Highlights
-
•
Vascular disease is a significant part of the clinical picture in common dementias.
-
•
Multiple connections link vascular risk, vascular disease and cognitive impairment.
-
•
This has inspired multiple therapeutic approaches, see this special issue.
Keywords: Vascular cognitive impairment, VCID, Vascular dementia, Treatments, Clinical trials, Drugs
Abbreviations: AD, Alzheimer's disease; ADRD, Alzheimer's disease and related dementias; FTD, frontotemporal dementia; LBD, dementia with Lewy bodies; NAPA, national plan to address Alzheimer's disease; NIA, national institute on aging; PD, Parkinson's disease; SVD, small vessel disease; VaD, vascular dementia; VCID, vascular contributions to cognitive impairment and dementia
Abstract
Vascular cognitive impairment (VCI), encompassing vascular dementia, has been claimed as the “second-most common dementia” after Alzheimer Disease. Whether or not this is true, the clinical picture of most dementia in older people includes vascular disease. There are no validated pharmacological targets for prevention or treatment of VCI. This has inspired a multitude of potential treatment approaches, reflected by the articles in this Special Issue. These include in vitro testing of the novel oral anticoagulant dabigatran for protection against β-amyloid neurotoxicity, and an overview of neuroinflammation in VCI and the role of circulating markers (PIGF, VEGF-D) identified by the MarkVCID study. There are reviews of potential therapeutics, including adrenomedullin and nootropic preparations (exemplified by cerebrolysin). The role of sleep is reviewed, with possible therapeutic targets (5HT2A receptors). There is a clinical study protocol (INVESTIGATE-SVD) and a feasibility analysis for a secondary prevention trial in small vessel disease. Clinical data include secondary analyses of blood pressure and cerebral blood flow from a longitudinal clinical trial (NILVAD), differences between methylphenidate and galantamine responders and non-responders (STREAM-VCI), appraisal of treatment approaches in India, and primary outcomes from a randomised trial of Argentine tango dancing to preserve cognition in African American women (ACT). Treating vascular disease has great potential to improve global cognitive health, with public health impacts alongside individual benefit. Vascular disease burden varies across populations, offering the possibility of proactively addressing health inequity in dementia using vascular interventions. The next 5–10 years will witness cost-effective lifestyle interventions, repurposed drugs and novel therapeutics.
1. Introduction
This short perspective article is an Introduction to a Special Issue of CCCB on the theme “Therapeutic Approaches to Vascular Cognitive Impairment”. Our aim is to attempt definitions, and to give an overview of the current therapeutic approaches, relevant clinical trials, and future prospects. Scanning the papers within this Special Issue (listed in Table 1), we are struck by the diversity of subject matter. They range from a protein biochemical analysis of the anticoagulant drug dabigatran in cell cultures (Bihaqi et al., this issue) through meta-analyses of the potential therapeutic effects of biopharmaceutical natural products (Alsuleimani and Quinn, this issue), to a protocol for a randomised clinical trial in cerebral small vessel disease (SVD) (Blair et al., this issue) and secondary analyses of completed trials (De Heus et al., this issue; Leijenaar et al., this issue). And on to the possible therapeutic effects of dancing the Argentine tango (Wharton et al., this issue). All these studies have the ultimate goal of providing more therapeutic options for prevention and treatment of dementia (and lesser cognitive impairment) caused by vascular disease. All come from dedicated researchers, working in expert teams around the globe, under the umbrella of vascular contributions to cognitive impairment and dementia (VCID) [1], [2], [3]. The diversity of topics highlights the multifaceted nature of VCID, and the creativity of researchers in this area.
Table 1.
Papers in the Special Issue Therapeutic Approaches to Vascular Cognitive Impairment.
