Abstract
Several studies reported acute symptomatic seizures as a possible neurological complication of COVID-19 pneumonia. Apart from metabolic imbalances, hypoxia, and fever, other ictogenic mechanisms are likely related to an immune-mediated damage. The same mechanisms are shared by other respiratory viruses. Since neurotropic properties of SARS-CoV-2 have been questioned, we investigated whether SARS-CoV-2 has a similar ictogenic potential to other respiratory non-neurotropic viruses.
We conducted a retrospective study identifying 1141 patients with SARS-CoV-2 pneumonia and 146 patients with H1N1/H3N2 pneumonia. We found a similar prevalence of seizures in the two viral pneumonia (1.05% with SARS-CoV-2 vs 2.05% with influenza; p = 0.26). We detailed clinical, electroencephalographic, and neuroradiological features of each patient, together with the hypothesized pathogenesis of seizures.
Previous epilepsy or pre-existing predisposing conditions (i.e., Alzheimer’s disease, stroke, cerebral neoplasia) were found in one-third of patients that experienced seizures, while two-thirds of patients had seizures without known risk factors other than pneumonia in both groups. The prevalence of pre-existing predisposing conditions and disease severity indexes was similar in SARS-CoV-2 and H1N1/H3N2 pneumonia, thus excluding they could act as potential confounders. Considering all the patients with viral pneumonia together, previous epilepsy (p < 0.001) and the need for ventilatory support (p < 0.001), but not the presence of pre-existing predisposing conditions (p = 0.290), were associated with seizure risk.
Our study showed that SARS-CoV-2 and influenza viruses share a similar ictogenic potential. In both these infections, seizures are rare but serious events, and can manifest without pre-existing predisposing conditions, in particular when pneumonia is severe, thus suggesting an interplay between disease severity and host response as a major mechanism of ictogenesis, rather than a virus-specific mechanism.
Keywords: SARS-CoV-2, Influenza, Pneumonia, Seizures, Ictogenic potential
1. Introduction
The spread of coronavirus-19 (COVID-19) disease has led to a growing amount of literature about neurological complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Among neurological manifestations, epileptic seizures have been frequently reported, also in patients without pre-existing predisposing factors, such as previous epilepsy or pre-existing structural brain abnormalities [1]. Moreover, the difficulties in identifying mild clinical seizure activity and non-convulsive status epilepticus could have led to an underestimation of seizure occurrence in the complex setting of SARS-CoV-2 infection [2]. Nevertheless, a precise assessment of the risk of developing seizures as a consequence of SARS-CoV-2 infection, and a comparison with other viral infections, lack.
Metabolic imbalances, fever and hypoxia – complications that may occur in the context of viral pneumonia – could indeed contribute to ictogenesis, but the possibility of a virus-induced brain damage has also been proposed for SARS-CoV-2 [3]. Therefore, the neuro-invasivity and the neurotropic properties of this virus have been investigated: several studies demonstrated that SARS-CoV-2 can reach the central nervous system (CNS) through binding to the angiotensin converting enzyme 2 (ACE2) receptors both on endothelial cells of the blood–brain barrier and on the olfactory epithelium in the nasal cavity, with a centripetal brain invasion [3], [4].
In this regard, a previously published post-mortem case series, investigating neuropathological features in the brain of forty patients who died for respiratory complications of SARS-CoV-2, documented the presence of viral RNA or proteins in the brain tissue of 53% of the subjects, even in the absence of obvious neurological symptoms [5]. This finding confirmed the hypothesis that SARS-CoV-2 can infiltrate the CNS, but the presence of SARS-CoV-2 was not associated with the severity of neuropathological changes, which occurred similarly in patients without SARS-CoV-2 brain infiltration [5]. On the other hand, the detection of SARS-CoV-2 in cerebrospinal fluid (CSF) of patients with seizures and COVID-19 disease is uncommon [6].
Therefore, the frequent failure to detect the virus in the CSF, together with the documented neuropathological changes occurring despite the absence of SARS-CoV-2 in the brain, suggested that an immune-mediated damage is more likely than a direct viral damage [4].
