Abstract
Low back pain (LBP) is a common aging-associated disease that can cause disability in old people. Previous research revealed that an imbalance in the extracellular matrix (ECM) via abnormal hypoxia-inducible factor-1alpha (HIF-1α) expression in nucleus pulposus (NP) cells was one of the key factors in the pathogenesis of intervertebral disc degeneration (IDD). However, the mechanism by which the ECM is reduced in patients with IDD is not fully understood. Here, we reported that a new member of the interleukin (IL)-1 family, IL-33, was positively correlated with HIF-1α and was decreased in the NP cells of individuals with IDD. IL-33 overexpression in degenerative NP cells decreased the levels of matrix metalloproteinase-3/13 (MMP-3/13), a disintegrin and metallo-proteinase with thrombospondin motifs-4/5 (ADAMTS-4/5), and promoted ECM expression in vitro. Furthermore, we preliminarily explored the antiapoptotic effects of IL-33, which could reduce the expression of Caspase-3 and promote the level of Bcl-2 in degenerative NP cells. Furthermore, when HIF-1α expression was silenced, IL-33-mediated upregulation of ECM expression was weakened. Thus, IL-33-induced HIF-1α upregulation may represent a novel therapeutic strategy to ameliorate IDD in patients with LBP.
Keywords: Low back pain, nucleus pulposus cells, IL-33, HIF-1α, ECM
Introduction
Low back pain (LBP) has become the leading cause of disability in the world, and the incidence rate in the past 20 years has continued to increase [1]. In the United States, nearly 40% of adults have reported LBP, and 20-33% of patients are unable to work. Thus, this disease has major socioeconomic impacts [2]. The management of LBP is hampered because the pathogenesis is unclear. Recent studies have shown that intervertebral disc degeneration (IDD) is one of the main causes of LBP [3].
Intervertebral disc (IVD) tissue is a soft tissue between vertebral bodies that bears load and provides for the physiological activities of the spine. The nucleus pulposus (NP), cartilaginous end plate (EP) and annulus fibrosis (AF) together form the IVD [4,5]. NP cells exist in an extracellular matrix (ECM) composed of collagen II and proteoglycans. Studies have shown that increased NP cell apoptosis, the loss of NP cell function, and ECM degradation are the most fundamental pathological factors in IDD. ECM accounts for 99% of the volume of IVD tissue and plays a prominent role in the IVD [6,7]. There is no blood supply to the NP and the inner layer of the AF, which mainly obtain nutrition through diffusion in the EP and are in a state of hypoxia [8]. Changes in the hypoxic environment may affect the activation or suppression of a series of steady-state genes related to cell adaptation and survival in the hypoxic environment, which may be an important mechanism leading to disc degeneration [9]. Among these genes, hypoxia-inducible factor-1α (HIF-1α) is a crucial transcription factor that responds to oxygen concentrations. Many studies have shown that abnormally low expression of HIF-1α leads to reduced ECM production in IDD [10-12]. However, the mechanism of HIF-1α downregulation is unclear.
In disc tissue, as degeneration proceeds, increased levels of inflammatory cytokines promote the downregulation of ECM, change the phenotype of IVD cells, and lead to spinal instability and structural changes [13]. Cytokines such as interleukin (IL)-1α, IL-1β, necrosis factor alpha (TNF-α), IL-2, IL-4, IL-8, IL-6, and interferon gamma (IFN-γ) have been investigated in many studies [14-16]. Among these, IL-1β has probably been studied the most. IL-1β and IL-1 receptors (IL-1R) have been confirmed in IDD pathology. IL-1β promotes an increase in genes encoding ECM-degrading enzymes, leading to the exacerbation of IDD [14]. IL-33, a novel member of the IL-1 family, was first identified as nuclear factor in high endothelial vein (NF-HEV) in cells and is widely expressed in various tissues. IL-33 is an alarm signal (alarmin) that is released when a cell or tissue is damaged, and it sends an alarm to immune cells expressing the suppression of tumorigenicity 2 (ST2) receptor [17,18]. At present, few reports have demonstrated the role of IL-33 in IDD. In our study, we first found reduced IL-33 expression in patients with IDD. However, the biological functions and precise mechanism of IL-33 in IDD need to be further examined.
