Abstract
Background: To investigate the clinical efficacy and safety of crizotinib and alectinib in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) treatment and the predictive value of serum carcinoembryonic antigen (CEA) and carbohydrate antigen 125 (CA125) for treatment efficacy. Methods: A total of 120 patients with ALK-positive NSCLC were enrolled and randomly assigned to receive crizotinib treatment (54 patients, the control group) or alectinib treatment (66 patients, the research group). Treatment efficacy, adverse reactions, survival, and quality of life of patients were compared between the two groups. Enzyme-linked immunosorbent assay was used to determine the serum CEA and CA125 concentrations and these levels were compared between patients with certain treatment responses or no responses. Receiver operating characteristic curve was used to assess the predictive value of CEA and CA125 for treatment efficacy. Results: The overall disease control rate, overall response rate, and number of 1-year survival patients were substantially higher in the research group compared with the control group. Moreover, the incidence of adverse reactions was significantly lower and progression-free survival and overall survival rates were higher in the research group compared with those in the control group. The area under the curve (AUC) for predicting treatment efficacy was 0.889 for CEA and 0.866 for CA125. Conclusion: Alectinib was clinically more efficacious and safer than crizotinib for ALK-positive NSCLC treatment. Both CEA and CA125 demonstrated excellent predictive value for treatment efficacy.
Keywords: Crizotinib, alectinib, ALK-positive non-small cell lung cancer, CEA, CA125
Introduction
Lung cancer (LC) is difficult to diagnose in the early stage and this delay leads to poor prognosis [1,2]. According to cancer statistics, LC is the leading cause of cancer deaths [3]. There are two histological types of LC: non-small cell LC (NSCLC) and small cell LC. In the United States, 80% of patients with LC are diagnosed with NSCLC [4,5]. Anaplastic lymphoma kinase (ALK) gene rearrangement occurs in 3%-5% of the patients with NSCLC [6]. The current main treatments for patients with advanced ALK-positive NSCLC are targeted therapy with oral ALK inhibitors and standard pemetrexed-plus-platinum chemotherapy [7]. However, despite constant improvements in NSCLC treatment, the overall cure and survival rates of patients with NSCLC remains extremely low [8]. Therefore, the development and investigation of highly effective drugs for treating NSCLC can improve the survival rate and quality of life of patients with NSCLC.
Crizotinib is an ALK inhibitor that inhibits the expression of ALK-rearranged oncogenes in NSCLC and controls systemic and intracranial diseases in patients with NSCLC [9]. A study reported that high doses of crizotinib induces immunogenic cell death and stimulates anti-tumor immune responses to suppress tumors [10]. In a study by Nishio et al. [11] conducted in an Asian patients with NSCLC, crizotinib was more efficacious for treating advanced ALK-positive NSCLC and improving progression-free survival compared with chemotherapy. Alectinib is a second-generation ALK inhibitor administered after first-line crizotinib that inhibits ALK activity in ALK-positive neuroblastoma cells [12,13]. Another study reported that alectinib, which functions as a central nervous system (CNS) penetrant as well, reduces the risk of CNS metastasis, improves the survival of patients with ALK-positive NSCLC, lessens the medical burden of patients [14]. CNS is a common metastatic site for the initial progression of NSCLC, suggesting that alectinib can reduce the risk of NSCLC progression [15].
To date, the efficacy of crizotinib and alectinib to treat ALK-positive NSCLC has been rarely compared. Therefore, the present study was conducted to compare the two drugs with respect to efficacy, safety, quality of life of patients, and survival.
Materials and methods
Patient information
We enrolled 120 patients with ALK-positive NSCLC who were admitted to our hospital from January 2017 to February 2018 and randomly assigned them to receive crizotinib (54 patients, the control group) or alectinib treatment (66 patients, the research group). The control group was comprised of 32 males and 22 females (aged 22-79 years, mean age 60.59±5.37 years). The research group was comprised of 37 males and 29 females (aged 21-77 years, mean age 59.88±6.16 years). The present study was approved by the Ethics Committee of Lanling County People’s Hospital. All the research participants and their families signed informed consent with complete knowledge of the study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: patients diagnosed with ALK-positive NSCLC by pathology, histology, or laboratory indicators; [16] those with stage IIIB or IV disease according to the International Association for the Study of LC TNM classification; [17] those with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) [18] score of 0-2 points; those with a life expectancy of >3 months; those with no previous ALK inhibitor administration; those with a Karnofsky score of ≥70 points; and those with measurable lesions. The exclusion criteria were as follows: patients with other malignant tumors or severe heart, kidney, and lung dysfunction; those with severe mental illness; those with ALK-negative NSCLC; those with a history of receiving >2 chemotherapy sessions; and those unable to cooperate with the terms of the study.