| Authors | Title | Affiliation | Countries |
|---|---|---|---|
| Alsulaimani RA, TJ Quinn | The Efficacy and Safety of Animal-Derived Nootropics in cognitive disorders: Systematic Review and Meta-Analysis. | Abdul-Aziz University, Jeddah; University of Glasgow | Saudi Arabia, UK |
| Arshad F, S Mondal, A Paplikar, S Rajendran, Y Kalkonde, S Alladi | Treatment approaches for Vascular Cognitive Impairment in India | National Institute of Mental Health and Neurosciences, Bengaluru; Action & Research in Community Health, Gadchiroli | India |
| Bihaqi SW, HV Rao, A Sen, P Grammas | Dabigatran reduces thrombin-induced neuroinflammation and AD markers in vitro: Therapeutic relevance for Alzheimer's disease | Ryan Inst for Neuroscience, University of Rhode Island; Thomas Jefferson University, Philadelphia; Rajendra Memorial Research Inst of Medical Sciences, Patna | USA, India |
| Blair GW, MS Stringer, MJ Thrippleton, FM Chappell, K Shuler, I Hamilton, DJ Garcia, FN Doubal, A Kopczak, M Duering, M Ingrisch, D Kerkhofs, J Staals, H van den Brink, T Arts, WH Backes, R van Oostenbrugge, GJ Biessels, M Dichgans, JM Wardlaw | Imaging NeuroVascular, Endothelial and STructural Integrity in prepAration to TrEat Small Vessel Diseases. The INVESTIGATE-SVDs study Protocol. Part of the SVDs@Target Project | University of Edinburgh; University Hospital, LMU Munich; Maastricht University; University Medical Center Utrecht; DZNE, Munich; SyNergy, Munich |
UK, The Netherlands, Germany |
| De Heus R, DLK De Jog, BL Lawlor, JAHR Claassen | Longitudinal changes in the control mechanisms for blood pressure and cerebral blood flow in Alzheimer's disease: secondary results of a randomized controlled trial | Radboud University Medical Center; Trinity College Dublin | The Netherlands, Ireland |
| Early F, EM Weekman, DM Wilcock | Pathologic sequelae of vascular cognitive impairment and dementia sheds light on potential targets for intervention. | University of Kentucky, Lexington | USA |
| Ihara M, K Washida, T Yoshimoto, S Saito | Adrenomedullin: a vasoactive agent for sporadic and hereditary vascular cognitive impairment | National Cerebral and Cardiovascular Center, Osaka | Japan |
| Leijenaar JF, S Ingala, CH Sudre, HJMM Mutsaerts, AE Leeuwis, WM van der Flier, P Scheltens, HC Weinstein, F Barkhof, J van Gerven, GJ Groeneveld, ND Prins | Differences between responders and non-responders to methylphenidate or galantamine in VCI | VU University Medical Center, Amsterdam; King's College London; Institute of Neurology, University College London; University Hospital Ghent; Onze Lieve Vrouwe Gasthuis West, Amsterdam; center for Human Drug Research, Leiden; Leiden University Medical Center | The Netherlands, UK, Belgium |
| Morrison E, DM Lyall, JP Pell, DF Mackay, FN Doubal, JM Wardlaw, TJ Quinn, SDJ Makin | Feasibility of trials of existing vascular prevention, as treatments for cerebrovascular small vessel disease in patients without symptomatic stroke. | University of Glasgow; University of Edinburgh; University of Aberdeen | UK |
| Wafford K | Aberrant waste disposal in neurodegeneration: why improved sleep could be the solution | Slowave Therapeutics Ltd, Guildford | UK |
| Wharton W, L Jeong, L Ni, AA Bay, RJ Shin, LE McCullough, H Silverstein, AR Hart, D Swieboda, W Hu, ME Hackney | A pilot randomized clinical trial of adapted tango to improve cognition and psychosocial function in african american women with family history of Alzheimer's disease (ACT trial) | Emory University, Atlanta; University of Georgia, Athens; Rutgers University; Birmingham/Atlanta VA Geriatric Research Clinical and Education Center | USA |
Papers are listed in alphabetical order of first author surname.
2. What is vascular dementia? And vascular cognitive impairment? And VCID?
The term vascular dementia (VaD) [4], [5], [6], [7], [8], [9] has a checkered history, with substantial prior debate as to what it means and its prevalence [5,8,9]. We take the view that pure VaD means clinical dementia that is primarily due to vascular disease, and that VaD is quite rare as a primary diagnosis that might be written by a care provider or neuropathologist. More common is vascular cognitive impairment, which embraces the fact that vascular disease can cause cognitive deficits beyond normal aging, yet not of sufficient severity to warrant a dementia diagnosis [8,10,11].
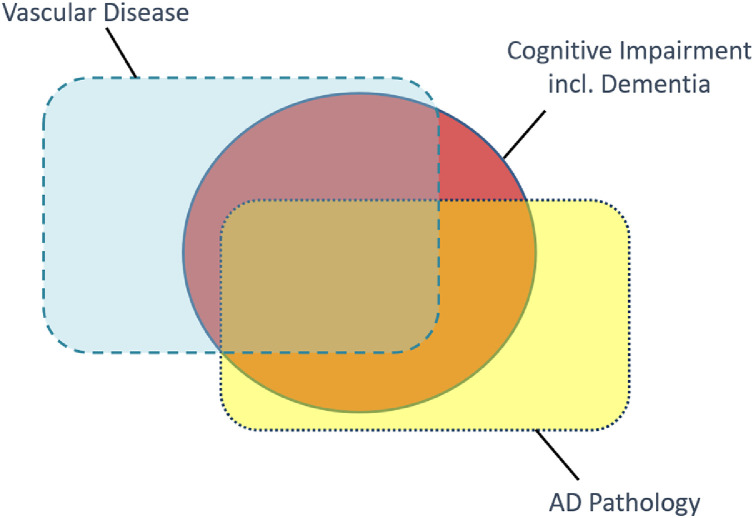
There are multiple vascular disease states underlying vascular cognitive impairment, the best known being SVD [4,12,13] (characterised by diffuse white matter hyperintensities, lacunes, microinfarcts and microbleeds) as well as atheroma, large vessel infarcts and intracerebral hemorrhage [[4], [5], [6],8]. The concept of VCID goes further, in recognizing that most patients with a clinical dementia diagnosis – usually due primarily to AD neurodegenerative pathology – are co-morbid with some degree of culprit vascular disease (see Fig. 1). VCID is a scientific framework that broadly defines a field of research, focused on vascular-centric pathways to cognitive impairment and dementia [1], [2], [3].
