Abstract
Objective: This study aimed to explore the effect of miR-451 on IVF/ICSI-ET outcome in endometriosis patients with infertility. Methods: Eighty patients with endometriosis and infertility who came to our hospital for IVF/ICSI-ET from February 2018 to November 2019 were collected as the research participants, and 66 healthy women at the same time were selected as the control group. The miR-451 and MIF expression levels in serum, tissues and cell lines of patients with endometriosis and infertility were quantitatively detected by qRT-PCR. Correlation between miR-451 and endometriosis complicated with infertility was analyzed. The effect of miR-451 on IVF/ICSI-ET outcome in those patients was assessed. Results: The miR-451 and MIF expression levels in endometriosis complicated with infertility tissues and cell lines were quantitatively detected by qRT-PCR. Compared with normal people, miR-451 was abnormally low in endometriosis complicated with infertility tissues and cell lines (P<0.001), while MIF was abnormally high (P<0.001), and the miR-451 expression was dramatically down-regulated and the MIF expression was markedly up-regulated in serum of endometriosis patients complicated with infertility. ROC analysis identified that the area under the miR-451 curve (AUC=0.9078) was >0.8, and the AUC (0.8606) of MIF was >0.8. Correlation analysis showed that the expression of miR-451 and MIF was negatively correlated in endometriosis complicated with infertility. According to miR-451 expression in endometriotic lesions, the subjects were divided into the miR-451 high expression group and miR-451 low expression group, with 40 cases in each group. The pregnancy rate after IVF/ICSI-ET in patients with endometriosis and infertility with high expression of miR-451 was higher than that in those with low expression (P>0.05). The incidence of complications during pregnancy after IVF/ICSI-ET in patients with endometriosis and infertility with high expression of miR-451 was lower than that in those with low expression (P>0.05). The pregnancy outcome after IVF/ICSI-ET in the miR-451 high expression group was better than that in the miR-451 low expression group (P<0.05). Conclusion: miR-451 was down-regulated in endometriosis patients complicated with infertility, and low expression of miR-451 after IVF/ICSI-ET indicated a poor outcome.
Keywords: miR-451, MIF, endometriosis complicated with infertility, IVF/ICSI-ET, pregnancy outcome
Introduction
Endometriosis complicated with infertility is a chronic gynecological disease with estrogen dependence. It can be defined by the existence of endometrial stroma and glands outside the normal part of endometrium [1-3]. Advanced endometriosis may lead to gynecological malignancies and infertility, affecting women of childbearing age [4]. Endometriosis complicated with infertility is related to ovarian clear cells and endometrioid carcinoma. However, its exact pathophysiology is not well known [5]. Recent studies on endometriosis complicated with infertility show that miRNA should be studied at the molecular signal transduction pathway for the condition and prognosis of endometriosis complicated with infertility. Biological targeted therapy provides a new direction for endometriosis complicated with infertility, it determines effective biomarkers, and explores the potential mechanism of development, which is conducive to finding a more appropriate clinical treatment scheme [6-8]. miRNA plays a vital role in the development of some gynecological diseases [9,10]. However, their functions in inhibiting or promoting cancer are still not fully understood. Some studies have found that specific miRNA can regulate the cell function of different tumors [11,12].
Some reports on miRNA show that miR-451 is abnormally expressed in cancers of the reproductive system [13]. Li Menghui et al. have pointed out that there is abnormal inhibitory expression in the ectopic endometrium [14]. However, there is no research on the correlation between miR-451 and endometriosis complicated with infertility, and its influence on the outcome of routine in vitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET).
Therefore, by detecting miR-451 expression in endometriosis complicated with infertility, we explored the correlation between miR-451 and endometriosis complicated with infertility and its influence on IVF/ICSI-ET outcome, in order to find a reliable diagnosis and prognostic gene targets for clinical treatment.