In particular, seizures occurring in the context of COVID-19-related encephalitides or encephalopathies, seem to be mainly associated with a systemic hyperinflammation syndrome, with a massive release of cytokines and chemokines, especially when neurologic symptoms appear concomitantly to respiratory symptoms [7].
Conversely, a smaller number of seizures occurring in the context of SARS-CoV-2-related encephalitis have been attributed to an autoimmune mechanism through antibodies against neuronal cell-surface or synaptic proteins triggered by the virus; in these cases a prolonged time interval between respiratory and neurological symptoms is generally observed, with neurological impairment appearing late, when respiratory symptoms are resolving [8], [9].
While receiving great attention with COVID-19 pandemic due to the huge number of infected subjects in a very short period, the above-cited pathogenic mechanisms are shared by other non-neurotropic viruses, for instance influenza viruses.
Influenza viruses represent the most frequent cause of viral pneumonia in adults [10], with a significant clinical and epidemiological impact. While some influenza strains are mainly responsible for pneumonia in immunocompromised patients or subjects with multiple underlying diseases, human influenza A viruses – with the most common strains H1N1 and H3N2 – affect not only elderly but also young subjects without comorbidities, as occurred in SARS-CoV-2 pneumonia.
Nonetheless, little is known about the ictogenic potential of influenza A viruses, with only few reports focusing on adults [11], [12], [13], [14].
Given this background, our main aim was to investigate whether SARS-CoV-2 has a similar ictogenic potential of H1N1 and H3N2 influenza viruses, and to estimate the risk of seizure occurrence in adult patients hospitalized with viral pneumonia.
As a secondary aim we analyzed clinical, radiological, and laboratory features of patients who presented seizures in both groups, exploring the possible ictogenic mechanisms and, more specifically, investigating whether seizures can occur in patients without pre-existing predisposing factors.
We think that this information could turn useful to understand the relationship between infections and ictogenesis, and to cope with the present and future epidemics.
2. Methods
We conducted a retrospective study on patients hospitalized at our tertiary care hospital in Pisa with influenza (H1N1 or H3N2) or SARS-CoV-2 pneumonia.
Data on H1N1 and H3N2 cases were collected from all the patients admitted between December 1st 2014 and February 29th 2020 (date set to avoid the possibility of double – SARS-CoV-2 and influenza – infection), while SARS-CoV-2 cases were collected from all the patients admitted between March 1st 2020 and December 31st 2020.
Since our interest was to estimate the risk of seizure occurrence specifically in patients with SARS-CoV-2 and H1N1/H3N2 pneumonia, who required hospitalization and who are more prone to develop systemic and neurological complications, we excluded all the hospitalized patients with a positive nasal swab in the absence of a diagnosis of viral pneumonia (i.e., asymptomatic or pauci-symptomatic patients).
All the hospitalized patients with a diagnosis of SARS-CoV-2 or H1N1/H3N2 pneumonia were included.
The diagnosis of viral pneumonia was based on: (1) the presence of clinical signs and symptoms of lower respiratory tract disease, (2) the positivity of the PCR assay for viral genome on nasal swab (SARS-CoV-2, H1N1 or H3N2), and (3) the parenchymal involvement on chest imaging (either CT scan or X-ray).
For each patient, we collected demographic and clinical data. Moreover, in order to extract a synthetic and reliable index of pneumonia severity – which could result in a higher systemic inflammation and a subsequent higher risk to develop seizures – we screened whether any ventilatory support was required.
Afterward, we identified: (1) all the patients for whom an electroencephalogram (EEG) or a neurological consult for suspected seizure was performed; (2) all the subjects for whom the evaluation of a Consultant Neurologist was conclusive for a diagnosis of seizure. In this latter group, we distinguished patients in which seizures developed during viral pneumonia for the first time, from patients who presented seizures having a previous diagnosis of epilepsy, pre-existing structural brain abnormalities, or any other possible pre-existing predisposing conditions to seizures [15].