Treatment methods
The control group received 250 mg crizotinib (Pfizer) twice daily. The research group received 300 mg crizotinib twice daily. All patients were treated for at least three courses, 3 weeks for each course. Further, all patients underwent regular examinations, including routine blood tests, electrocardiograms, liver and kidney function tests, and CT scans, during the treatment period. Appropriate mitigation measures were employed when adverse reactions occurred during the treatment period.
Efficacy evaluation
Treatment efficacy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [19]. A complete response (CR) was defined as the complete disappearance of the lesion, with no lesion being detected for at least 4 weeks. A partial response (PR) was defined as a ≥30% decrease in lesion diameter that was confirmed for at least 4 weeks. Progressive disease (PD) was defined as new lesions or a ≥20% increase in lesion diameter, and stable disease (SD) was defined as a <30% decrease or <20% increase in lesion diameter. CR and PR were classified as effective responses and SD and PD were considered ineffective responses. Patients with CR, PR, and SD were considered to be in remission and those with PD were considered not to be in remission. The overall response rate and overall disease control rate were calculated.
Outcome measures
The control and research groups were compared for efficacy, adverse reactions (alopecia, peripheral neuritis, abnormal liver function, nausea and vomiting, visual impairment, edema, thrombocytopenia, and leukopenia), the number of 1-year survival patients, progression-free survival, overall survival, the quality of life, and serum CEA and CA125 concentrations and were further stratified according to patients with effective or ineffective treatment responses. The quality of life was assessed according to the Karnofsky Performance Status Scale [20]. Improved quality of life was defined as a score increase of 10 points compared with that before treatment. Stable quality of life was defined by a score increase of <10 points and decline in the quality of life was defined as a score decrease of 10 points.
Detection
Blood (5 mL) from the medial cubital vein was obtained from patients at 8:00 am at 4 weeks after the end of treatment and placed in a vacuum-free blood collection tube. Following centrifugation at 3500 RPMs for 8 min, the separated serum was collected in an EP tube and stored at -80°C. Thereafter, the serum was thawed in a refrigerator at 4°C, followed by complete thawing at room temperature. Serum CEA and CA125 concentrations were measured using enzyme-linked immunosorbent assay (ELISA) [21] in strict accordance with the human CEA ELISA and human CA125 ELISA kit instructions (Shanghai Hengfei Biotechnology Co., Ltd., CSB-E04767h-1, CSB-E04771h-1). The sample, standard, and blank wells were identified. The optical density of each well was measured using a fully automatic enzyme label analyzer (Shanghai Fuze Trading Co., Ltd., AMR-100) and CEA and CA125 concentrations were determined.
Follow-up
The patients were followed up every 3 months via telephone correspondence or a visit to determine the final treatment outcome of patients. The overall survival period was defined as the period from the start of the treatment to the time of death or the end of follow-up.
Statistical analysis
Data were visualized using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). SPSS 21.0 was used for statistical analysis. The count data were expressed as number and percentage [n (%)] and were compared between the control and research groups using the chi-squared test. The continuous variables were expressed as mean ± SD and were compared between the two groups using independent sample t-tests. ROC curves were employed to assess the predictive value of CEA and CA125 for treatment efficacy. A statistical difference was indicated by P<0.05.
Results
Baseline data
There were no significant differences between the control and research groups in terms of sex, mean age, body mass index (BMI), ECOG PS score, pathological type, TNM stage, smoking habits, alcohol consumption, marital status, diet, or place of residence (P>0.05, Table 1).
Table 1.