Fig. 1.
Two common age-related pathologies contribute to clinical dementia diagnosis. The orange circle symbolises cognitive impairment, including dementia, in terms of clinical diagnoses. The two rectangles symbolize the overlap of hypothesized contributions from two major pathological contributions to these clinical diagnoses. Vascular disease pathology is shown in blue with dashed outline, Alzheimer's disease pathology (β-amyloid deposits and neurofibrillary tangles) in yellow, dotted outline. The size of the shapes and relative overlaps are indicative rather than quantitative. Nevertheless, for most cognitive impairment seen in older adults, the individual patient would lie within the “Vascular” rectangle (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
VCID is not a disease or a diagnosis. Rather, it is a concept that frames hypotheses and experiments to pursue understanding of the spectrum of cerebrovascular and cardiovascular changes that are associated with cognitive impairment including (but not exclusively) dementia. VCID is intentionally independent of any syndromic clinical diagnosis (VaD, vascular cognitive impairment, “mixed dementia” etc.). An important corollary is that VCID encompasses the vascular pathology that is thought to contribute to overall cognitive deficit in patients with a primary clinical diagnosis of dementia, including AD dementia. From a molecular and cellular standpoint, VCID research is perhaps best represented as the aging neurovascular unit integrating, and failing to cope with, biological challenges due to vascular disease, misfolded proteins of neurodegeneration, metabolic disease and immune signaling [1].The pathobiology underlying VCID includes multiple mechanisms, including hypoxic/ischemic stress, BBB dysfunction, oxidative stress, neuroimmune dysregulation, vascular fibrosis and incomplete perivascular clearance [14]. We are aware that the papers in this Special Issue may not all conform to these definitions.
The United States National Plan to Address Alzheimer's disease (NAPA; https://aspe.hhs.gov/system/files/pdf/264206/NatlPlan2020.pdf) states that Alzheimer's Disease and Related Dementias (ADRDs) includes VCID. The NINDS leads triennial ADRD summits that define national priorities for VCID research [2]. Research milestones from the ADRD Summits together congressionally added resources that have made robust and targeted implementation possible. As a result, the NIH funded portfolio in VCID has increased almost 5-fold since 2015 ($362 million in FY 2020). The added funds together with NINDS-NIA collaboration have also allowed the NINDS to publish 13 VCID funding initiatives and support several large VCID programs and consortia that address VCID biomarkers (launched in 2016, MarkVCID: https://markvcid.partners.org/), post-stroke VCID (launched in 2019, DISCOVERY: https://discoverystudy.org/), and a clinical research effort to determine that characteristics of white matter hyperintensities and synergies with comorbidities that may cause cognitive decline and dementia outcomes (launched 2020, DiverseVCID). Each of these projects is mandated to address health inequity in VCID. The NINDS aims, via its VCID program and its AD/ADRD program overall, to seek fundamental knowledge about AD/ADRD and to use that knowledge to reduce the burden of dementia.
Clearly there is considerable variation in the funding landscape for VCID across other countries. For example, the United Kingdom has government-funded networks with vascular themes: Dementias Platform UK (https://www.dementiasplatform.uk/) and Dementia Research Institute (https://ukdri.ac.uk/). We are encouraged by the global diversity in affiliations represented in this Special Issue (see Table 1) suggesting strong international research support.
3. Current therapeutic options in vascular cognitive impairment
Treatment of patients diagnosed with VaD or vascular cognitive impairment is currently based on management of risk factors: blood pressure control, cigarette smoking cessation, managing body weight, blood sugar, lipids etc [7,15,16].. For a comprehensive review of previous clinical trials related to vascular cognitive impairment and VaD, see a previous review [17].
The importance of B-group vitamins (folate, B6, B12) in cognitive impairment is apparent from numerous clinical studies, notably the OPTIMA trial [18]. The functional status of these three B vitamins is reflected by plasma concentrations of the biomarker homocysteine [19]. In addition to being a biomarker, homocysteine may have harmful effects on cognitive health, likely mediated by vascular actions [20]. A recent consensus concluded that “elevated plasma total homocysteine is a modifiable risk factor for development of cognitive decline, dementia, and Alzheimer's disease in older persons” [19].
Two concepts stand out with respect to therapeutic options in vascular cognitive impairment, making clinical trials in this area especially promising but also challenging. The first is the diversity of pathologies on micro and macro scales, and their associations with cognitive impairment and dementia diagnoses, echoed throughout this Special Issue. Since VCID is not related to a single specific disease entity, what qualifies as a therapeutic could be either very specific for a particular vascular target or disease (for example, PF07265803 being tested in cardiomyopathy patients with a familial LMNA mutation, see Table 2), or quite broad, encompassing any therapy affecting overall brain vascular function relevant to neuronal function and cognition, or the connection between vasculature and neurons (such as a NO-donor, as in the LACI-2 trial) [21]. The second concept is the critical “window of opportunity” for maximal impact on clinical symptomatology. This brings up the important aspect of precision in trials: whom, and when, to treat. It is becoming clear that for implementation of successful clinical trials (i.e. with a clear positive or negative outcome), in vivo biomarkers are necessary for selecting individuals with a favourable benefit-to-risk ratio. Ultimately, as in AD dementia, use of biomarkers for patient selection, for risk stratification and as surrogate outcomes will improve trial design, and hasten development of precision therapeutic approaches. This is especially important to clinical trials in vascular cognitive impairment, given the diverse landscape of related molecules and cells and associated clinical symptoms. Chemical biomarkers of vascular origin in blood and CSF are likely to be informative, not only in clinical trials relevant to VCID but also in AD trials [22,23].