Materials and methods
Eighty patients with endometriosis and infertility who came to Hengshui people’s Hospital for IVF/ICSI-ET from February 2018 to November 2019 were collected as the research participants, and 66 healthy women at the same time were selected as the control group. Inclusion criteria: It was in line with the guidelines of the American Society of Reproductive Medicine [15]; endometriosis complicated with infertility was confirmed by pathology, cytology and imaging; patients with endometriosis and infertility did not receive preoperative chemotherapy, immunotherapy and radiotherapy. Exclusion criteria: patients complicated with liver cirrhosis, coagulation dysfunction, or incomplete clinical data, those who did not cooperate with follow-up or who were lost to follow up, were excluded. The research was approved by the Ethics Committee of our hospital, and informed consent was signed in advance by the study participants.
Main instruments and reagents
ABI Stepone Plus real-time fluorescence quantitative PCR instrument and Trizol extraction kit were used. All primer sequences were synthesized by Shanghai Sangon Bioengineering Co., Ltd. The test tissue was taken from the endometriotic lesion tissue of women with endometriosis; and Immortalized cells (hEM15A cells) of eutopic endometrial stromal cells in patients with human endometriosis were purchased from BeiNa Biotech, BNCC102159.
Detection methods
qRT-PCR detection: The mRNA expression in tissues and cells was detected by qRT-PCR. Total RNA of tissues was extracted according to Trizol reagent operating instructions and dissolved in 20 μL DEPC water (Table 1). Then, it was reverse transcribed using a reverse transcription kit: pre-denaturation at 95°C for 15 min, denaturation at 95°C for 15 s, annealing at 58°C for 30 s, 35 cycles in total, extension at 72°C for 15 min. Each sample was provided with three multiple holes for three repeated tests. After that, the amplification curve and melting curve of Real-Time PCR were confirmed, and the relative quantity of target genes was calculated based on the result parameters. U6 was employed as internal reference in miR-451, GAPDH was employed as internal reference in MIF, and the relative quantification of target genes was calculated via 2-ΔCt.
Table 1.
MIF, miR-451 and their internal reference primer sequences
| Genes | 3’UTR-F | 3’UTR-R |
|---|---|---|
| MIF | 5’-CTAGAGCCCACCCCAACCTTCTGGTGGGGAGAAATAAACGGTTTAGAGACTGC-3’ | 5’-GGCCGCAGTCTCTAAACCGTTTATTTCTCCCCACCAGAAGGTTGGGGTGGGCT-3’ |
| miR-451 | 5’-AAACCGUUACCAUUACUGAGUU-3’ | 5’-UUCUCCGAACGUGUCACGUTT-3’ |
| GAPDH | 5’-CAAAGGTGGATCAGATTCAAG-3’ | 5’-GGTGAGCATTATCACCCAGAA-3’ |
| U6 | 5’-CTCGCTTCGGCAGCACA-3’ | 5’-AACGCTTCACGAATTTGCGT-3’ |
Statistical methods
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The normal distribution data were expressed by means ± standard deviation (meas ± SD), and the comparison of measurement data between groups adopted independent-samples t test. Data at multiple time points were compared through repeated measures analysis of variance (ANOVA), and back testing was done with the Bonferroni method. The comparison of mean values among multiple groups was done under one-way ANOVA, and back testing was performed with LSD-t test. The diagnostic value was evaluated by receiver operating characteristics (ROC) curve. The correlation was assessed via Pearson test. The difference was statistically remarkable when P<0.05.
Results
General information
There was no statistically significant difference in the age, BMI, household registration and other general information of the two groups of subjects (P>0.05). See Table 2.
Table 2.
