrid hydrogel has a major advantage over fibrin glue in preventing adhesion formation.
3.6. Enhanced regeneration after partial liver resection
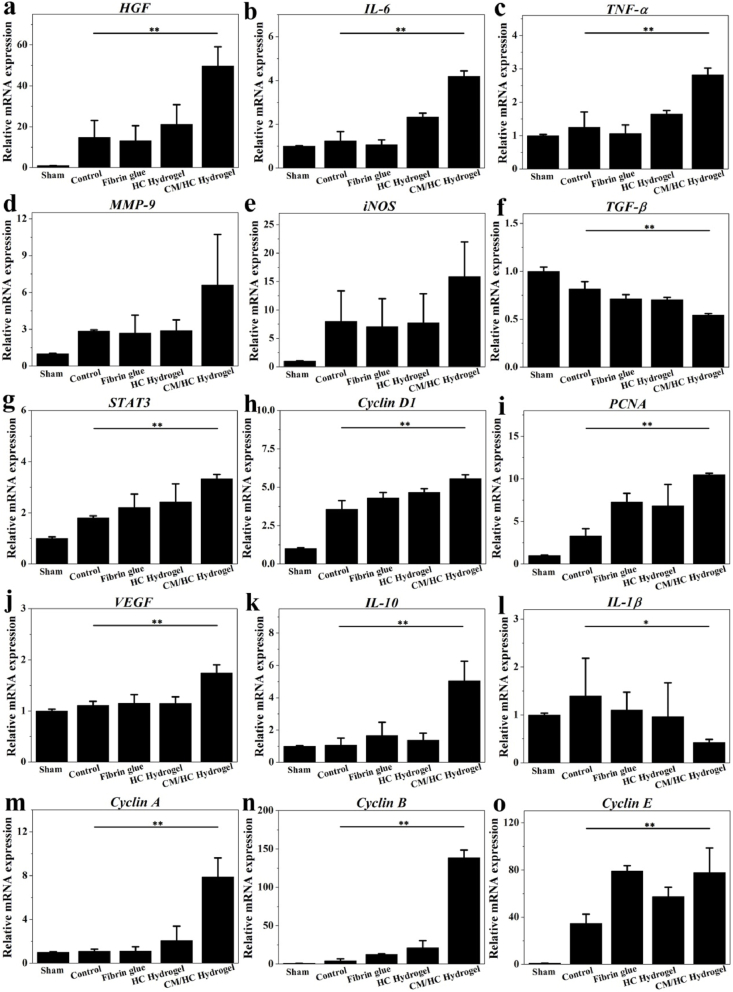
Rapid tissue regeneration and restoration of liver function are critical after partial liver resection, especially for patients with chronic liver disease. To explore the benefits from CM/HC hydrogel for liver regeneration, related gene expression in this process were investigated by qRT-PCR. Initiation of mitogenic response is triggered by cytokine stimulation after liver injuries. Tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and hepatocyte growth factor (HGF) are crucial priming factors for hepatocytes to enter cell cycle for proliferation [20]. CM can bring higher mRNA expression of TNF-α, IL-6 and HGF in CM/HC hydrogel-treated group (Fig. 5a, b, c). In addition, some other factors are also important to participate in early events involved in liver regeneration. Matrix metalloprotease 9 (MMP-9) plays an important role in extracellular matrix remodeling and angiogenesis, which is likely to up-regulate TNF-α, HGF and vascular endothelial growth factor (VEGF) [21]. There was a elevation of MMP-9 expression after CM/HC hydrogel treatment compared with other groups (Fig. 5d). Inducible nitric oxide synthase (iNOS) mediates the formation of cyclooxygenase-2 and the synthesis of prostaglandins that control the regenerative process [22]. The mRNA expression of iNOS increased in CM/HC hydrogel-treated group than that in control and Fibrin glue groups (Fig. 5e). Transforming growth factor β (TGF-β) is one of the growth-terminating signals during liver regeneration [23]. Interleukin-1β (IL-1β) is another mito-inhibitor factor leading to diminished hepatocyte DNA synthesis [24]. CM/HC hydrogel treatment can decrease the mRNA up-regulation of TGF-β and IL-1β (Fig. 5f, l). Downstream events of initial signal transduction cascades involve the activation of intracellular signals and cytokines, such as signal transducer and activator of transcription 3 (STAT3), Cyclin D1, Cyclin A, Cyclin B, Cyclin E, proliferating cell nuclear antigen (PCNA) and VEGF. STAT3 is an important signaling molecule associated with cell cycle events [25]. Cyclin D1, Cyclin A, Cyclin B, Cyclin E are essential regulators for both S-phase and mitosis [26]. PCNA is a biological marker in cell nucleus to assess cell proliferation. VEGF is mitogenic for liver sinusoidal endothelial cells to reconstruct liver tissues. Up-regulation of mRNA expression for STAT3, Cyclin D1, Cyclin A, Cyclin B, Cyclin E, PCNA and VEGF were observed in CM/HC hydrogel-treated group than that in other groups (Fig. 5g, h, i, j, m, n, o). Moreover, induction of anti-inflammatory response was shown by elevated mRNA expression of interleukin-10 (IL-10) after CM/HC hydrogel treatment (Fig. 5k). Therefore, loading and controllable release of CM in hybrid hydrogel significantly enhanced gene expressions for regenerative response and anti-inflammation, while depressed expressions of mito-inhibitor factors to accelerate tissue repair.