Table 2.
Candidates in cardiovascular or related disease areas. Pipelines of the 10 biggest Pharma companies.
| Company | Drugs | Company website |
|---|---|---|
| J&J |
|
www.investor.jnj.com/pharmaceutical-pipeline |
| Roche |
|
https://www.roche.com/research_and_development/who_we_are_how_we_work/pipeline.htm |
| Pfizer |
|
https://www.pfizer.com/science/drug-product-pipeline |
| Bayer |
|
https://pharma.bayer.com/development-pipeline |
| Novartis |
|
https://www.novartis.com/our-science/novartis-global-pipeline |
| Merck |
|
https://www.merck.com/research-and-products/product-pipeline/ |
| GSK |
|
https://www.gsk.com/en-gb/research-and-development/our-pipeline/ |
| Sanofi |
|
https://www.sanofi.com/en/science-and-innovation/research-and-development/specialty-care-rd-pipeline |
| AbbVie |
|
https://www.abbvie.com/our-science/pipeline.html |
| Abbott |
|
https://www.abbottcdx.com/biomarkers-and-pipeline.html |
Companies listed in decreasing order of net asset value. Websites were accessed 23/04/2021.
Some interventions may impact multiple functions. These have historically been classified under the rubric of “lifestyle,” including physical exercise, diet, stress-reduction, and cognitive activity. Sleep is another potential area of holistic intervention that has a vascular component, since brain dynamics and physical forces exerted on brain structures are altered both by head and body positioning, as well as by neural activity and brain blood flow during sleep. Obstructive sleep apnea is suggested as a vascular risk factor for dementia. Sleep deprivation may alter clearance of molecules from the brain, as well as enhance leakage at the BBB [24].
Pharmaceutical modification of vascular risk factors, such as blood pressure lowering, glycemic control and lipid homeostasis, are presenting as opportunities for prevention and treatment of vascular cognitive impairment. To this end, more trials with vascular biomarkers and cognition as primary outcome are warranted, in order to determine optimal ranges and risk thresholds, which may differ from those associated with disease in other organs, notably the heart [17,25]. An alternative approach – which may be more palatable to the pharmaceutical industry - is to ensure that cognitive outcomes and related biomarkers are included as secondary outcomes in definitive phase III trials of cardiovascular drugs (see recent guidelines on post-stroke cognitive impairment) [26].
In our view there are three pragmatic strategies for therapeutic investigations in VCID. The first entails the re-purposing of specific licensed drugs from other disease categories, such as donepezil from the AD cabinet, angiotensin receptor blockers, angiotensin converting enzyme inhibitors, or dabigatran from systemic cardiovascular disease, and testing their effects on cognition as primary outcome. The second is that of testing known vascular interventions for benefit on cognition as outcome, without pre-existing knowledge of the underlying molecular and cellular mediators of the effect or focus on a specific drug. This could include lifestyle interventions as well as pharmaceutical interventions on vascular risk factors, or intermediate phenotypes such as hypertension, without focus on specific drugs. A topical example is the intensive blood pressure lowering approach in SPRINT-MIND [27]. The third strategy is identification of specific molecular pathophysiology connecting vascular cellular disease to cognitive impairment, and targeted disease-modification. Recent genetic candidates for risk of SVD [28,29] may yield molecular targets for drug discovery in this category. An example is anti-fibrinogen immunotherapy, in light of recent pre-clinical mechanistic studies demonstrating connections between brain penetration of fibrin(ogen), with CNS immune activation and neurodegeneration [30].
Notwithstanding the variety of approaches and potential interventions in vascular cognitive impairment, there are few unambiguous clinical trials in this area. A recent encouraging result is from SPRINT-MIND (target systolic blood pressure of 120 mmHg) [27] which showed significant evidence of benefit in MCI and the combined outcome of MCI and dementia [27]. A similar large trial, with cholesterol-lowering to test cognitive benefit as one of the primary endpoints, PREVENTABLE, is currently in progress. This will test whether treatment with atorvastatin, in older adults without known dementia, or known obstructive cardiovascular, peripheral vascular or cerebral vascular disease, can prevent cognitive impairment.
4. Current clinical trials in vascular cognitive impairment
Reviewing the clinical trials that are currently active in vascular cognitive impairment confirms a diversity of approaches across the international research community. We recently screened the www.clinicaltrials.gov database for all currently active trials of interventions (embracing any intervention, not restricted to drug molecules) in this area. These interventions include not only putative treatments but also therapies for possible prevention, to promote good vascular health and to avoid future diagnoses related to vascular cognitive impairment. The resulting 45 trials are listed in supplementary Table S1.