General clinical data of patients
| Group | Endometriosis complicated with infertility (80 cases) | Healthy women (66 cases) | X2 | P |
|---|---|---|---|---|
| Age (years) | 0.000 | 1.000 | ||
| ≤32.00 | 32 (40.00) | 32 (40.00) | ||
| >32.00 | 48 (60.00) | 48 (60.00) | ||
| BMI (Kg/m2) | 0.197 | 0.657 | ||
| ≤21.20 | 27 (33.75) | 20 (30.30) | ||
| >21.20 | 53 (66.25) | 46 (69.70) | ||
| Place of domicile | 0.120 | 0.730 | ||
| Countryside | 35 (43.75) | 27 (40.91) | ||
| Towns | 45 (56.25) | 39 (59.09) | ||
| Family history of infertility | - | - | ||
| Yes | 8 (10.00) | - | ||
| No | 72 (90.00) | - | ||
| Smoking | 0.000 | 1.000 | ||
| Yes | 0 (0.00) | 0 (0.00) | ||
| No | 80 (100.00) | 66 (100.00) | ||
| Years of infertility (years) | - | - | ||
| ≤4 | 10 (12.50) | - | ||
| >4 | 70 (87.50) | - | ||
| EMS staging | - | - | ||
| Stages I/II | 60 (75.00) | - | ||
| Stages III/Stages IV | 20 (25.00) | - |
miR-451 expression in endometriosis complicated with infertility
(1) miR-451 expression in tissues and cell lines: The miR-451 and MIF expression levels in endometriosis complicated with infertility tissues and cell lines were quantitatively detected via qRT-PCR (Figures 1, 2). Compared with normal people, the miR-451 expression was abnormally low in endometriosis complicated with infertility tissues and cell lines (P<0.001), while the MIF expression was abnormally high (P<0.001).
Figure 1.
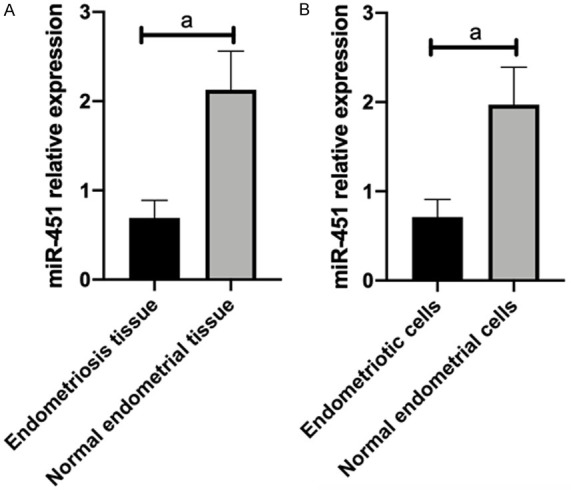
miR-451 expression in tissues and cell lines. Compared with normal people, miR-451 has abnormally low expression in endometriosis and infertility tissues (A) and cell lines (B). Note: ameans P<0.001.
Figure 2.
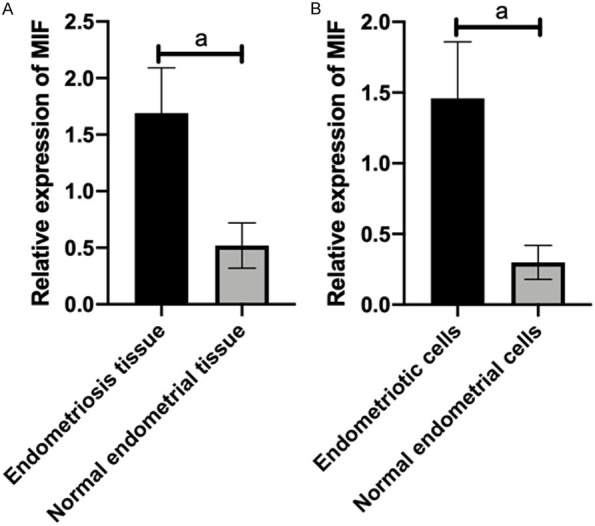
MIF expression in tissues and cell lines. Compared with normal people, MIF was abnormally high in endometriosis complicated with infertility tissues (A) and cell lines (B). Note: ameans P<0.001.
(2) miR-451 expression in serum and its diagnostic value: Compared with normal people, the miR-451 expression was dramatically down-regulated and MIF was markedly up-regulated in serum of patients with endometriosis and infertility. ROC curve showed that the AUC (0.9078) of miR-451 was >0.8, and the AUC (0.8606) of MIF was >0.8, as shown in Figures 3 and 4.
Figure 3.