Fig. 5.
Relative mRNA expression associated with liver regeneration under different treatments at 24 h after partial liver resection. Data represent mean ± SD, n = 6; *p < 0.05, **p < 0.01. HGF: hepatocyte growth factor; TNF-α: tumor necrosis factor α; IL-6: interleukin 6; MMP-9: matrix metalloprotease 9; VEGF: vascular endothelial growth factor; iNOS: inducible nitric oxide synthase; TGF-β: transforming growth factor β; IL-1β: interleukin-1β; STAT3: signal transducer and activator of transcription 3; PCNA: proliferating cell nuclear antigen; IL-10: interleukin 10.
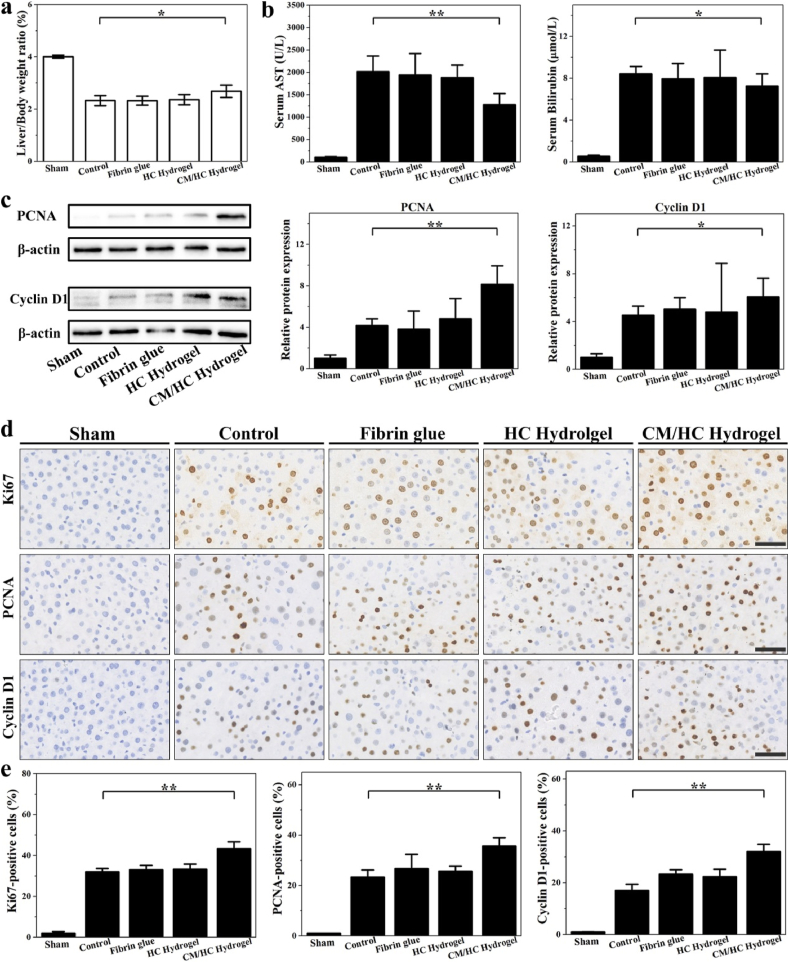
The liver to body weight ratio was increased in rats treated with CM/HC hydrogel compared to other groups (Fig. 6a). Partial liver resection caused remarkable elevation of AST, ALT and bilirubin in serum. After different treatments, AST, ALT and bilirubin in CM/HC hydrogel group showed lower serum levels, indicating the accelerated recovery of liver function (Figs. 6b and S7). As mentioned above, PCNA and Cyclin D1 are well-known biological markers for proliferating cells. Elevation of protein levels of PCNA and Cyclin D1 were observed in CM/HC hydrogel group through Western blot analysis (Fig. 6c). To further investigate the effects of CM/HC hydrogel treatment on liver regeneration after partial liver resection, immune-histochemical staining was performed (Fig. 6d and e). The stained cells of Ki67, PCNA and Cyclin D1were examined to evaluate the tissue regeneration. Ki67 antigen is a nuclear protein associated with ribosomal RNA transcription, and is expressed exclusively in proliferating cells. The results clearly demonstrated that the number of positive cells of proliferating markers was significantly higher in CM/HC hydrogel-treated groups, suggesting active cell replication in regenerative tissue.