In this study, we determined that IL-33 is a key nuclear cytokine that induces ECM production by upregulating the expression of HIF-1α. In addition, we found that the level of IL-33 in the NP tissue of IDD patients was abnormally decreased. Therefore, we hypothesized that IL-33 was a reason for the decreased HIF-1α expression in degenerative NP cells, resulting in LBP.
Materials and methods
Human tissue collection and grading
The management of the Changzheng Hospital affiliated with the Second Military Medical University provided the ethical approval of human IVD tissue collection, and the approval number is 13071002114. Human lumbar IVD tissue was collected during IDD surgery. Control samples were obtained from patients who suffered from trauma. All tissues were collected after obtaining patient consent. All patients underwent magnetic resonance imaging (MRI), and the Pfirrmann score was used to score patients as I or II, III or IV and V for the normal group, mild degeneration group and severe degeneration group, respectively. Patient details are shown in Table 1.
Table 1.
Demographic data of human tissue donors in experimental studies
| Subject No. | Gender | Age (years) | Thompson Grade |
|---|---|---|---|
| 1 | M | 31 | I |
| 2 | M | 65 | III |
| 3 | F | 29 | II |
| 4 | M | 33 | II |
| 5 | F | 56 | IV |
| 6 | F | 36 | III |
| 7 | M | 41 | IV |
| 8 | M | 53 | IV |
| 9 | F | 66 | V |
| 10 | M | 32 | I |
| 11 | F | 28 | II |
| 12 | F | 67 | V |
| 13 | F | 35 | III |
| 14 | M | 29 | I |
| 15 | M | 30 | II |
| 16 | M | 42 | I |
| 17 | F | 55 | III |
| 18 | F | 58 | IV |
| 19 | F | 60 | V |
| 20 | F | 33 | II |
| 21 | M | 64 | V |
| 22 | M | 59 | IV |
| 23 | M | 31 | II |
| 24 | F | 36 | VI |
Isolation of human nucleus pulposus cells
The obtained human NP tissue was rinsed 3 times with D-HANKS solution. Scissors were used to cut the NP tissue (approximately 1 mm3 in size), which was placed in a sterile centrifuge tube and digested with 0.25% trypsin at 37°C for 20 min. Then, the tissues were centrifuged at 800 R/min for 5 min, aspirated, digested with 0.2% type II collagenase at 37°C for 4 h, and filtered with 200 mesh screens. The filtrate was centrifuged at 800 r/min at a low speed for 5 min, the supernatant was discarded, the tissue was washed with DMEM/F12 medium and centrifuged at 800 R/min at low speed for 5 min, and the process was repeated 3 times. The cells were inoculated into culture flasks and cultured with DMEM/F12 medium containing 15% fetal bovine serum and 1% penicillin/streptomycin. The cells were cultured in an incubator containing 5% CO2 at 37°C. The culture medium was changed twice a week for follow-up research.
Real-time PCR (qRT-PCR)
Total RNA was extracted from cells and tissue samples with TRIzol reagent (Invitrogen). According to the manufacturer’s instructions, PCR analysis was performed using gene-specific primers (Table 2) and purified total RNA (1 μg) was reverse-transcribed into cDNA with a PrimeScript RT reagent kit (Takara). qRT-PCR was performed using a SYBR green PCR kit and a MyiQ Single Color Real-time PCR Detection System (Bio-Rad Laboratories). The reaction was repeated using RNA samples from three independent experiments. Then, 18S rRNA was used as the internal control, and the 2-ΔΔCT method was used to calculate the fold change in the expression of each gene.
Table 2.