Therefore, considering all the patients with viral pneumonia (SARS-CoV-2 vs H1N1/H3N2), we further investigated if the same pre-existing predisposing conditions and disease severity indexes were more associated with one or another pneumonia, acting as potential confounders for our analysis.
Finally, considering all the patients with viral pneumonia together (either SARS-CoV-2 and H1N1/H3N2) and distinguishing patients with seizures from patients without seizures, we evaluated if pre-existing predisposing conditions and disease severity indexes were related to a higher seizure risk or, alternatively, were equally represented in the seizure and non-seizure groups.
Data were compared employing the statistical software SPSS. Comparisons were made by t-test for continuous variables and chi-square or Fisher exact test for categorical variables, as appropriate. The null hypothesis was rejected for p < 0.05
The study was approved by our local ethics committee, and all patients gave their consent for the analysis of anonymized data. We obtained an additional informed consent to review clinical records, employing pseudonymization of data, from the patients who experienced seizures.
3. Results
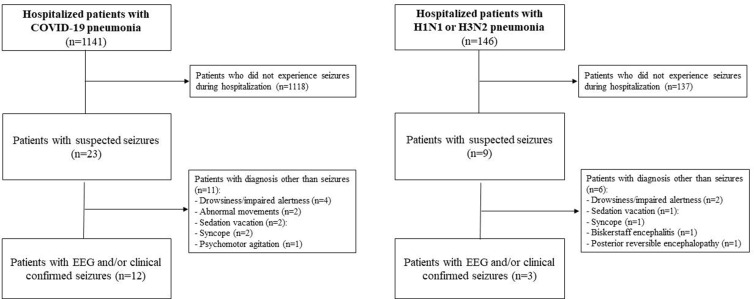
We identified 1141 patients with SARS-CoV-2 pneumonia and 146 with influenza pneumonia, of which 99 with H1N1 and 47 with H3N2 (Fig. 1 ).
Fig. 1.
Study flowchart.
Demographic variables did not differ between the two groups (Table 1 ).
Table 1.
Comparison of demographic and clinical features between patients with H1N1/H3N2 pneumonia and patients with SARS-CoV-2 pneumonia. Results are expressed as mean ± standard deviation for continuous variables and as absolute value with relative frequency for categorical variables. Abbreviation: ECMO = extracorporeal membrane oxygenation.
| SARS-CoV-2 pneumonia (n = 1141) | H1N1/H3N2 pneumonia (n = 146) | P value | ||
|---|---|---|---|---|
| Age | 68.69 ± 16.80 | 67.83 ± 18.91 | p = 0.66 | |
| Sex | males = 61.84% | males = 54.80% | p = 0.101 | |
| Ventilatory support | 225 (19.72%) | 24 (16.44%) | p = 0.345 | |
| Type of ventilatory support | Only non-invasive ventilatory support | 109 (9.95%) | 12 (8.22%) | p = 0.603 |
| Invasive ventilation without tracheotomy | 73 (6.40%) | 7 (4.79%) | p = 0.450 | |
| Invasive ventilation with tracheotomy | 39 (3.42%) | 4 (2.74%) | p = 0.811 | |
| ECMO | 4 (0.35%) | 1 (0.68%) | p = 0.453 | |
| Previous diagnosis of epilepsy | 13 (11.39%) | 2 (13.70%) | p = 0.810 | |
| Other seizure-predisposing factors | 182(15.95%) | 23 (15.75%) | p = 0.951 | |
| Type of seizure-predisposing factor | Alzheimer’s disease | 60 (5.26%) | 6 (4.11%) | p = 0.555 |
| Previous stroke | 103 (9.03%) | 12 (8.22%) | p = 0.749 | |
| Cerebral neoplasia (primitive or metastatic) | 19 (1.67%) | 5(3.42%) | p = 0.139 | |
An EEG was recorded for suspected seizures in 23 patients with SARS-CoV-2 pneumonia, and 9 with influenza pneumonia. Seizures were confirmed in 12 and 3 patients, respectively (Fig. 1) and comparison between proportions by Fisher exact test did not reveal any statistical difference (SARS-CoV-2 pneumonia = 1.05%; H1N1/H3N2 pneumonia = 2.05%, p = 0.26). Post hoc analysis, performed with G Power software [16], found reasonable power for the statistical test with our sample size (1-beta = 93%).