In terms of conventional drugs (small organic molecules) these include the AChE inhibitor donepezil (licensed for symptomatic treatment of AD), the PDE3 inhibitor cilostazol, as well as the anti-CGRP antibody fremanezumab, currently used to treat migraine (Table S1). The Australia-based TRIDENT-MRI trial administers a triple cocktail of an angiotensin receptor blocker, calcium channel blocker and thiazide diuretic (telmisartan, amlodipine, indapamide combination) that resembles the treatment regimen in the SPRINT-MIND trial. More broadly, there are herbal products such as butyl‑phthalide (a component of celery oil) and the Chinese herbal medicine Sai-Luo Tong. Though a herbal formulation may be less amenable to regulatory processes than a laboratory-derived synthetic drug, we recall that many widely-used medicines began as herbal remedies (aspirin, digitalis, atropine, warfarin, paclitaxel, to name a few).
In terms of interventions that are not drugs, there are dietary supplements that are formulations of ferrous iron or of omega-3 poly-unsaturated fatty acids, as well as an integrated dietary intervention (the MIND diet). There are wearable devices to administer remote ischemic conditioning, a smartphone-based reminder application (the SmartPrompt App) and behavioural strategies including cognitive stimulation therapy and music therapy. And, with an indication of “Vascular Dementia” alongside an impressive array of other neurological disease states, there are bone marrow-derived stem cells (for intravenous or intranasal delivery). Other potential non-drug interventions include non-invasive brain stimulation [31]. Overall, this is a relatively sparse field in terms of active clinical trials.
5. Drug company pipelines relevant to vascular cognitive impairment
In pharma company pipelines there are relatively few current trials in the cardiovascular space by comparison with other disease areas, for example Oncology or Infectious Diseases. Table 2 lists the agents in phase 1, 2 or 3 with the world's ten largest drug-makers for Cardiovascular Disease or for a related vascular indication (such as diabetic macular degeneration). An underlying trend is that many of the agents in Table 2 are being progressed for primary indications in other disease areas. Testing for an alternative indication in cardiovascular disease may represent a calculated gamble, as part of a larger plan to commercialize a candidate molecule.
There are recurring indications in Table 2, including heart failure, pulmonary hypertension and diabetic macular disease. Heart failure (HF) has a mineralocorticoid receptor antagonist (finenerone) in phase-3 for HF with preserved ejection fraction (HFpEF), as well as HF with reduced ejection fraction, and a guanylyl cyclase activator in phase-2 for HFpEF (vericiguat). For pulmonary hypertension, there is a dual endothelin receptor antagonist in phase-3 (macitentan) as well as a CDK inhibitor being tested for tolerability in phase-1. For diabetic macular edema there is a decoy antagonist for VEGF and the VEGF family member PIGF in phase-3 (aflibercept) and an anti-VEGF agent in phase-2 (abicipar). In addition, there are several agents targeted to lipid handing pathways, for example an antisense oligonucleotide targeted to lipoprotein-A, being tested for efficacy in secondary prevention of cardiovascular disease (pelacarsen).
Some agents are being tested in highly focussed trials for relatively rare “orphan” diseases. Examples are the galactosidase inhibitor venglustat in phase-2 for Fabry disease, a rare genetic disorder caused by mutations in the gene encoding alpha-galactosidase-A. Another is a p38-MAPK inhibitor (PF07265803) in phase-3 for those patients with dilated cardiomyopathy who carry a particular point mutation in the gene encoding lamin. These programs have likely arisen from the increase in genotypic screening in the past 20 years – meaning increased precision, as more mutations are catalogued, and also increasingly widespread use of genetic screening (reflecting reduced cost).
6. Drug re-purposing for vascular cognitive impairment
Re-purposing means taking a drug that is already licensed for treatment of some other disease indication and testing it for therapeutic use in a disease state relevant to vascular cognitive impairment. Multiple cellular processes and molecular pathways are implicated in these vascular disease states - cerebral small vessel disease, diabetes mellitus, large vessel atheroma, to name a few. A major attraction of re-purposing is that the safety and tolerability, and adverse effect profile, of the drug entity are known before it enters a re-purposing trial. A salient example of successful re-purposing is memantine [32], now widely-used in AD dementia. Another advantage is that the molecular target (enzyme, ion channel, transcription factor) is also generally well understood, prior to re-purposing. Several druggable pathways appear promising for possible re-purposing in vascular cognitive impairment. One example is the renin-angiotensin system, targeted by multiple ACE inhibitors and angiotensin receptor AR1 blockers (e.g. RADAR trial) [33]. Another example is the microvascular nitric oxide signaling pathway, which modulates vasodilator tone. Here, relevant drugs are NO donors [21], PDE5 inhibitors[34] and guanyl cyclase activators (e.g. vericiguat) [35]. A third example of a druggable pathway is vessel fibrosis and remodeling. This appears more challenging in terms of drug targets, but with some optimism for ROCK inhibitors and endothelin ET1A antagonists [25]. Other possible targets may be found in cholesterol-lipid handling (PREVENTABLE trial; https://www.preventabletrial.org/) insulin and glucose homeostasis (GLP-1 agonists) [36], cellular aging and “senescence”, and perivascular clearance pathways [37]. The GLP-1 agonist semaglutide, which is licensed for use in type-2 diabetes mellitus, is currently in a phase-3 trial for efficacy in dementia. The EVOKE trial aims to recruit 1800 people with MCI or mild AD dementia, to test semaglutide versus placebo for up to 3.3 years (NCT04777409).