Primer sequences used for RT-PCR
| Gene | Primer name | Sequence |
|---|---|---|
| GAPDH | GAPDH-F | ACAACTTTGGTATCGTGGAAGG |
| GAPDH-R | GCCATCACGCCACAGTTTC | |
| MMP-3 | MMP-3-F | ACATGGAGACTTTGTCCCTTTTG |
| MMP-3-R | TTGGCTGAGTGGTAGAGTCCC | |
| MMP-13 | MMP-13-F | TTGAGCTGGACTCATTGTCG |
| MMP-13-R | CGCGAGATTTGTAGGATGGT | |
| ADAMTS-4 | ADAMTS-4-F | ACACTGAGGACTGCCCAACT |
| ADAMTS-4-R | GTGTAGCGAGGAACCCAGTC | |
| ADAMTS-5 | ADAMTS-5-F | CAAGGACAAGAGCCTGGAAG |
| ADAMTS-5-R | CTGCATCGTAGTGCTCCTCA | |
| Caspase-3 | Caspase-3-F | TGTTTGTGTGCTTCTGAGCC |
| Caspase-3-R | CACGCCATGTCATCATCAAC | |
| Bcl-2 | Bcl-2-F | ATGTGTGTGGAGACCGTCAA |
| Bcl-2-R | GCCGTACAGTTCCACAAAGG |
All sequences are given in 5’-3’ direction.
Immunohistochemistry
Human NP tissue sections (5 μm) were deparaffinized in ethanol and xylene. The sections were incubated with 3% H2O2 for 10 min to remove endogenous peroxidase. The sections were blocked with 1.5% normal goat or rabbit blocking serum at room temperature for 45 min and incubated with anti-il-33 (1:200; Sigma-Aldrich) and anti-HIF-1α (1:200; Cell Signaling) primary antibodies at 4°C overnight. One day later, goat anti-rabbit immunoglobulin G (1:3000, ab205718 Abcam) secondary antibodies were added and incubated for 30 min. Diaminobenzidine was added, and the sample was developed for 5 min, counterstained with hematoxylin, differentiated with 1% hydrochloric acid alcohol, returned to blue, and mounted. Finally, pictures of the slices were taken with an optical microscope. Cells with a pale brown cytoplasm or membrane were noted as positive cells.
Western blotting
Proteins were prepared as previously reported [19], and the protein content was measured using a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts (20 μg) of protein were separated by SDS-PAGE, and immunoblotting was performed with primary antibodies against IL-33 (Sigma-Aldrich, 1:1000), HIF-1α (Cell Signaling, 1:1000), Collagen II (Affinity, 1:1000), Aggrecan (Affinity, 1:1000), or actin (Servicebio, 1:1000). Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, Myc-HRP (Thermo, 1:5000) and Flag-HRP (Sigma, 1:5000). Bands were detected using an enhanced chemiluminescence system (EMD Millipore, Billerica, MA, USA), and Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used for quantification.
Luciferase assays
Lipofectamine 2000 reagent (Invitrogen) was used to transfect NP cells in a 12-well plate. The cells were collected and analyzed for luciferase activity using a dual luciferase detection system (Promega) after 48 h. Then, the luciferase activity was measured using a luciferase detection kit (RG005, Beyotime Institute of Biotechnology, Shanghai, China).
Statistical analysis
The data were analyzed using SPSS 10 statistical software and are expressed as the mean ± standard deviation. Comparisons between groups were performed by Student’s t test or one-way ANOVA. A value of P<0.05 indicates that the difference is statistically significant.
Results
IL-33 promotes the expression of Collagen II and Aggrecan in NP cells
To investigate whether IL-33 regulates ECM expression, an IL-33-overexpressing adenovirus was used in NP cells (Figure 1A). At 48 h after adenovirus administration, the mRNA levels of Collagen II and Aggrecan were higher than those observed in cells treated with Ad-LacZ alone (Figure 1B and 1C). Moreover, IL-33 overexpression significantly increased Collagen II and Aggrecan protein expression (Figure 1D). In addition, when the expression of the IL-33 receptor ST2 was silenced by siST2 (Figure 1E), IL-33-induced ECM production was not affected (Figure 1F). These results illustrated that IL-33 promoted ECM expression independent of ST2.
Figure 1.