On this basis, we estimated a risk of seizure occurrence during viral pneumonia (either SARS-CoV-2 or influenza) of 1.20% (confidence interval 95%=0.60%-1.81%).
The detailed clinical features of the patients who experienced seizures during viral pneumonia are reported in Table 2 , together with the hypothesized mechanisms of ictogenesis.
Table 2.
Clinical, radiological, and laboratory features of patients with seizures occurred during H1N1/H3N2 and SARS-CoV-2 pneumonia.
| n° | Age, gender | Pre-existing risk factors for seizures* (yes/no) | Possible mechanisms of ictogenesis * | Pattern and description of seizures | Other associated neurological symptoms | Significant comorbidities | EEG features (ictal/interictal) • | Brain imaging (CT and/or MRI) | Serum levels of IL-6 (normal values: 0.9–6.6 pg/ml) | CSF § (cells/mm3, proteins mg/dL) | ASM ∞ | Maximal ventilatory support | Length of hospitalization (days) | Pre and post morbid status (mRS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with influenza pneumonia | ||||||||||||||
| 1 | 75, F | No | Metabolic | Repetitive FBTCS | None | Acute myocardial infarction, COPD, DM | Diffuse delta waves | Mild chronic leukoencephalopathy | NP | NP | BDZ, propofol | Tracheotomy | 36 | 2 ⟶ 6 |
| 2 | 86, F | Yes (previous epilepsy) | Alzheimer-related epilepsy | Focal motor SE | Dementia | COPD, HBP | Interictal: sharp delta activity on left-T region with bilateral spreading | Severe cortical atrophy and moderate chronic leukoencephalopathy | NP | NP | BDZ, LEV 1000 mg/die | High-flow oxygen therapy | 10 | 5 ⟶ 5 |
| 3 | 55, M | No | Encephalitis MRI - | One single FBTCS | AMS | HBP | Interictal: delta waves on right F-T regions | Normal | NP | NP | BDZ | High-flow oxygen therapy | 12 | 0 ⟶ 0 |
| Patients with COVID-19 pneumonia | ||||||||||||||
| 4 | 79, M | No | Metabolic | One single FBTCS | None | HBP | Interictal: slight global slowing | Mild chronic leukoencephalopathy | NP | NP | BDZ | High-flow oxygen therapy | 4 | 1 ⟶ 1 |
| 5 | 54, M | Yes (brain metastasis) | Neoplastic | One single FBTCS | None | Lung cancer | Interictal: sharp waves on right F-T areas | Right cortical frontal metastasis | NP | NP | BDZ, LEV 1000 mg/die | High-flow oxygen therapy | 22 | 2 ⟶ 3 |
| 6 | 74, F | No | Autoimmune limbic encephalitis | Repetitive FIAS | AMS | None | Ictal: rhythmic theta activity starting on the left-T area, with bilateral spreading | T2/DWI hyperintensity on both hippocampi | 49.6 pg/ml | 2 cells/mm3, 104 mg/dL | LEV 1000 mg/die, VPA 1500 mg/die | C-PAP | 35 | 0 ⟶ 2 |
| 7 | 74, F | No | Encephalitis MRI + | Focal motor SE | AMS | HBP | Ictal: continuous spikes and sharp waves on the left-T region | T2/DWI hyperintensity on bilateral hippocampi, left occipito-parietal cortex and left thalamus | 41.6 pg/ml | 1 cells/mm3, 30 mg/dL | VPA 1500 mg/die, LCS 200 mg/die, BDZ | High-flow oxygen therapy | 52 | 0 ⟶ 5 |