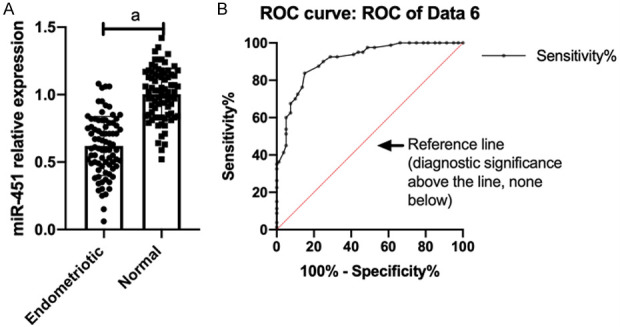
miR-451 expression in serum and its diagnostic value. Compared with normal people, the miR-451 expression (A) in serum of patients with endometriosis and infertility was remarkably down-regulated, and the AUC (0.9078) (B) of miR-451 was >0.8. Note: ameans P<0.001.
Figure 4.
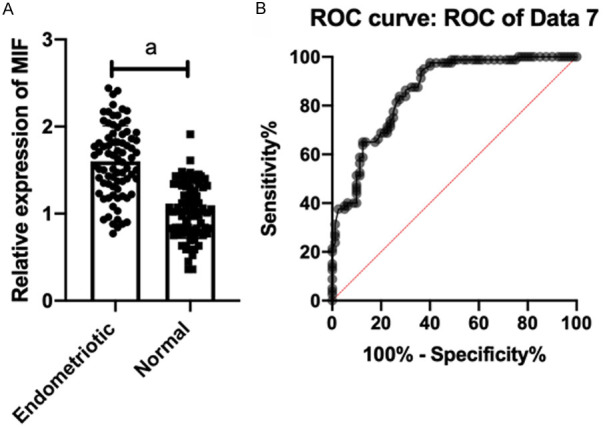
MIF expression in serum and its diagnostic value. Compared with normal people, the MIF expression (A) in serum of patients with endometriosis and infertility increased markedly, and the AUC (0.8606) (B) of MIF was >0.8. Note: ameans P<0.001.
Correlation analysis between miR-451 and endometriosis complicated with infertility
Correlation analysis revealed that the miR-451 and MIF expression levels were negatively correlated in endometriosis patients complicated with infertility. Thus, we believe that the miR-451 expression is lower with the aggravation of endometriosis complicated with infertility (Figure 5).
Figure 5.
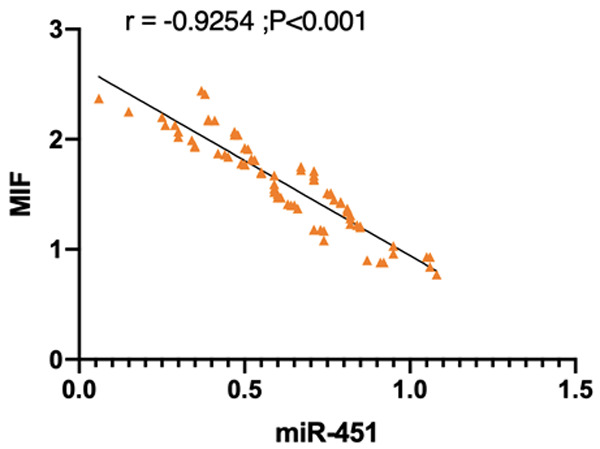
Correlation analysis between miR-451 and endometriosis complicated with infertility. Correlation analysis manifested that the miR-451 and MIF expression levels were negatively correlated in endometriosis complicated with infertility.
Effect of miR-451 on IVF/ICSI-ET outcome in patients with endometriosis and infertility
Compared with those before surgery, the miR-451 expression in patients with endometriosis and infertility after IVF/ICSI-ET increased markedly (P<0.05), as shown in Figure 6.
Figure 6.