IL-33 induced the expression of COLL II and aggrecan in vitro. A. NP cells were infected with Ad-Flag-IL-33 or Ad-LacZ. IL-33 mRNA levels were measured by qRT-PCR. B and C. NP cells were infected with Ad-Flag-IL-33 or Ad-LacZ for 48 h. COLL II and Aggrecan mRNA levels were measured by qRT-PCR. D. NP cells were infected with Ad-Flag-IL-33 or Ad-LacZ for 48 h. COLL II and Aggrecan protein levels were measured by Western blotting. E. NP cells were transfected with siST2 for 48 h. ST2 mRNA levels were measured by qRT-PCR. F. NP cells were transfected with siST2 (100 nM) or siCTL (100 nM) for 24 h, and then the cells were infected with Ad-Flag-IL-33 or Ad-LacZ for an additional 48 h. CoLL II and Aggrecan protein levels were measured by Western blotting. **P<0.01, ***P<0.001, ****P<0.0001.
IL-33 regulates matrix-degrading enzymes and apoptosis-related gene expression in NP cells
To investigate the inhibitory effect of IL-33 on matrix metabolism and apoptosis in disc degeneration, IL-33-overexpressing adenovirus was administered to degenerative NP cells. At 48 h after adenovirus administration, the mRNA levels of MMP-3/13 and ADAMTS-4/5 were higher than those observed in the control group (Figure 2A-D). Moreover, IL-33 overexpression significantly decreased Caspase-3 expression and increased the expression of bcl-2 in degenerative NP cells (Figure 2E and 2F). These results illustrated that IL-33 has antimetastatic and antiapoptotic effects on disc degeneration.
Figure 2.
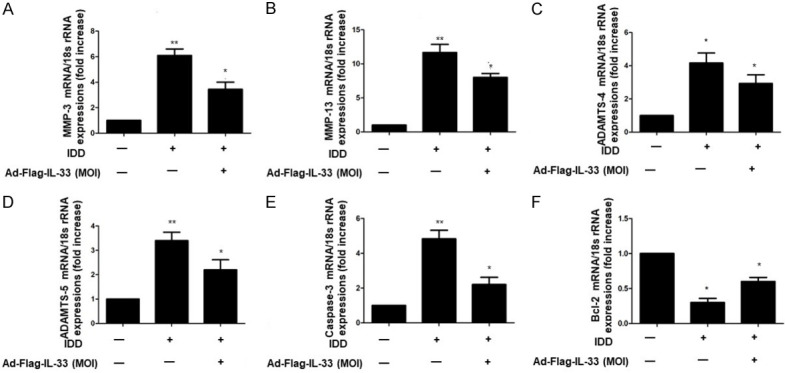
IL-33 regulated matrix-degrading enzymes and apoptosis-related gene expression in NP cells. A-D. Ad-Flag-IL-33 (50 MOI) overexpression inhibits the expression of matrix-degrading enzymes in IVD degenerative (IDD) NP cells. MMP-3 MMP-13 and ADAMTS-4/5 mRNA levels were analyzed by qRT-PCR. E and F. Ad-Flag-IL-33 (50 MOI) overexpression inhibited Caspase-3 expression in intervertebral disc degeneration (IDD) NP cells and stimulated Bcl-2 expression in IDD NP cells. Caspase-3 and Bcl-2 mRNA levels were measured by qRT-PCR. *P<0.05, **P<0.01.
IL-33 promotes the expression of Collagen II and Aggrecan via HIF-1α
To confirm the role of IL-33 in ECM expression, we found that IL-33 significantly upregulated the mRNA and protein expression of HIF-1α, which had previously been shown to promote ECM expression (Figure 3A and 3B). To provide further evidence of the involvement of IL-33 in HIF-1α-mediated ECM production, we showed that IL-33 could promote the transcriptional activity of HIF-1α (Figure 3C). By using RNA interference in loss-of-function experiments, we demonstrated that siHIF-1α could decrease the level of HIF-1α mRNA in NP cells (Figure 3D), and compared with the IL-33-enhanced group, the downregulation of HIF-1α decreased collagen II and aggrecan mRNA and protein levels (Figure 3E and 3F).
Figure 3.