| 8 | 56, F | No | Encephalitis MRI - | One single FBTCS | AMS | None | Interictal: global slowing | Normal | 51.9 pg/ml | 8 cells/mm3, 63 mg/dL | LEV 1250 mg/die | C-PAP | 16 | 0 ⟶ 0 |
| 9 | 83, M | No | Ischemic stroke | Repetitive FIAS | Visual deficit | COPD, previous myocardial infarction | Ictal: rhythmic slow and sharp abnormalities on anterior regions | Acute ischemic stroke in occipital regions, bilaterally | 80.1 pg/ml | NP | BDZ, VPA 600 mg/die | High-flow oxygen therapy | 23 | 1 ⟶ 3 |
| 10 | 41, M | Yes (previous epilepsy) | Epileptic encephalopathy | Focal motor SE | Deaf-mutism since childhood | None | Ictal: sharp rhythmic theta activity and burst of delta activity on the right hemisphere | Moderate cortical atrophy and multifocal subcortical gliosis | 4.1 pg/ml | 0 cells/mm3, 31 mg/dL | propofol, BDZ, DPH 300 mg/die, LCS 300 mg/die | Tracheotomy | 51 | 3 ⟶ 5 |
| 11 | 71, M | No | Ischemic stroke | One single FBTCS | Left emianopsia | Limb critical ischemia, COPD, bladder cancer | Interictal: slow activity mixed with diffuse sharp waves | Acute ischemic stroke in right occipital lobe | 35.1 pg/ml | NP | LEV 3000 mg/die | Tracheotomy | 45 | 2 ⟶ 6 |
| 12 | 51, M | No | Encephalitis MRI + | One single FBTCS | AMS | None | Interictal: isolated diffuse delta waves superimposed to normal alfa rhythm | Limited laminar cortical necrosis in occipital regions and mild chronic leukoencephalopathy | 68.5 pg/ml | 7 cells/mm3, 64 mg/dL | VPA 1000 mg/die | ECMO | 63 | 0 ⟶ 0 |
| 13 | 71, M | Yes (previous epilepsy) | Focal symptomatic pharmaco-resistant epilepsy | Focal motor SE | Dementia | COPD, DM, renal and hepatic diseases | Ictal: rhythmic delta activity and spikes on right F-T area spreading bilaterally | Severe cortical atrophy and right frontal gliosis | NP | NP | PB 200 mg/die, LEV 2000 mg/die, LCS 200 mg/die | High-flow oxygen therapy | 25 | 5 ⟶ 6 |
| 14 | 68, M | No | Encephalitis MRI - | NCSE | AMS | Asthma | Ictal: continuous spikes/polispikes and waves on anterior regions | Mild cortical atrophy | NP | 1 cells/mm3, 35 mg/dL | VPA 1500 mg/die, LEV 2500 mg/die | Tracheotomy | 49 | 0 ⟶ 0 |
| 15 | 73, M | Yes (previous ischemic stroke) | Post-anoxic | Repetitive FBTCS | Coma | Cardiac arrest, acute endocarditis, previous stroke, COPD, DM | Burst suppression | Diffuse brain oedema | 240.6 pg/ml | NP | Midazolam, propofol | Intubation | 32 | 1 ⟶ 6 |
Abbreviation: AMS: altered mental status; ASM: anti-seizure medications; BDZ: benzodiazepines; COPD: chronic obstructive pulmonary disease; CSF: cerebrospinal fluid; CT: computed tomography; DM: diabetes mellitus; DPH: phenytoin; DWI: diffusion weighted imaging; EEG: electroencephalogram; FBTCS: focal to bilateral tonic-clonic seizure; FIAS: focal impaired awareness seizure; F-T: fronto-temporal; HBP: high blood pressure; IL: interleukin; LCS: lacosamide; LEV: levetiracetam; MRI, magnetic resonance imaging; n°: number; NCSE: non convulsive status epilepticus; NP: not performed; SE: status epilepticus; T: temporal; VPA: valproic acid.