Comparison of baseline characteristics between the control and research groups [n (%), mean ± SD]
| Factors | n | Control group (n=54) | Research group (n=66) | χ2/t | P |
|---|---|---|---|---|---|
| Sex | 0.124 | 0.724 | |||
| Male | 69 | 32 (59.26) | 37 (56.06) | ||
| Female | 51 | 22 (40.74) | 29 (43.94) | ||
| Mean age (year) | 120 | 60.59±5.37 | 59.88±6.16 | 0.665 | 0.507 |
| BMI (kg/m2) | 120 | 21.50±2.53 | 21.37±2.15 | 0.304 | 0.762 |
| ECOG PS score | 0.425 | 0.809 | |||
| 0 | 61 | 29 (53.70) | 32 (48.48) | ||
| 1 | 55 | 23 (42.59) | 32 (48.48) | ||
| 2 | 4 | 2 (3.71) | 2 (3.04) | ||
| Pathological type | 0.367 | 0.833 | |||
| Squamous cell carcinoma | 54 | 25 (46.30) | 29 (43.94) | ||
| Adenocarcinoma | 60 | 27 (50.00) | 33 (50.00) | ||
| Other | 6 | 2 (3.70) | 4 (6.06) | ||
| TNM stage | 1.061 | 0.303 | |||
| IIIB | 64 | 26 (48.15) | 38 (57.58) | ||
| IV | 56 | 28 (51.85) | 28 (42.42) | ||
| Smoking | 0.081 | 0.776 | |||
| No | 45 | 21 (38.89) | 24 (36.36) | ||
| Yes | 75 | 33 (61.11) | 42 (63.64) | ||
| Alcohol consumption | 1.028 | 0.311 | |||
| No | 43 | 22 (40.74) | 21 (31.82) | ||
| Yes | 77 | 32 (59.26) | 45 (68.18) | ||
| Marital status | 0.182 | 0.670 | |||
| Unmarried | 22 | 9 (16.67) | 13 (19.70) | ||
| Married | 98 | 45 (83.33) | 53 (80.30) | ||
| Diet | 0.003 | 0.956 | |||
| Light diet | 73 | 33 (61.11) | 40 (60.61) | ||
| Heavy diet | 47 | 21 (38.89) | 26 (39.39) | ||
| Place of residence | 0.030 | 0.862 | |||
| Rural area | 41 | 18 (33.33) | 23 (34.85) | ||
| Urban area | 79 | 36 (66.67) | 43 (65.15) |
Comparison of clinical efficacy between the two groups
In the control group, 16 patients experienced CR, 22 demonstrated PR, 6 demonstrated SD, and 10 demonstrated PD, with an overall disease control rate of 81.48% and overall response rate of 70.37%. In the research group, 33 patients experienced CR, 18 demonstrated PR, 10 demonstrated SD, and 5 demonstrated PD, with an overall disease control rate of 92.42% and overall response rate of 77.27%. The overall disease control and overall response rates were lower in the control group than in the research group and the difference in the overall response rate was significant (P<0.05, Table 2).
Table 2.
Comparison of clinical efficacy between the control and research groups [n (%)]
| Group | n | CR | PR | SD | PD | Overall disease control rate | Overall response rate |
|---|---|---|---|---|---|---|---|
| Control group | 54 | 16 (29.63) | 22 (40.74) | 6 (11.11) | 10 (18.52) | 81.48 | 70.37 |
| Research group | 66 | 33 (50.00) | 18 (27.27) | 10 (15.15) | 5 (7.58) | 92.42 | 77.27 |
| χ2 | - | - | - | - | - | 3.252 | 4.495 |
| P | - | - | - | - | - | 0.071 | 0.034 |
Adverse reactions in the two groups
The incidence of alopecia, peripheral neuritis, abnormal liver function, nausea and vomiting, visual impairment, edema, thrombocytopenia, and leukopenia was significantly lower in the research group than that in the control group (P<0.05, Table 3).
Table 3.