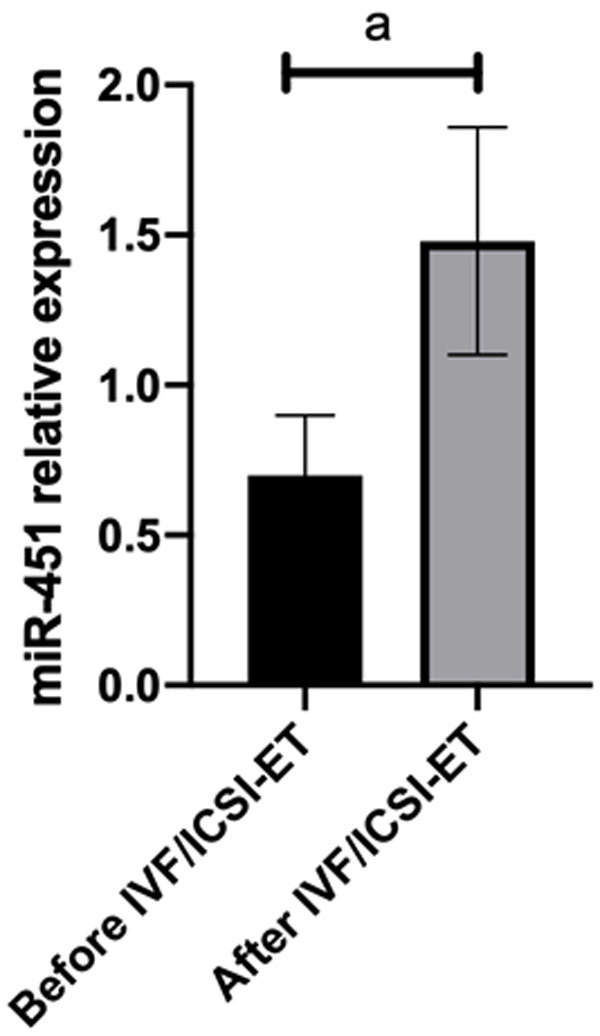
miR-451 expression after IVF/ICSI-ET. Compared with those before surgery, the miR-451 expression in patients with endometriosis and infertility after IVF/ICSI-ET was significantly down-regulated. Note: ameans P<0.001.
Based on miR-451 expression in endometriotic lesions, the subjects were divided into a miR-451 high expression group and a miR-451 low expression group, with 40 cases in each group. Comparison of pregnancy rate, complications during pregnancy, and pregnancy outcome after IVF/ICSI-ET were shown in Tables 3, 4 and 5.
Table 3.
Pregnancy rate after IVF/ICSI-ET
| Group | High expression group (40) | Low expression group (40) | χ2 | P |
|---|---|---|---|---|
| One month after operation | 6 (15.00) | 10 (25.00) | 20.09 | <0.001 |
| Six months after operation | 15 (37.50) | 20 (50.00) | 1.270 | 0.260 |
| Twelve months after operation | 30 (75.00) | 34 (85.00) | 1.250 | 0.264 |
Table 4.
Complications during pregnancy after IVF/ICSI-ET
| Group | High expression group (40) | Low expression group (40) | χ2 | P |
|---|---|---|---|---|
| Pregnancy-induced hypertension syndrome | 0 (0.00) | 1 (2.50) | 0.000 | 1.000 |
| Placenta previa | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
| Eclampsia | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
| Placental abruption | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
| Cholestasis | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
| Threatened uterus rupture | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
Table 5.
Comparison of pregnancy outcomes after IVF/ICSI-ET
| Group | High expression group (40) | Low expression group (40) | χ2 | P |
|---|---|---|---|---|
| Abortion in early pregnancy | 2 (5.00) | 3 (7.50) | 0.213 | 0.644 |
| Abortion in advanced pregnancy | 2 (5.00) | 3 (7.50) | 0.213 | 0.644 |
| Premature delivery | 6 (15.00) | 8 (20.00) | 0.346 | 0.556 |
| Singleton | 32 (80.00) | 24 (60.00) | 3.810 | 0.051 |
| Twins | 4 (10.00) | 2 (5.00) | 0.721 | 0.396 |
| Cesarean section | 28 (70.00) | 35 (87.50) | 3.660 | 0.056 |
(1) Pregnancy rate after IVF/ICSI-ET: The pregnancy rate of patients with endometriosis complicated with infertility after IVF/ICSI-ET in the miR-451 high expression group was higher than that of the miR-451 low expression group (P<0.05), as shown in Table 3.
(2) Complications during pregnancy after IVF/ICSI-ET: The incidence of complications during pregnancy after IVF/ICSI-ET in patients with endometriosis and infertility in the miR-451 high expression group was lower than that in the miR-451 low expression group (P>0.05), as shown in Table 4.