* Pre-existing risk factors included previous structural brain damage (tumor or stroke) or previous epileptic history.
Autoimmune limbic encephalitis was diagnosed based on Graus criteria [27]. Metabolic etiology was defined based on serum detection of electrolyte imbalances or hypoglycemia.
• All these patients underwent at least two EEG recording, the first with a 9-electrode montage, the second using 19 electrodes, both according to the 10–20 international system.
∞ Concomitant drugs other than anti-seizure medications are not listed (available upon request), but none of the patients took therapies with high ictogenic potential.
The diagnosis of encephalitis was defined based on the Criteria of International Encephalitis Consortium, 2013[26]: presence of AMS lasting ≥ 24 h and presence of two or more of the following: i) generalized or partial seizures not fully attributable to a pre-existing epilepsy; ii) new onset of focal neurologic findings; iii) CSF white blood cell count ≥ 5/mm3; iv) abnormality of brain parenchyma on neuroimaging suggestive of encephalitis; v) abnormality on EEG consistent with encephalitis and not attributable to other causes.
CSF examination also included PCR for SARS-CoV-2, Herpes Simplex Virus 1–2, Human Herpes Virus 6, enterovirus, parechovirus, cytomegalovirus, Varicella-Zoster Virus, Cryptococcus, Streptococci, Hemophilus Influenzae, Listeria Monocytogenes, Escherichia Coli and autoimmune panel for anti LGI1, CASPR2, NMDAr. All the patients had negative results.
Here we highlight that 8 patients from SARS-CoV-2 group and 2 patients from influenza group experienced their first seizure during pneumonia, in the absence of pre-existing predisposing conditions to seizures.
Concerning pre-existing predisposing conditions [15], [17], [18] in the remaining patients with seizures, we identified previous stroke in one patient (case n° 15), Alzheimer’s disease in 2 patients (cases n° 2 and 13) and cerebral neoplasia in one patient (case n° 5). Three patients had a previous diagnosis of epilepsy (cases n° 2, 10, 13).
The comparison between SARS-CoV-2 (n = 1141) and H1N1/H3N2 (n = 146) pneumonia did not show any association between clinical pre-existing conditions or disease severity indexes and one of the two viral pneumonia (Table 1).
Comparing patients with seizures (n = 15) and patients without seizures (n = 1272) regardless of the etiology of pneumonia (either SARS-CoV-2 or H1N1/H3N2), we observed a higher requirement of ventilatory support in the seizure group overall (seizure group 53.33% vs non-seizure group 19.19%, p < 0.001) and, more specifically, of invasive ventilation with tracheotomy (seizure group 26.67% vs non-seizure group 3.38%, p = 0.002). Moreover, a previous diagnosis of epilepsy was strongly associated with the seizure group (seizure group 20.00% vs non-seizure group 1.02%, p < 0.001). Conversely, the presence of pre-existing predisposing factors was not significantly associated with seizure or non-seizure group (seizure group 26.67% vs non-seizure group 16.35%, p = 0.290).
4. Discussion
Our retrospective analysis did not show any difference in the frequency of seizures in patients hospitalized with SARS-CoV-2 pneumonia compared to patients hospitalized with H1N1 or H3N2 pneumonia. The post hoc power analysis further confirmed this finding, ruling out the hypothesis of a reduced statistical power due to the sample size. Both in SARS-CoV-2 and in H1N1/H3N2 pneumonia, seizures represent a rare complication, and this is in line with previously published studies in COVID-19 patients [19].
Among patients with seizures 33.3% of patients with SARS-CoV-2 and 33.3% with influenza had pre-existing predisposing factors (cases n° 2, 5, 10, 13, 15, Table 2), such as structural brain damage or previous epileptic history. In these cases, viral pneumonia could be considered as a contributing factor, but not a causative one.
In the remaining patients, we could assume that both SARS-CoV-2 and influenza viruses induced acute symptomatic seizures, manifesting with various clinical presentation and outcomes, thus suggesting different pathophysiological mechanisms.