7. Overview of the papers in this special issue
The papers in this Special Issue are listed in Table 1. Several of these concern clinical trials. One of the challenges for any clinical trial in vascular cognitive impairment is that older people are likely to be taking medications for other indications. These co-meds may confound the response to an experimental interventional drug. Makin and colleagues performed a pragmatic analysis of a large cohort of individuals (UKBiobank; https://www.ukbiobank.ac.uk/) to determine the numbers eligible for inclusion in a clinical trial relevant to VCID, for example with a statin, antiplatelet or blood pressure-lowering medication (Morrison et al., this issue). Another paper presents a protocol for a study of people with SVD, as part of a UK-EU multi-center network (SVD@Target) (Blair et al., this issue). That study will combine participants with sporadic SVD and monogenic familial SVD (CADASIL), and will recruit across 3 sites: Edinburgh, Maastricht and Munich. The study is not interventional, rather a test for associations between MRI biomarkers of SVD and brain vascular function (BBB leakage, cerebrovascular reactivity, vessel pulsatility and CSF flow) (Blair et al., this issue). In secondary analysis of data from a completed interventional trial (NILVAD) Claassen and colleagues examined whether CBF autoregulation and baroreflex sensitivity changed in older people with clinical diagnosis of AD (De Heus et al., this issue). Perhaps surprisingly, both mechanisms showed no decline over an 18 months timescale. This allays some safety concerns around blood pressure lowering, which is a feature of several therapeutic approaches being considered for dementia in older patients [27,33,34].
Another secondary analysis explored data from a completed trial in VCI patients (STREAM-VCI). Prins, van der Flier and colleagues investigated severity of vascular disease (from brain MRI measures) and of AD pathology (cerebrospinal fluid biomarkers and β-amyloid PET), and tested for differences between patients who responded to treatment with methylphenidate and galantamine and those who did not (Leijenaar et al., this issue).
In terms of novel candidates, adrenomedullin is an endogenous 52-amino acid peptide, released from endothelial cells. With anti-inflammatory, pro-angiogenic and oligodendrocyte trophic actions, adrenomedullin has passed through phase 1 testing in humans. Intravenous delivery of adrenomedullin is currently in a randomised trial for acute ischemic stroke. Ihara and colleagues give an expert review of the data portfolio for adrenomedullin as a possible therapeutic agent for use in vascular cognitive disorders, including CADASIL (Ihara et al., this issue). Nootropics are natural or synthetic preparations (e.g. cerebrolysin, actovegin and cortexin) that may enhance cognition. Cerebrolysin, a peptide fraction isolated from pig brain, is already used therapeutically in stroke, TBI and dementia in Russia, China and forty other countries. Here Alsuleimani and Quinn performed a systematic review of the available evidence for therapeutic use of nootropics in cognitive disorders (Alsuleimani and Quinn, this issue).
The merit of repurposing existing drugs extends to testing “vascular drugs” for possible use in AD. Dabigatran, classed as a novel oral anticoagulant (NOAC), binds thrombin directly. Dabigatran is widely prescribed in atrial fibrillation for prevention of stroke and other major cardiovascular disease. In a cell culture platform, Bihaqi and colleagues present biochemical evidence that exposure to thrombin increases APP expression and release of Aβ1–42, both effects being inhibited by dabigatran (Bihaqi et al., this issue).
Physical exercise is one of the few therapeutic interventions with evidenced cognitive benefit in older people. It appears likely that whatever the mechanism underlying this beneficial action, it is at least in part vascular. Hackney and colleagues present the results from another trial of a non-pharmacological intervention, specifically the Argentine tango (registered on ct.gov: NCT03269149) (Wharton et al., this issue). The participants were “young older” African American women, average age 60 y, and the intervention was a series of 20 dance classes over a 12 week period, while controls had no intervention. Participants in the active arm had significantly altered circulating inflammatory cytokines (IFN-γ, TNFα, and MCP-1) and executive function (Wharton et al., this issue).
Wilcock and her colleagues review evidence for the possible involvement of neuro-inflammatory pathways, chronic hypoperfusion and angiogenesis in brain pathology relevant to VCID (Early et al., this issue). Matrix metalloproteinase cascades are identified as potential therapeutic targets, and the role of vascular endothelial growth factor (VEGF) and placental growth factor (PIGF) discussed. PIGF is a member of the VEGF family, released by numerous tissues (not only placenta). PIGF was recently identified as a potential blood biomarker in data from the MarkVCID consortium [23].