Adverse reactions in the control and research groups
| Events | Control group (n=54) | Research group (n=66) | χ2 | P |
|---|---|---|---|---|
| Alopecia | 5.057 | 0.025 | ||
| Yes | 4 (7.41) | 0 (0.00) | ||
| No | 50 (92.59) | 66 (100.00) | ||
| Peripheral neuritis | 8.485 | 0.004 | ||
| Yes | 12 (22.22) | 3 (4.55) | ||
| No | 42 (77.78) | 63 (95.45) | ||
| Abnormal liver function | 4.207 | 0.040 | ||
| Yes | 11 (20.37) | 5 (7.58) | ||
| No | 43 (79.63) | 61 (92.42) | ||
| Nausea and vomiting | 7.916 | 0.005 | ||
| Yes | 10 (18.52) | 2 (3.03) | ||
| No | 44 (81.48) | 64 (96.97) | ||
| Visual impairment | 3.153 | 0.076 | ||
| Yes | 10 (18.52) | 3 (4.55) | ||
| No | 44 (81.48) | 63 (95.45) | ||
| Edema | 5.399 | 0.020 | ||
| Yes | 8 (14.81) | 2 (3.03) | ||
| No | 46 (85.19) | 64 (96.97) | ||
| Thrombocytopenia | 6.377 | 0.012 | ||
| Yes | 5 (9.26) | 0 (0.00) | ||
| No | 49 (90.74) | 66 (100.00) | ||
| Leukopenia | 10.476 | 0.001 | ||
| Yes | 8 (14.81) | 0 (0.00) | ||
| No | 46 (85.19) | 66 (100.00) |
Survival of patients in the two groups
In the control group, 13 patients survived for 1 year; the progression-free survival was 8.50±2.13 months and overall survival was 10.15±3.41 months. In the research group, 28 patients survived for 1 year; the progression-free survival was 9.91±2.27 months and overall survival was 12.66±4.58 months. The number of 1-year survival patients, progression-free survival, and overall survival were notably higher in the research group than those in the control group (P<0.05, Table 4).
Table 4.
Comparison of postoperative survival between the control and research groups
| Group | n | 1-year survival patients [n (%)] | Progression-free survival (month) | Overall survival (month) |
|---|---|---|---|---|
| Control group | 54 | 13 (24.07) | 8.50±2.13 | 10.15±3.41 |
| Research group | 66 | 28 (42.42) | 9.91±2.27 | 12.66±4.58 |
| χ2/t | - | 4.446 | 3.480 | 3.340 |
| P | - | 0.035 | 0.007 | 0.001 |
Quality of life of patients in the two groups
In the control group, the quality of life after treatment had declined in 15 patients, was stable in 29, and had improved in 10; the stability rate was 72.22%. In the research group, the quality of life after treatment had declined in 8 patients, was stable in 43, and had improved in 15; the stability rate was 87.88%. The stability rate was considerably higher in the research group than in the control group (P<0.05, Table 5).
Table 5.
Quality of life of patients in the control and research groups [n (%)]
| Group | n | Declined quality of life | Stable quality of life | Improved quality of life | Stability rate |
|---|---|---|---|---|---|
| Control group | 54 | 15 (25.93) | 29 (55.56) | 10 (18.51) | 72.22 |
| Research group | 66 | 8 (12.12) | 43 (65.15) | 15 (22.73) | 87.88 |
| χ2/t | - | - | - | - | 4.699 |
| P | - | - | - | - | 0.030 |
Serum expression of CEA and CA125 in patients
Among the 120 patients enrolled, 89 showed effective treatment responses, whereas 21 showed ineffective responses. Serum CEA concentration was 9.69±1.98 μg/mL and 12.77±2.03 μg/mL in patients with effective and ineffective treatment responses, respectively. Serum CA125 concentration was 65.64±9.73 μg/mL and 83.11±10.36 μg/mL in patients with effective and ineffective treatment responses, respectively. The patients with ineffective treatment responses showed increased serum CEA and CA125 concentrations compared with the effective treatment responses (Figure 1).
Figure 1.

Serum CEA and CA125 expressions in patients. A. The patients with effective treatment responses showed decreased serum CEA concentration compared with the ineffective treatment responses. B. Serum CA125 concentration was significantly lower in patients with effective treatment responses than in those with ineffective treatment responses. Note: ***P<0.001.
Predictive value of serum CEA and CA125 concentrations for treatment efficacy
According to the ROC curves for predicting treatment efficacy, CEA showed an AUC of 0.889 (95% CI: 0.821-0.957), a cutoff value of 11.18, sensitivity of 90.48%, and specificity of 74.16%, whereas CA125 showed an AUC of 0.866 (95% CI: 0.798-0.934), a cutoff value of 75.69, sensitivity of 95.24%, and specificity of 73.03% (Table 6 and Figure 2).