Fig. 6.
a) Evaluation of liver regeneration by liver to body weight ratio under different treatments at 24 h after partial liver resection. Data represent mean ± SD, n = 6; *p < 0.05, **p < 0.01. b) Serum levels of AST and bilirubin in different groups at 24 h after partial liver resection. Data represent mean ± SD, n = 6; *p < 0.05, **p < 0.01. c) Western blot analysis of PCNA and Cyclin D1 for cell proliferation in different groups at 24 h after partial liver resection. Data represent mean ± SD, n = 6; *p < 0.05, **p < 0.01. d) Immuno-histochemical staining of Ki67, PCNA and Cyclin D1 in liver tissues under different treatments at 24 h after partial liver resection. Scale bar: 50 μm. e) Percentage of Ki67-, PCNA- and Cyclin D1-positive cells in each group at 24 h after partial liver resection. Data represent mean ± SD, n = 6; *p < 0.05, **p < 0.01.
4. Conclusion
A multifunctional hybrid hydrogel, consisting of O-HA, GC and MenSCs-derived CM, was developed for hemostasis, adhesion prevention and promoting regeneration after partial liver resection. Due to the reversible Schiff-base reaction between aldehyde and amino groups in O-HA and GC, the hybrid hydrogel was imparted with injectability and self-healing capability. The hybrid hydrogel also obtained the abilities of hemostasis, tissue adhesion, and anti-bacterial activities because of dense positive charges, Schiff base, swelling property or inherent viscosity. In addition, loading and controllable release of MenSCs-derived CM were achieved in hybrid hydrogel. Upon the proposed multifunctional hybrid hydrogel, the bleeding of partial liver resection can be quickly stopped owing to the hemostatic property. Moreover, post-operatively intra-abdominal adhesion formation was significantly reduced in hybrid hydrogel-treated resection surface. Furthermore, controllably released cytokines of CM from hybrid hydrogel significantly enhanced hepatic cell proliferation and tissue regeneration after partial liver resection.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Zuhong Li: Conceptualization, Methodology, Investigation. Yalei Zhao: Conceptualization, Methodology, Investigation. Xiaoxi Ouyang: Methodology, Formal analysis, Investigation. Ya Yang: Investigation, Resources. Yangjun Chen: Conceptualization, Methodology, Supervision. Qixia Luo: Investigation. Yanhong Zhang: Resources. Danhua Zhu: Resources. Xiaopeng Yu: Resources. Lanjuan Li: Conceptualization, Supervision, Project administration, Funding acquisition.
Acknowledgements
This work was supported by the Independent Project Fund of the State Key Laboratory for Diagnosis and Treatment of Infectious Disease, Zhejiang Provincial Natural Science Foundation of China (LQ19C120001), Zhejiang Provincial Natural Science Foundation of China (LY17H030005), National Key Research and Development Program of China (2019YFC0840600 & 2019YFC0840609).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.10.001.
Contributor Information
Yangjun Chen, Email: chenyj@wmu.edu.cn.