(3) Comparison of pregnancy outcomes after IVF/ICSI-ET: The pregnancy outcome after IVF/ICSI-ET in patients with endometriosis and infertility in the miR-451 high expression group was better than that in the miR-451 low expression group (P>0.05), as shown in Table 5.
Discussion
The study of the pathogenesis of endometriosis complicated with infertility is the focus of current research [16]. The pathogenesis of endometriosis is still unclear. However, miRNA, as a short non-coding RNA, participates in the pathogenesis of endometriosis by silencing the expression of target genes and the transcription of gene expression [17,18]. Although some studies have confirmed the mechanism of miR-451 in gynecological diseases, the research on whether miRNAs can be used for non-invasive diagnosis of endometriosis in infertile women is incomplete [19]. The purpose of this study is to monitor the miR-451 expression in tissues, cell lines and serum of patients with endometriosis and infertility, and to explore the related effects of miR-451 on IVF/ICSI-ET outcome, so as to provide a new theoretical basis for the diagnosis and treatment of endometriosis complicated with infertility in molecular biology.
We monitored the miR-451 expression in endometriosis complicated with infertility. The miR-451 and MIF expression levels in serum, tissues and cell lines of endometriosis complicated with infertility were quantitatively detected by qRT-PCR: compared with normal people, miR-451 was abnormally low in serum, tissues and cell lines of endometriosis complicated with infertility, while MIF was abnormally high. ROC curve showed that the AUC of miR-451 was >0.8, and the AUC of MIF was >0.8. miRNA and its target mRNA may cause changes in the normal physiological state of endometrial tissues and trigger pathological processes. The expression of related miRNA in ectopic endometrial tissues is also tied to related infertility symptoms in women with endometriosis [20,21]. It has been reported in related studies of miRNA and endometriosis that miR-92a promotes the development of progesterone-resistant endometriosis by inhibiting PTEN expression, so miR-92a regulation may be a potential medical method for treating endometriosis [22]. Li et al. also verified that miR-451 was down-regulated in the follicular fluid of women with endometriosis by miRNA analysis and quantitative reverse transcription polymerase chain reaction analysis, which affected the embryonic development potential of mice and humans [23], in line with this study. Hence, we believe that the miR-451 expression is low in serum, tissues and cell lines of patients with endometriosis and infertility. It is suggested that the decreased miR-451 expression in our in situ endometrium is the pathogenesis of endometriosis.
Correlation analysis showed that the expression of miR-451 and MIF was negatively correlated in endometriosis complicated with infertility. Therefore, we believe that the miR-451 expression is lower with the aggravation of endometriosis complicated with infertility. Macrophage migration inhibitory factor (MIF) is a key proinflammatory cytokine over-expressed in endometriosis [24]. However, MIF, as a potential biomarker of endometriosis, cannot be used alone, and it has higher diagnostic accuracy when used in combination [25]. In this study, miR-451 is negatively correlated with MIF in lesion tissues, and it is a tumor suppressor microRNA with multiple target genes. For example, it may regulate the proliferation and migration of human colorectal cancer cells by targeting MIF [26]; it also may play an anti-cancer role in osteosarcoma. miR-451 inhibits cell proliferation, migration and angiogenesis partially by inhibiting MIF expression [27]. Therefore, we believe that it can be used to monitor the changes of patients with endometriosis and infertility simultaneously. Finally, we focused on the effect of miR-451 on IVF/ICSI-ET outcome in endometriosis patients with infertility. The pregnancy rate after IVF/ICSI-ET in the miR-451 high expression group was dramatically higher than that in the miR-451 low expression group. In addition, the rate of complications such as pregnancy-induced hypertension syndrome, placenta previa, eclampsia and uterine rupture in the miR-451 high expression group was reduced markedly. Thus, we believe that the pregnancy outcome after IVF/ICSI-ET in patients with endometriosis and infertility in the miR-451 high expression group is better than that in the miR-451 low expression group.
In this experiment, due to the limited medical resources and the small number of selected subjects, there may be some contingency in the possible results. It is not excluded that BIM has different effects on IVF/ICSI-ET in different patients. We will conduct a longer follow-up investigation on this subject, and constantly improve our experiment to achieve the best experimental results.