Table 6.
Predictive value of serum CEA and CA125 concentrations for treatment efficacy
| Group | AUC | 95% CI | S.E | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| CEA | 0.889 | 0.821-0.957 | 0.035 | 11.18 | 90.48 | 74.16 |
| CA125 | 0.866 | 0.798-0.934 | 0.034 | 75.69 | 95.24 | 73.03 |
Figure 2.
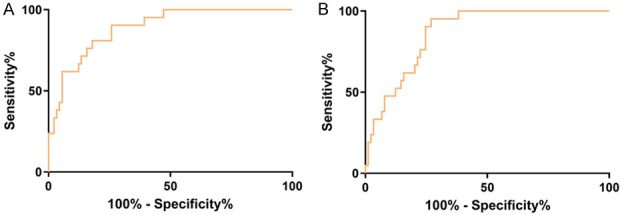
ROC curves of serum CEA and CA125 for predicting treatment efficacy. A. ROC curve of serum CEA concentration for predicting treatment efficacy. B. ROC curve of serum CA125 concentration for predicting treatment efficacy.
Discussion
Both crizotinib and alectinib are ALK inhibitors used to treat patients with ALK-positive NSCLC. However, acquired mutations in NSCLC increase the resistance to crizotinib, thereby resulting in lower chemosensitivity to crizotinib [22]. The resistance to alectinib may be attributable to the expression of an adenosine triphosphate-binding cassette transporter ABCC11 that is activated in vivo [23]. The resistance to crizotinib and alectinib in patients with ALK-positive NSCLC is different. The present study did not investigate the mechanism of drug resistance to crizotinib and alectinib; instead, we compared the efficacy of the two drugs.
Numerous researchers have studied the efficacy of crizotinib and alectinib in patients with ALK-positive NSCLC. Ou et al., [24] indicated that patients with ALK-positive NSCLC who experienced poor efficacy after treatment with crizotinib showed good tolerance and treatment outcomes after treatment with alectinib, with grade 3-5 adverse reactions, including dyspnea and pulmonary embolism, occurring in only 27.5% of the patients. In a study by Gandhi et al. [25] involving alectinib-treated patients with ALK-positive NSCLC having CNS metastasis who were tolerant to crizotinib, alectinib greatly relieve the CNS metastasis and controlled the disease, with or without radiotherapy. In the present study, patients treated with alectinib showed significantly higher overall disease control rates and overall response rates compared with those treated with crizotinib. Furthermore, adverse reactions, such as alopecia, peripheral neuritis, abnormal liver function, nausea and vomiting, visual impairment, edema, thrombocytopenia, and leukopenia, were less common in patients treated with alectinib than in those treated with crizotinib, suggesting that alectinib was superior to crizotinib in disease control, efficacy, and safety. ALK inhibitors reportedly induce ocular toxicity that manifest as side effects including spots, adaptation disorder, presbyopia, decreased vision, and blurred vision [26]. A previous study reported that abnormal liver function was the most common side effect of alectinib treatment [27], which is similar to the results of the present study. In a study by Shaw et al. [28] of alectinib-treated patients with stage III ALK-positive NSCLC, alectinib treatment resulted in longer progression-free survival and overall survival, better efficacy, and superior tolerability compared with crizotinib. In the present study, the number of 1-year survival patients, progression-free survival, overall survival, and proportion of stable quality of life patients were significantly higher in the research group than in the control group, indicating that alectinib can substantially reduce the mortality risk in patients with ALK-positive NSCLC and effectively improve their quality of life and survival.
CEA is a prognostic serum tumor marker for patients with NSCLC. High CEA concentrations are often associated with disease progression and poor prognosis [29,30]. Zhang et al. [31] demonstrated that CA125, a serum tumor marker involved in NSCLC occurrence and progression, was higher in the NSCLC group than in the benign lung lesion group and healthy control group, indicating the potential of serum CA125 concentration to predict NSCLC. At the end of the study, we determined the serum CEA and CA125 concentrations to assess their predictive value for treatment efficacy in patients with ALK-positive NSCLC and observed that these concentrations were significantly lower in patients with effective treatment responses than in those with ineffective treatment responses. The AUC was 0.889 for CEA and 0.866 for CA125 in predicting efficacy, suggesting that serum CEA and CA125 concentrations have a good predictive value for treatment efficacy in patients with ALK-positive NSCLC.