Firstly, seizures could be the result of metabolic derangement or hypoxia due to the process of pneumonia, as it occurs also during systemic infections caused by other agents or sepsis (cases n° 1, 4, Table 2) [20].
Secondly, seizures could be symptomatic of thrombotic events, in particular acute ischemic stroke (cases n° 9, 11, Table 2), the risk of which is increased both in the setting of SARS-CoV-2 and of influenza pneumonia [21], [22]. Possible mechanisms driving thrombosis during these infections include increased platelet activation, alterations in the balance of pro-coagulant and anti-coagulant factors and vascular endothelial dysfunction or activation [23]. Moreover, an excessive production and release of pro-inflammatory cytokines could promote blood coagulation, thus increasing the risk of ischemic stroke [24].
Thirdly, seizures could be directly related to an excessive systemic inflammatory reaction that is thought to be one the most common causes of virus-related encephalitides. Cytokine storm is induced by several immunity triggers, including SARS-CoV-2 and H1N1/H3N2 infections [25]. In our cohort cases n° 3, 7, 8, 12, and 14 developed seizures probably as a result of cytokine-mediated mechanisms. IL-6 levels were elevated in serum of all these patients, except case n° 14, for whom IL-6 level was not available. Of note, in these cases seizures were not the only neurological manifestation, since altered mental status and confusion were also associated, together with abnormal EEG, brain imaging and/or abnormal CSF examination, allowing the diagnosis of virus-related encephalitides (Table 2).
Finally, another possible pathogenic mechanism responsible of ictogenesis during viral pneumonia involves adaptive immunity, with post-viral autoimmune encephalitides. In our cohort, patient n° 6 developed seizures in the context of an autoimmune limbic encephalitis COVID-19 related (see also [9] for detailed case description and literature review), while one patient with H1N1 pneumonia developed a Bickerstaff encephalitis and underwent EEG recording for altered mental status despite not having seizures (Fig. 1).
A strength of our study was indeed to provide clinical features of the large groups of patients with pneumonia (1141 SARS-CoV-2 and 146 H1N1/H3N2) in the attempt to clarify the prevalent contribution of pneumonia itself or pre-existing predisposing conditions in the ictogenesis. Our analysis showed that disease severity indexes and pre-existing predisposing factors were comparable between the two viral pneumonia groups, thus excluding they could act as potential confounders for our analysis (Table 1). In both infections, seizure risk was significantly associated with pneumonia severity but not with pre-existing predisposing factors, except for previous epilepsy.
Concerning the hypothesized mechanisms of seizures, our study has indeed some limitations: first, the presence of the viruses in the CSF was not ruled out for all the patients experiencing seizures; secondly, interleukin levels were dosed only in a subset of patients and on serum samples, therefore elevated values reflected a systemic – and not specifically neurologic – inflammatory response.
In conclusion, regardless of the specific mechanism responsible for seizures, our study demonstrated that SARS-CoV-2 and H1N1/H3N2 pneumonia have a similar ictogenic potential. In both these infections, seizures are rare (prevalence = 1.20%, range = 0.60–1.81%) but serious events, often associated with severe respiratory involvement, and affect also subjects without pre-existing predisposing conditions. This suggests that the interplay between pneumonia severity and the host response is the major driver of this clinical picture. Other viruses that cause severe respiratory diseases, without directly targeting the CNS are likely to have a similar ictogenic potential. Future studies, aimed at understanding the relationship between genetics, inflammation, and immunity, could better clarify why specific subgroups of patients are prone to develop these severe complications.
Declarations of conflict of interest
None.
Disclosure of conflicts of interest
None of the authors has any conflict of interest to disclose.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Michelangelo Bartolotta, Fabio Cignoni, Rossella Buscemi and Marta Barsotti from the EEG Lab of the Neurology Unit are gratefully acknowledged for their technical contribution to the EEG recordings and data collection.