To sum up, miR-451 is down-regulated in patients with endometriosis and infertility, and low expression of miR-451 after IVF/ICSI-ET indicates a poor outcome.
Disclosure of conflict of interest
None.
References
- 1.Kolanska K, Cohen J, Bendifallah S, Selleret L, Antoine JM, Chabbert-Buffet N, Darai E, d’Argent EM. Pregnancy outcomes after controlled ovarian hyperstimulation in women with endometriosis-associated infertility: GnRH-agonist versus GnRH-antagonist. J Gynecol Obstet Hum Reprod. 2017;46:681–686. doi: 10.1016/j.jogoh.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Miller JE, Ahn SH, Marks RM, Monsanto SP, Fazleabas AT, Koti M, Tayade C. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. 2020;11:108. doi: 10.3389/fimmu.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian RY, Wu X, Sheng J, Zheng P, Zhou Q, Duan AH, Zhang JP, Zhang YL, Lu D. Evaluation of endometriosis fertility index in follow-up treatment of endometriosis combined with infertility patients after laparoscopic surgery. Zhonghua Fu Chan Ke Za Zhi. 2017;52:233–238. doi: 10.3760/cma.j.issn.0529-567X.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Radzinsky VY, Orazov MR, Ivanov II, Khamoshina MB, Kostin IN, Kavteladze EV, Shustova VB. Implantation failures in women with infertility associated endometriosis. Gynecol Endocrinol. 2019;35:27–30. doi: 10.1080/09513590.2019.1632089. [DOI] [PubMed] [Google Scholar]
- 5.Bakun OV, Yurkiv OI, Slobodian KV, Kolesnik OV, Maruschak AV. The level of some hormones in the blood women with endometriosis which associated with infertility. Wiad Lek. 2019;72:654–656. [PubMed] [Google Scholar]
- 6.Malvezzi H, Hernandes C, Piccinato CA, Podgaec S. Interleukin in endometriosis-associated infertility-pelvic pain: systematic review and meta-analysis. Reproduction. 2019;158:1–12. doi: 10.1530/REP-18-0618. [DOI] [PubMed] [Google Scholar]
- 7.Hui Y, Zhao S, Gu J, Hang C. Analysis of factors related to fertility after endometriosis combined with infertility laparoscopic surgery. Medicine (Baltimore) 2020;99:e20132. doi: 10.1097/MD.0000000000020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali AH, Haider G, Ahmed K, Hashmi M, Kerio P, Irshad R. Correlation between E-cadherin and hormone receptor status among breast cancer patients. J Coll Physicians Surg Pak. 2020;30:1030–1034. doi: 10.29271/jcpsp.2020.10.1030. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Liu N, Wu X, Wu P, Song N, Ma J. Identification of differentially expressed circular RNAs in keloid and normal skin tissue by high-throughput sequencing. Dermatol Ther. 2021;34:e14745. doi: 10.1111/dth.14745. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Wang D, Chen S, Yang Q. The role of miR-34c-5p/Notch in epithelial-mesenchymal transition (EMT) in endometriosis. Cell Signal. 2020;72:109666. doi: 10.1016/j.cellsig.2020.109666. [DOI] [PubMed] [Google Scholar]
- 11.Takebayashi K, Nasu K, Okamoto M, Aoyagi Y, Hirakawa T, Narahara H. hsa-miR-100-5p, an overexpressed miRNA in human ovarian endometriotic stromal cells, promotes invasion through attenuation of SMARCD1 expression. Reprod Biol Endocrinol. 2020;18:31. doi: 10.1186/s12958-020-00590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Chen Y, Gao J, Shankar S, Zhang G. Diagnostic value of circulating MicroRNAs for endometriosis: a meta-analysis. Reprod Sci. 2020;27:793–805. doi: 10.1007/s43032-019-00024-5. [DOI] [PubMed] [Google Scholar]