IL-33 enhanced COLL II and aggrecan expression via HIF-1α. A. NP cells were infected with Ad-Flag-IL-33 or Ad-LacZ for 48 h. qRT-PCR was used to measure HIF-1α mRNA levels. B. NP cells were infected with Ad-Flag-IL-33 or Ad-LacZ for 48 h. Western blotting was used to measure HIF-1α protein levels. C. Analysis of IL-33-mediated modulation of HRE-Luc luciferase reporter activity. D. NP cells were transfected with siIL-33 or siCTL for 48 h. HIF-1α mRNA levels were measured by qRT-PCR. E. NP cells were transfected with siHIF-1α or a siCTL control, and HIF-1α mRNA levels were measured by qRT-PCR. F. NP cells were transfected with siHIF-1α (100 nM) or siCTL (100 nM) for 24 h, and then the cells were infected with Ad-Flag-IL-33 or Ad-LacZ (MOI=50) for an additional 48 h. COLL II and aggrecan protein levels were analyzed by Western blotting. *P<0.05, **P<0.01, ***P<0.001.
IL-33 inhibition compromises ECM expression
NP cells were transfected with synthetic IL-33 antisense oligonucleotides to confirm its regulatory effect on ECM expression. Figure 4A shows that siIL-33 specifically suppressed endogenous IL-33 expression by 50%. The Collagen II and Aggrecan mRNA levels in siIL-33-treated NP cells were reduced (Figure 4B and 4C). Moreover, IL-33 inhibition in NP cells decreased the protein levels of HIF-1α, Collagen II and Aggrecan (Figure 4D). However, HIF-1α supplementation in NP cells compensated for the decrease in ECM induced by the loss of IL-33 (Figure 4E).
Figure 4.

IL-33 silencing attenuated COLL II and aggrecan expression in vitro. A. NP cells were transfected with siIL-33 or a siCTL control, and IL-33 mRNA levels were measured by qRT-PCR. B and C. NP cells were transfected with siIL-33 or a siCTL control. COLL II and Aggrecan mRNA levels were measured by qRT-PCR. D. NP cells (from controls, n=5) were transfected with siIL-33 or a siCTL control (50 or 100 nM). Western blotting was used to measure COLL II and aggrecan protein levels. E. NP cells were transfected with siIL-33 (100 nM) or siCTL (100 nM) for 24 h, and then the cells were infected with Ad-Myc-HIF-1α or Ad-LacZ (MOI=50) for an additional 48 h. COLL II and aggrecan protein levels were analyzed. **P<0.01, ***P<0.001.
Aberrant expression of IL-33 and HIF-1α in the NP tissues of IDD patients
Since the decrease in HIF-1α expression in human or murine NP cells causes the downregulation of ECM expression [20], we further assessed the expression of HIF-1α and IL-33 in NP samples from IDD patients. IL-33 transcript and protein levels decreased gradually as the degeneration level increased (Figure 5A and 5B). The mRNA and protein levels of HIF-1α were, as expected, seriously decreased by 45% in NP tissues with IDD (Figure 5C). Moreover, as shown in Figure 5D, the transcription levels of both genes were positively correlated (r=0.534, P=0.05). In addition, immunolocalization analysis showed higher protein levels of IL-33 and HIF-1α in NP cells from normal individuals than those from IDD patients (Figure 5E). These results suggested that the downregulation of IL-33 decreased HIF-1α expression in IDD patients, resulting in LBP.
Figure 5.
Abnormal expression of IL-33 and HIF-1α in IDD patients. A. IL-33 and HIF-1α protein expression in normal (n=24) and IDD patients (grade II III IV V, each grade included 6 patients) (n=24) was examined by Western blotting. B. IL-33 expression was quantified in 48 samples, and the intensities were normalized to GAPDH. C. HIF-1α expression was quantified in 48 samples, and the intensities were normalized to GAPDH. D. Correlation between IL-33 and HIF-1α protein expression (r=0.534, P=0.015). E. Immunohistochemical analysis of NP tissues from control and IDD patients. The arrows indicate decreased IL-33 and HIF-1α conjugates in NP cells. **P<0.01, ***P<0.001, ****P<0.0001. Scale bar, 100 µm, represents 40 times magnification; Scale bar, 40 µm, represents 100 times magnification.