References
- 1.Vohora D., Jain S., Tripathi M., Potschka H. COVID-19 and seizures: Is there a link? Epilepsia. 2020 doi: 10.1111/epi.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dono F., Nucera B., Lanzone J., Evangelista G., Rinaldi F., Speranza R., et al. Status epilepticus and COVID-19: a systematic review. Epilepsy Behav. 2021;118 doi: 10.1016/j.yebeh.2021.107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020 doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 4.Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat Rev Neurol. 2021 doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll E., Melmed K.R., Frontera J., Placantonakis D.G., Galetta S., Balcer L., et al. Cerebrospinal fluid findings in patients with seizure in the setting of COVID-19: a review of the literature. Seizure Eur J Epilepsy. 2021;89:99–106. doi: 10.1016/j.seizure.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. 2021 doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilotto A., Masciocchi S., Volonghi I., Crabbio M., Magni E., De-Giuli V., et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2–related encephalitis: the ENCOVID multicenter study. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzanelli C., Milano C., Canovetti S., Tagliaferri E., Turco F., Verdenelli S., et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: case-report and review of the literature. Brain, Behav Immun - Heal. 2021 doi: 10.1016/j.bbih.2021.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu B., Wu Z., Huang L., Chai Z., Zheng P., Sun Q., et al. A comparison of demographic, epidemiological and clinical characteristics of hospital influenza-related viral pneumonia patients. BMC Infect Dis. 2021;21 doi: 10.1186/s12879-021-06485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekstrand J.J., Herbener A., Rawlings J., Turney B., Ampofo K., Korgenski E.K., et al. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol. 2010 doi: 10.1002/ana.22184. [DOI] [PubMed] [Google Scholar]
- 12.Chen L.W., Teng C.K., Tsai Y.S., Wang J.N., Tu Y.F., Shen C.F., et al. Influenza-associated neurological complications during 2014–2017 in Taiwan. Brain Dev. 2018 doi: 10.1016/j.braindev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Farooq O., Faden H.S., Cohen M.E., Ramanathan M., Barrett H., Farkas M.K., et al. Influenza - A H1N1 infection in children. J Child Neurol. 2012:431–438. doi: 10.1177/0883073811417873. [DOI] [PubMed] [Google Scholar]
- 14.Ruisanchez-Nieva A., Martinez-Arroyo A., Gomez-Beldarrain M., Bocos Portillo J., Garcia-Monco J.C. Influenza-associated seizures in healthy adults: report of 3 cases. Epilepsy Behav Case Rep. 2017;8:12–13. doi: 10.1016/j.ebcr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 17.Costa C., Romoli M., Liguori C., Farotti L., Eusebi P., Bedetti C., et al. Alzheimer’s disease and late-onset epilepsy of unknown origin: two faces of beta amyloid pathology. Neurobiol Aging. 2019;73:61–67. doi: 10.1016/J.NEUROBIOLAGING.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Menon B., Shorvon S.D. Ischaemic stroke in adults and epilepsy. Epilepsy Res. 2009;87:1–11. doi: 10.1016/J.EPLEPSYRES.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., et al. A retrospective multicenter study. Epilepsia. 2020 doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eidelman L.A., Putterman D., Putterman C., Sprung C.L. The spectrum of septic encephalopathy: definitions, etiologies, and mortalities. J Am Med Assoc. 1996 [PubMed] [Google Scholar]
- 21.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004 doi: 10.1056/nejmoa041747. [DOI] [PubMed] [Google Scholar]
- 22.Wijeratne T., Sales C., Karimi L., Crewther S.G. Acute ischemic stroke in COVID-19: a case-based systematic review. Front Neurol. 2020 doi: 10.3389/fneur.2020.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph L., Fink L.M., Hauer-Jensen M. Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis. 2002 doi: 10.1097/00001721-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ryabkova V.A., Churilov L.P., Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: cytokine storm – The common denominator and the lessons to be learned. Clin Immunol. 2021 doi: 10.1016/j.clim.2020.108652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013 doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T., et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]