Lanjuan Li, Email: ljli@zju.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jamagin W.R., Gonen M., Fong Y.M., DeMatteo R.P., Ben-Porat L., Little S., et al. Improvement in Perioperative outcome after hepatic resection - analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clavien P.-A., Petrowsky H., DeOliveira M.L., Graf R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 3.Wei A.C., Poon R.T.P., Fan S.T., Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.C., Jeong H.J., Lee S.K., Kim S.J. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling. STEM CELLS Translational Medicine. 2016;5:816–825. doi: 10.5966/sctm.2015-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonstra E.A., Molenaar I.Q., Porte R.J., de Boer M.T. Topical haemostatic agents in liver surgery: do we need them? HPB. 2009;11:306–310. doi: 10.1111/j.1477-2574.2009.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukushima K., Tanaka H., Srinivasan P.K., Pawlowsky K., Kogel B., Uemoto S., et al. Hemostatic efficacy and safety of the novel medical adhesive, MAR VIVO-107, in a rabbit liver resection model. Eur. Surg. Res. 2018;59:48–57. doi: 10.1159/000481818. [DOI] [PubMed] [Google Scholar]
- 7.Flegeau K., Pace R., Gautier H., Rethore G., Guicheuex J., Le Visage C., et al. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017;247:589–609. doi: 10.1016/j.cis.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Dimatteo R., Darling N.J., Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018;127:167–184. doi: 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G.P., Yu Y.R., Wu X.W., Wang G.F., Ren J.N., Zhao Y.J. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018;28:1801386. [Google Scholar]
- 10.Le Blanc K., Pittenger M.F. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 11.Alcayaga-Miranda F., Cuenca J., Luz-Crawford P., Aguila-Diaz C., Fernandez A., Figueroa F.E., et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Xiang B.Y., Wang X.J., Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 2017;8:9. doi: 10.1186/s13287-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan H.P., Chu C.R., Payne K.A., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du X., Liu Y., Wang X., Yan H., Wang L., Qu L., et al. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C. 2019;104:109930. doi: 10.1016/j.msec.2019.109930. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H., Mei X., He Y., Mao H., Tang W., Liu R., et al. Fast and high strength soft tissue bioadhesives based on a peptide dendrimer with antimicrobial properties and hemostatic ability. ACS Appl. Mater. Interfaces. 2020;12:4241–4253. doi: 10.1021/acsami.9b18720. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Wang X., Wang Y., Hao J. Rapid-forming and self-healing agarose-based hydrogels for tissue adhesives and potential wound dressings. Biomacromolecules. 2018;19:980–988. doi: 10.1021/acs.biomac.7b01764. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z., Ni J., Chen L., Yu L., Xu J.W., Ding J.D. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials. 2011;32:4725–4736. doi: 10.1016/j.biomaterials.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed F.F., Khokha R. Thinking outside the cell: proteases regulate hepatocyte division. Trends Cell Biol. 2005;15:555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann N.E., Siddiqui S.A., Agarwal S., McKellar S.H., Kurtz H.J., Gettman M.T., et al. Choice of hemostatic agent influences adhesion formation in a rat cecal adhesion model. J. Surg. Res. 2009;155:77–81. doi: 10.1016/j.jss.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Michalopoulos G.K., Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021;18:40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim T.H., Mars W.M., Stolz D.B., Michalopoulos G.K. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 22.Hortelano S., Dewez B., Genaro A.M., Diazguerra M.J.M., Bosca L. Nitric-oxide is released in regenerating liver after partial-hepatectomy. Hepatology. 1995;21:776–786. [PubMed] [Google Scholar]
- 23.Oh S.H., Swiderska-Syn M., Jewell M.L., Premont R.T., Diehl A.M. Liver regeneration requires Yap1-TGF beta-dependent epithelial-mesenchymal transition in hepatocytes. J. Hepatol. 2018;69:359–367. doi: 10.1016/j.jhep.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulton R., Woodman A., Calnan D., Selden C., Tam F., Hodgson H. Nonparenchymal cells from regenerating rat liver generate interleukin-1 alpha and -1 beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49–58. doi: 10.1053/jhep.1997.v26.pm0009214451. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Liang X.P., Kellendonk C., Poli V., Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J. Biol. Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht J.H., Hansen L.K. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397–404. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.