Discussion
IDD is associated with patients who suffer from LBP. However, the exact pathogenesis of IDD is unclear. Inadequate IVD functions, including a reduction in NP cells, impaired ECM expression, elevated matrix enzymes and apoptosis, are known to be the main reasons for backache or limited labor associated with LBP [21,22]. In this study, we demonstrated that the downregulation of nuclear IL-33 impaired ECM production by decreasing HIF-1α expression in NP cells, leading to IDD.
In previous studies, cytokines produced by NP cells have been studied. IL-1 expression is abnormally increased in IDD tissues and has been shown to suppress the expression of important ECM genes [23,24]. In this study, we first confirmed that IL-33, a novel member of the IL-1 family, could induce ECM expression and prevent the IDD process by regulating matrix-degrading enzymes and apoptosis. IL-33 was first identified as NF-HEV in cells, and was later shown to be widely expressed in various tissues. Human IL-33 is a protein consisting of 270 amino acids and includes N-terminal and C-terminal domains. The N-terminal structure (AA 1-65) includes nuclear localization sequences and chromosome binding regions; the C-terminal domain (AA 112-270) is a cytokine domain like that of IL-1. Since IL-33 lacks a signal peptide, it is localized in the nucleus. However, when the body encounters trauma or infection, IL-33 is released from the cell as a danger signal and plays a role as a cytokine like IL-1 [17,25]. When IL-33 exerts cytokine activity, it forms a heterodimer by binding to its specific receptor ST2 to transmit signals downstream [26,27]. As an active cytokine, IL-33 abnormalities participate in the development of many diseases, including airway inflammation, arthritis, atherosclerosis, and others [28,29]. However, in the present study, we found that IL-33 in NP cells induced ECM expression independent of ST2. These results illustrated that IL-33 did not promote ECM formation through the same cytokine activity as IL-1. IL-33 is localized in the nucleus and plays a dominant role.
Unlike IL-33 outside the cells, IL-33 in the nucleus plays a role that does not rely on ST2. There are several physiological mechanisms associated with nuclear IL-33: it affects the function of chromosomes through protein-protein interactions [30]; it binds to the transcriptional repressor histone methyltransferase SUV39H1 as a transcription factor [31]; it inhibits the activity of the transcription factor NF-κB through physical interactions; and it promotes the signal transduction of proinflammatory factors. In our study, we demonstrated that IL-33 affected ECM expression by upregulating HIF-1α expression. Many studies have focused on examining the regulatory effects of cytokines on HIF-1α: NF-κB binds to sites in the HIF-1α promoter and regulates the transcriptional activity of HIF-1α [32]. Transforming growth factor beta1 (TGF-β1) induces the stabilization of HIF-1α through the phosphorylation of mothers against decapentaplegic homolog 3 (Smad3) in periodontal ligament stem cells (PDLSCs) [33], and IL-4 downregulates HIF-1α expression, leading to the proangiogenic effects of macrophages [34]. As a transcription factor, HIF-1α has been shown to participate in IDD development. When HIF-1α expression was knocked down, NP cells suffered from death, and ECM expression decreased, leading to IDD. Previously, we pointed out that several studies considered nuclear IL-33 to be a regulator of transcription factors. This prompted us to examine whether IL-33 regulated the transcriptional activity of HIF-1α. In the future, further research is needed to elucidate the mechanism by which IL-33 regulates HIF-1α.