Wafford gives an overview of the importance of sleep in brain function, particularly in the essential processes of fluid clearance via “glymphatic” or perivascular pathways (Wafford, this issue). He reviews the central role of slow wave sleep in brain clearance, and deduces a possible role in age-related protein accumulation and neurodegeneration. He identifies central aminergic pathways, in particular 5-HT2A receptors, as possible therapeutic targets for improving sleep and hence, possibly, clearing unwanted protein deposits.
Alladi and her colleagues give an appraisal of vascular cognitive impairment in India (Arshad et al., this issue). The authors estimate that 11% of all people with dementia worldwide are located in India. They spell out the challenges posed by vascular cognitive impairment in the setting of an LMIC with over 1380 million inhabitants, and the opportunities for clinical trial delivery and healthcare impacts (Arshad et al., this issue).
8. Conclusions
Vascular cognitive impairment is not a single disease, rather a syndromic spectrum with multiple clinical profiles and potential causes. Though molecular targets are lacking, genetic candidates are emerging [28,29] and these may stimulate drug development interest. Three novel areas for future therapeutics stand out: (1) communication of blood vessels with neuronal activity or neurovascular coupling; (2) orchestration of molecular clearance and immune cell trafficking to and from the brain, and the related glymphatic function; (3) BBB function, capturing the related entry of molecules and cells, in close cross-talk with exit functions of brain vasculature. Current pharma industry activity is limited, largely relegated to additional indications for candidate molecules that are being developed in other disease areas. Repurposing of existing licensed medicines [32] may also yield progress in terms of drugs for vascular cognitive impairment. The multiplicity of vascular disease underlying vascular cognitive impairment [8] is reflected in the range of studies in this Special Issue, and this multi-disciplinary approach can be seen as a strength. Though pure VaD is rare, vascular disease is now recognised as a contributing factor in most dementia (leading to the concept of VCID) [1], [2], [3]. Hence, treating brain vascular disease has potential for broad clinical benefit, including AD dementia and other age-related dementias. In the context of real-world dementia, typically including multiple potential aetiologies and co-morbidities in given individual, therapies to address brain vascular health will have considerable merit [38]. Off-licence drugs and non-pharmacological lifestyle interventions also have potential to be highly cost-effective. Dancing the Argentine tango may ultimately salvage more patient-years than expensive prescribed medications. The diversity of topics in this special issue highlights the multifaceted nature of vascular cognitive impairment, and the creativity of researchers in this area.
Disclosures
AHH has received honoraria from Eli-Lilly and from NIA. He is chair of the Dementias Platform UK Vascular Experimental Medicine group. AHH and FME are both members of the executive committee for the Vascular Cognitive Disorders Professional Interest Area within The Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART). RAC is a full-time employee of NIH. The views expressed in this paper are those of the individual authors and do not represent the position or policies of the NIH or host institutions.
Acknowledgments
We thank Jeremy D Isaacs for helpful comments on the manuscript. Research in Dr Hainsworth's group is funded by the UK Medical Research Council (MR/R005567/1, MR/T033371/1), British Heart Foundation (PG/20/10397), UK Alzheimer's Society and Alzheimer's Drug Discovery Foundation (20140901). Research in Dr Elahi's group is supported by a Larry L. Hillblom start‐up grant (2019A012SUP) and an American Academy of Neurology fellowship.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2021.100033.
Contributor Information
Atticus H Hainsworth, Email: ahainsworth@sgul.ac.uk.
Fanny M Elahi, Email: fanny.elahi@ucsf.edu.
Roderick A Corriveau, Email: roderick.corriveau@nih.gov.
Appendix. Supplementary materials
References
- 1.Corriveau R.A., Bosetti F., Emr M., et al. The Science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell. Mol. Neurobiol. 2016;36(2):281–288. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corriveau R.A., Koroshetz W.J., Gladman J.T., et al. Alzheimer's disease-related dementias summit 2016: national research priorities. Neurology. 2017;89(23):2381–2391. doi: 10.1212/WNL.0000000000004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick P.B., Scuteri A., Black S.E., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esiri M.M., Wilcock G.K., Morris J.H. Neuropathological assessment of the lesions of significance in vascular dementia. J. Neurol. Neurosurg. Psychiatry. 1997;63(6):749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger K.A. The pathology of ischemic-vascular dementia: an update. J. Neurol. Sci. 2002;203-204:153–157. doi: 10.1016/s0022-510x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 6.McAleese K.E., Alafuzoff I., Charidimou A., et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016:14. doi: 10.1186/s12916-016-0676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. 2017;131(11):1059–1068. doi: 10.1042/CS20160607. (Lond.) [DOI] [PubMed] [Google Scholar]