The present study confirmed that alectinib was superior to crizotinib in efficacy, safety, quality of life, and survival of patients with ALK-positive NSCLC and demonstrated the ability of serum CEA and CA125 concentrations to predict treatment efficacy. However, this study also has limitations. First, the sample size should be expanded to validate the study results. Second, the molecular mechanisms of both ALK inhibitors that affect the biological function of NSCLC cells as well as their specific regulatory mechanisms should be investigated. Such investigations will be performed in the future.
In summary, alectinib is more clinically valuable for treating ALK-positive NSCLC compared with crizotinib. Further, the study showed that CEA and CA125 can be used as tumor markers for predicting treatment efficacy in patients with ALK-positive NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Yu H, Guan Z, Cuk K, Brenner H, Zhang Y. Circulating microRNA biomarkers for lung cancer detection in Western populations. Cancer Med. 2018;7:4849–4862. doi: 10.1002/cam4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok T, Reck M, Van Schil PE, Hellmann M, Peters S ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 3.Torre L, Siegel R, Jemal A. Lung cancer and personalized medicine. Lung Cancer Statistics. 2016:11–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wu L, Xu Y, Zhang B, Wu X, Wang Y, Pang Z. Trends in the incidence rate of lung cancer by histological type and gender in Sichuan, China, 1995-2015: a single-center retrospective study. Thorac Cancer. 2018;9:532–541. doi: 10.1111/1759-7714.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fashoyin-Aje LA, Fernandes LL, Sridhara R, Keegan P, Pazdur R. Demographic composition of lung cancer trials: FDA analysis. J. Clin. Oncol. 2018;15(Suppl):9088. [Google Scholar]
- 6.Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, Gridelli C, Delmonte A, Garcia Campelo R, Kim DW, Bearz A, Griesinger F, Morabito A, Felip E, Califano R, Ghosh S, Spira A, Gettinger SN, Tiseo M, Gupta N, Haney J, Kerstein D, Popat S. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 7.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 9.Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, Schnell P, Wilner KD, Wiltshire R, Camidge DR, Crinò L. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 2015;33:1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10:1486. doi: 10.1038/s41467-019-09415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishio M, Kim DW, Wu YL, Nakagawa K, Solomon BJ, Shaw AT, Hashigaki S, Ohki E, Usari T, Paolini J, Polli A, Wilner KD, Mok T. Crizotinib versus chemotherapy in asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat. 2018;50:691–700. doi: 10.4143/crt.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herden M, Waller CF. Springer; 2018. Alectinib. Small molecules in oncology; pp. 247–256. [Google Scholar]
- 13.Alam MW, Borenas M, Gustafsson DE, Cervantes D, Umapathy G, Palmer R, Hallberg B. Alectinib, an anaplastic lymphoma kinase inhibitor, abolishes ALK activity and growth in ALK-positive neuroblastoma cells. Front Oncol. 2019;9:579. doi: 10.3389/fonc.2019.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burudpakdee C, Wong W, Seetasith A, Corvino F, Yeh W, Gubens M. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer. 2018;119:103–111. doi: 10.1016/j.lungcan.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, Shaw AT, Huber RM, Hochmair MJ, Lee DH, Bazhenova LA, Gold KA, Ou SI, West HL, Reichmann W, Haney J, Clackson T, Kerstein D, Gettinger SN. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J. Clin. Oncol. 2018;36:2693–2701. doi: 10.1200/JCO.2017.77.5841. [DOI] [PubMed] [Google Scholar]