Interestingly, there is feedback regulation between some cytokines and HIF-1α. IL-1β inhibits the differentiation of human FOXP3+ T cells by inducing HIF-1α [35]. On the other hand, the promotor region of IL-1β contains the transcriptional binding site of HIF-1α [36]. Therefore, HIF-1α regulates IL-1β expression and activity in many physiological contexts. Whether such feedback exists between IL-33 and HIF-1α in IDD is unknown. A previous study demonstrated that in vascular endothelial cells, IL-33 initiated vascular remodeling in hypoxic pulmonary hypertension by upregulating HIF-1α expression [37]. In inflammatory bowel disease, HIF-1α could induce IL-33 expression in intestinal epithelial cells, contributing to mucosal homeostasis [38]. Furthermore, some researchers have proposed that in rheumatoid arthritis, a regulatory circuit exists between HIF-1α and IL-33 [39]. These studies suggest that the regulatory relationship between HIF-1α and IL-33 in IDD is complicated and requires further research. In addition, we only studied the mechanism in vitro, which is a limitation. In future research, we should perform in vivo experiments to clarify the pathogenesis of IDD more clearly.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81901560).
Disclosure of conflict of interest
None.
References
- 1.Will JS, Bury DC, Miller JA. Mechanical low back pain. Am Fam Physician. 2018;98:421–428. [PubMed] [Google Scholar]
- 2.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 3.Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3:e1076. doi: 10.1002/jsp2.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kos N, Gradisnik L, Velnar T. A brief review of the degenerative intervertebral disc disease. Med Arch. 2019;73:421–424. doi: 10.5455/medarh.2019.73.421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54–67. doi: 10.1016/j.biomaterials.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zheng Z, Wang J, Tang C, Khor S, Chen J, Chen X, Zhang Z, Tang Q, Wang C, Lou Y, Wang Z, Xiao J, Wang X. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int J Biol Sci. 2018;14:682–692. doi: 10.7150/ijbs.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang W, Zhao P, Zhang X. Apelin promotes ECM synthesis by enhancing autophagy flux via TFEB in human degenerative np cells under oxidative stress. Biomed Res Int. 2020;2020:4897170. doi: 10.1155/2020/4897170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon WK, Moon HJ, Kwon TH, Park YK, Kim JH. Influence of rabbit notochordal cells on symptomatic intervertebral disc degeneration: anti-angiogenic capacity on human endothelial cell proliferation under hypoxia. Osteoarthritis Cartilage. 2017;25:1738–1746. doi: 10.1016/j.joca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Choi H, Merceron C, Mangiavini L, Seifert EL, Schipani E, Shapiro IM, Risbud MV. Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling. Autophagy. 2016;12:1631–1646. doi: 10.1080/15548627.2016.1192753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Zhao Q, Li Y, Zhang J. Moxibustion alleviates intervertebral disc degeneration via activation of the HIF-1α/VEGF pathway in a rat model. Am J Transl Res. 2019;11:6221–6231. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu WJ, Zhang XK, Zheng XF, Yang YH, Jiang SD, Jiang LS. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int J Immunopathol Pharmacol. 2013;26:601–609. doi: 10.1177/039463201302600304. [DOI] [PubMed] [Google Scholar]
- 12.Madhu V, Boneski PK, Silagi E, Qiu Y, Kurland I, Guntur AR, Shapiro IM, Risbud MV. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF-1α-BNIP3 axis. J Bone Miner Res. 2020;35:1504–1524. doi: 10.1002/jbmr.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun ZY, Liu YT, Liang H, Li Y, Wang DG, Tian JW. Interleukin-1beta exacerbates the catabolic effects of human nucleus pulposus cells through activation of the nuclear factor kappa B signaling pathway under hypoxic conditions. Eur Rev Med Pharmacol Sci. 2018;22:7129–7139. doi: 10.26355/eurrev_201811_16244. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Yu XH, Wang C, He WS, Zhang SJ, Yan YG, Zhang J, Xiang YX, Wang WJ. Interleukin-1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–272. doi: 10.1016/j.cca.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Li Z, Chen F, Liu H, Wang H, Li X, Liu X, Wang J, Zheng Z. TGF-beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp Mol Med. 2017;49:e379. doi: 10.1038/emm.