- 13.Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, Lessey BA, Fazleabas AT. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod. 2015;30:2881–2891. doi: 10.1093/humrep/dev229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Zhang W, Fu J, Xu Y, Gu R, Qu R, Li L, Sun Y, Sun X. MicroRNA-451 is downregulated in the follicular fluid of women with endometriosis and influences mouse and human embryonic potential. Reprod Biol Endocrinol. 2019;17:96. doi: 10.1186/s12958-019-0538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pessoa de Farias Rodrigues M, Lima Vilarino F, de Souza Barbeiro Munhoz A, da Silva Paiva L, de Alcantara Sousa LV, Zaia V, Parente Barbosa C. Clinical aspects and the quality of life among women with endometriosis and infertility: a cross-sectional study. BMC Womens Health. 2020;20:124. doi: 10.1186/s12905-020-00987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang R, An J, Zheng Y, Li J, Wang Y, Jia Y, Zhang J, Lu Q. predicting and improving the probability of live birth for women undergoing frozen-thawed embryo transfer: a data-driven estimation and simulation model. Comput Methods Programs Biomed. 2021;198:105780. doi: 10.1016/j.cmpb.2020.105780. [DOI] [PubMed] [Google Scholar]
- 17.Lin MM, Niu ZR, Zhang H, Li R. Pregnancy outcomes of the first thawing cycle in “freeze-all” strategy of infertility patients with fever during oocyte recruitment: a matched-pair study. Chin Med J (Engl) 2020;134:800–805. doi: 10.1097/CM9.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Broi MG, Navarro PA. Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016;364:1–7. doi: 10.1007/s00441-015-2339-9. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Li Z, Liu J, Yu S, Wei Z. MicroRNA expression profiling in endometriosis-associated infertility and its relationship with endometrial receptivity evaluated by ultrasound. J Xray Sci Technol. 2017;25:523–532. doi: 10.3233/XST-17286. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Wu Z, Ma C, Pan N, Wang Y, Yan L. Endometrial miR-543 is downregulated during the implantation window in women with endometriosis-related infertility. Reprod Sci. 2019;26:900–908. doi: 10.1177/1933719118799199. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Liu CM, Cui LL, Zhou L, Li N, Wei XD. Expression and significance of MiR-126 and VEGF in proliferative diabetic retinopathy. Eur Rev Med Pharmacol Sci. 2019;23:6387–6393. doi: 10.26355/eurrev_201908_18518. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Peng J, Shi Y, Sun P. miR-92a promotes progesterone resistance in endometriosis through PTEN/AKT pathway. Life Sci. 2020;242:117190. doi: 10.1016/j.lfs.2019.117190. [DOI] [PubMed] [Google Scholar]
- 23.El-Mahdy RI, Saleem TH, Essam OM, Algowhary M. Functional variants in the promoter region of macrophage migration inhibitory factor rs755622 gene (MIF G173C) among patients with heart failure: association with echocardiographic indices and disease severity. Heart Lung. 2021;50:92–100. doi: 10.1016/j.hrtlng.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Chekini Z, Shahhoseini M, Aflatoonian R, Afsharian P. The relationship between functional promoter variants of macrophage migration inhibitory factor and endometriosis. Cell J. 2021;22:450–456. doi: 10.22074/cellj.2021.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues BL, Mazzaro MC, Nagasako CK, Ayrizono MLS, Fagundes JJ, Leal RF. Assessment of disease activity in inflammatory bowel diseases: non-invasive biomarkers and endoscopic scores. World J Gastrointest Endosc. 2020;12:504–520. doi: 10.4253/wjge.v12.i12.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YG, Han YZ, Zhang ZS, Yu Y, Xu XF, Yuan L. MiR-451 regulates proliferation and migration of colorectal cells by targeting MIF. Zhonghua Zhong Liu Za Zhi. 2020;42:312–318. doi: 10.3760/cma.j.cn112152-20190924-00622. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Liu SY, He YB, Huang RL, Deng SY, Ni GX, Yu B. MiR-451 suppresses proliferation, migration and promotes apoptosis of the human osteosarcoma by targeting macrophage migration inhibitory factor. Biomed Pharmacother. 2017;87:621–627. doi: 10.1016/j.biopha.2016.12.121. [DOI] [PubMed] [Google Scholar]