- 16.Dietel M, Bubendorf L, Dingemans AM, Dooms C, Elmberger G, García RC, Kerr KM, Lim E, López-Ríos F, Thunnissen E, Van Schil PE, von Laffert M. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax. 2016;71:177–184. doi: 10.1136/thoraxjnl-2014-206677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball D, Rami-Porta R International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 18.Käsmann L, Taugner J, Eze C, Roengvoraphoj O, Dantes M, Gennen K, Karin M, Tufman A, Belka C, Manapov F. The role of patient performance status and its changes before and after completion of multimodal treatment for inoperable stage III NSCLC. Ann Oncol. 2019;30(Suppl 2):36. [Google Scholar]
- 19.Nishino M. Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. Am Soc Clin Oncol Educ Book. 2018;38:1019–1029. doi: 10.1200/EDBK_201441. [DOI] [PubMed] [Google Scholar]
- 20.Khalid MA, Achakzai IK, Khan SA, Majid Z, Hanif FM, Iqbal J, Laeeq SM, Luck NH. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11:301–305. [PMC free article] [PubMed] [Google Scholar]
- 21.Hornbeck P. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0201s01. Chapter 2: Unit 2.1. [DOI] [PubMed] [Google Scholar]
- 22.Katayama R, Kobayashi Y, Friboulet L, Lockerman EL, Koike S, Shaw AT, Engelman JA, Fujita N. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funazo TY, Ozasa H, Tsuji T, Furugaki K, Yoshimura Y, Ajimizu H, Yasuda Y, Nomizo T, Sakamori Y, Yoshida H. ABCC11 is involved in resistance to alectinib. Cancer Research. 2019;79 [Google Scholar]
- 24.Ou SHI, Ahn JS, De Petris L, Govindan R, Yang JCH, Hughes BGM, Lena H, Moro-Sibilot D, Bearz A, Ramirez SV. Efficacy and safety of the ALK inhibitor alectinib in ALK+ non-small-cell lung cancer (NSCLC) patients who have failed prior crizotinib: an open-label, single-arm, global phase 2 study (NP28673) ResearchGate. 2015 [Google Scholar]
- 25.Gandhi L, Ou SI, Shaw AT, Barlesi F, Dingemans AC, Kim DW, Camidge DR, Hughes BG, Yang JC, de Castro J, Crino L, Léna H, Do P, Golding S, Bordogna W, Zeaiter A, Kotb A, Gadgeel S. Efficacy of alectinib in central nervous system metastases in crizotinib-resistant ALK-positive non-small-cell lung cancer: comparison of RECIST 1.1 and RANO-HGG criteria. Eur J Cancer. 2017;82:27–33. doi: 10.1016/j.ejca.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Chelala E, Hoyek S, Arej N, Kattan J, Kourie HR, Baakliny J, Antoun J. Ocular and orbital side effects of ALK inhibitors: a review article. Future Oncol. 2019;15:1939–1945. doi: 10.2217/fon-2018-0608. [DOI] [PubMed] [Google Scholar]
- 27.Ohe Y, Yamamoto N, Gemma A, Kusumoto M, Yamada I, Ishii T, Masuda N. 1393P safety profile and effectiveness of alectinib in the real-world surveillance study of 1251 Japanese patients with ALK-positive non-small cell lung cancer. Ann Oncol. 2018;29:mdy292.016. doi: 10.1111/cas.13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ou SHI, Perol M, Dziadziuszko R, Kim DW, Rosell R. Alectinib versus crizotinib in treatment-naive advanced ALK-positive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. ResearchGate. 2017 [Google Scholar]
- 29.Hanagiri T, Sugaya M, Takenaka M, Oka S, Baba T, Shigematsu Y, Nagata Y, Shimokawa H, Uramoto H, Takenoyama M, Yasumoto K, Tanaka F. Preoperative CYFRA 21-1 and CEA as prognostic factors in patients with stage I non-small cell lung cancer. Lung Cancer. 2011;74:112–117. doi: 10.1016/j.lungcan.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Qiu Y, Yang H, Chen H, Ge L, Xu X, Xiong X, He J. Detection of CEA mRNA, p53 and AE1/AE3 in haematoxylin-eosin-negative lymph nodes of early-stage non-small cell lung cancer may improve veracity of N staging and indicate prognosis. Jpn J Clin Oncol. 2009;40:146–152. doi: 10.1093/jjco/hyp144. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Mingdong L, Shan L. Expression of serum sB7-H4 in patients with non small cell lung cancer and its relationship with clinical pathological characteristics. Chinese Journal of Primary Medicine and Pharmacy. 2017;24:1965–1969. [Google Scholar]