- 8.Kalaria R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol. 2016;131(5):659–685. doi: 10.1007/s00401-016-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roman G.C., Erkinjuntti T., Wallin A., Pantoni L., Chui H.C. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien J.T., Erkinjuntti T., Reisberg B., et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 11.Moorhouse P., Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7(3):246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 12.Ighodaro E.T., Abner E.L., Fardo D.W., et al. Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J. Cereb. Blood Flow Metab. 2017;37(1):201–216. doi: 10.1177/0271678X15621574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erkinjuntti T., Kurz A., Gauthier S., Bullock R., Lilienfeld S., Damaraju C.V. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359(9314):1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 14.Iadecola C., Duering M., Hachinski V., et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J. Am. Coll. Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 16.Pantoni L. Treatment of vascular dementia: evidence from trials with non-cholinergic drugs. J. Neurol. Sci. 2004;226(1–2):67–70. doi: 10.1016/j.jns.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Smith E.E., Cieslak A., Barber P., et al. Therapeutic strategies and drug development for vascular cognitive impairment. J. Am. Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.117.005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jager C.A., Oulhaj A., Jacoby R., Refsum H., Smith A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int. J. Geriatr. Psychiatry. 2012;27(6):592–600. doi: 10.1002/gps.2758. [DOI] [PubMed] [Google Scholar]
- 19.Smith A.D., Refsum H., Bottiglieri T., et al. Homocysteine and dementia: an international consensus statement. J. Alzheimers Dis. 2018;62(2):561–570. doi: 10.3233/JAD-171042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainsworth A.H., Yeo N.E., Weekman E.M., Wilcock D.M. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID) Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862(5):1008–1017. doi: 10.1016/j.bbadis.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw J., Bath P.M.W., Doubal F., et al. Protocol: the lacunar intervention trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur. Stroke J. 2020;5(3):297–308. doi: 10.1177/2396987320920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake J.D., Chambers A.B., Ott B.R., Daiello L.A. Alzheimer's Disease neuroimaging I. peripheral markers of vascular endothelial dysfunction show independent but additive relationships with brain-based biomarkers in association with functional impairment in Alzheimer's disease. J. Alzheimers Dis. 2021;80(4):1553–1565. doi: 10.3233/JAD-200759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winder Z., Sudduth T.L., Fardo D., et al. Hierarchical clustering analyses of plasma proteins in subjects with cardiovascular risk factors identify informative subsets based on differential levels of angiogenic and inflammatory biomarkers. Front. Neurosci. 2020;14:84. doi: 10.3389/fnins.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eide P.K., Vinje V., Pripp A.H., Mardal K.A., Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144(3):863–874. doi: 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 25.Berry C., Sidik N., Pereira A.C., et al. Small-vessel disease in the heart and brain: current knowledge, unmet therapeutic need, and future directions. J. Am. Heart Assoc. 2019;8(3) doi: 10.1161/JAHA.118.011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn T.J., Richard E., Teuschl Y., et al. European stroke organisation and European academy of neurology joint guidelines on post-stroke cognitive impairment. Eur. J. Neurol. 2021;6(3):I–XXXVIII. doi: 10.1177/23969873211042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson J.D., Pajewski N.M., Auchus A.P., et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung J., Marini S., Pera J., et al. Genome-wide association study of cerebral small vessel disease reveals established and novel loci. Brain. 2019;142(10):3176–3189. doi: 10.1093/brain/awz233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persyn E., Hanscombe K.B., Howson J.M.M., Lewis C.M., Traylor M., Markus H.S. Genome-wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat. Commun. 2020;11(1):2175. doi: 10.1038/s41467-020-15932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu J.K., Rafalski V.A., Meyer-Franke A., et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 2018;19(11):1212–1223. doi: 10.1038/s41590-018-0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantone M., Lanza G., Ranieri F., Opie G.M., Terranova C. Editorial: non-invasive brain stimulation in the study and modulation of metaplasticity in neurological disorders. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.721906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shineman D.W., Alam J., Anderson M., et al. Overcoming obstacles to repurposing for neurodegenerative disease. Ann. Clin. Transl. Neurol. 2014;1(7):512–518. doi: 10.1002/acn3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kehoe P.G., Blair P.S., Howden B., et al. The rationale and design of the reducing pathology in Alzheimer's disease through angiotensin targeting (RADAR) trial. J. Alzheimers Dis. 2018;61(2):803–814. doi: 10.3233/JAD-170101. [DOI] [PubMed] [Google Scholar]
- 34.Pauls M.M.H., Clarke N., Trippier S., et al. Perfusion by Arterial Spin labelling following Single dose Tadalafil In Small vessel disease (PASTIS): study protocol for a randomised controlled trial. Trials. 2017;18(1) doi: 10.1186/s13063-017-1973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong P.W., Pieske B., Anstrom K.J., et al. Vericiguat in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2020;382(20):1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 36.Femminella G.D., Livingston N.R., Raza S., et al. Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer's subjects? Alzheimers Res. Ther. 2021;13(1):47. doi: 10.1186/s13195-021-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carare R.O., Aldea R., Agarwal N., et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group-part of Vascular professional interest area (PIA): cerebrovascular disease and the failure of elimination of Amyloid-beta from the brain and retina with age and Alzheimer's disease-Opportunities for Therapy. Alzheimers Dement. 2020;12(1):e12053. doi: 10.1002/dad2.12053. (Amst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K.Y., Schneider L.S., Howard R. The need to show minimum clinically important differences in Alzheimer's disease trials. Lancet Psychiatry. 2021;8(11):1013–1016. doi: 10.1016/S2215-0366(21)00197-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.