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281:154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 18.Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Yan Q, Shi C, Tian Y, Cao P, Yuan W. BMSC paracrine activity attenuates interleukin-1β-induced inflammation and apoptosis in rat AF cells via inhibiting relative NF-κB signaling and the mitochondrial pathway. Am J Transl Res. 2017;9:79–89. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Li C, Meng X, Bai Y, Qi J, Wang J, Zhou Q, Zhang W, Zhang X. Hypoxia-inducible factor-lα mediates aggrecan and collagen Π expression via NOTCH1 signaling in nucleus pulposus cells during intervertebral disc degeneration. Biochem Biophys Res Commun. 2017;488:554–561. doi: 10.1016/j.bbrc.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 21.Phillips KL, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK, Le Maitre CL. The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc. Arthritis Res Ther. 2013;15:R213. doi: 10.1186/ar4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J, Li B, Zhang P, Wang L, Lu H, Song X. Osteogenic protein-1 attenuates the inflammatory cytokine-induced NP cell senescence through regulating the ROS/NF-κB pathway. Biomed Pharmacother. 2018;99:431–437. doi: 10.1016/j.biopha.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wang X, Liu H, Li Z, Chen F, Wang H, Zheng Z, Wang J. TNF-alpha enhances apoptosis by promoting chop expression in nucleus pulposus cells: role of the MAPK and NF-kappaB pathways. J Orthop Res. 2018;37:697–705. doi: 10.1002/jor.24204. [DOI] [PubMed] [Google Scholar]
- 24.Gorth DJ, Shapiro IM, Risbud MV. A new understanding of the role of IL-1 in age-related intervertebral disc degeneration in a murine model. J Bone Miner Res. 2019;34:1531–1542. doi: 10.1002/jbmr.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefrançais E, Cayrol C. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur Cytokine Netw. 2012;23:120–127. doi: 10.1684/ecn.2012.0320. [DOI] [PubMed] [Google Scholar]
- 26.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 Axis in Organ Fibrosis. Front Immunol. 2018;9:2432. doi: 10.3389/fimmu.2018.02432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt SE. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016;113:E7572–E7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Grove KC, Provoost S, Braun H, Blomme EE, Teufelberger AR, Krysko O, Beyaert R, Brusselle GG, Joos GF, Maes T. IL-33 signalling contributes to pollutant-induced allergic airway inflammation. Clin Exp Allergy. 2018;48:1665–1675. doi: 10.1111/cea.13261. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Chen B, Wen Z, Huang Z, Ye L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget. 2017;8:32407–32418. doi: 10.18632/oncotarget.16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao D, Perros F, Caramori G, Meng C, Dormuller P, Chou PC, Church C, Papi A, Casolari P, Welsh D, Peacock A, Humbert M, Adcock IM, Wort SJ. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem Biophys Res Commun. 2014;451:8–14. doi: 10.1016/j.bbrc.2014.06.111. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Zhu Y, Wang X, Gong J, Hu C, Guo B, Zhu B, Li Y. Temporal regulation of HIF-1 and NF-κB in hypoxic hepatocarcinoma cells. Oncotarget. 2015;6:9409–9419. doi: 10.18632/oncotarget.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Guo L, Li R, Xu Q, Yang J, Chen J, Deng M. Transforming growth factor-β1 and hypoxia inducible factor-1α synergistically inhibit the osteogenesis of periodontal ligament stem cells. Int Immunopharmacol. 2019;75:105834. doi: 10.1016/j.intimp.2019.105834. [DOI] [PubMed] [Google Scholar]
- 34.Dehne N, Tausendschön M, Essler S, Geis T, Schmid T, Brüne B. IL-4 reduces the proangiogenic capacity of macrophages by down-regulating HIF-1α translation. J Leukoc Biol. 2014;95:129–137. doi: 10.1189/jlb.0113045. [DOI] [PubMed] [Google Scholar]
- 35.Feldhoff LM, Rueda CM, Moreno-Fernandez ME, Sauer J, Jackson CM, Chougnet CA, Rupp J. IL-1β induced HIF-1α inhibits the differentiation of human FOXP3(+) T cells. Sci Rep. 2017;7:465. doi: 10.1038/s41598-017-00508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talreja J, Talwar H, Bauerfeld C, Grossman LI, Zhang K, Tranchida P, Samavati L. HIF-1α regulates IL-1β and IL-17 in sarcoidosis. Elife. 2019;8:e44519. doi: 10.7554/eLife.44519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Wang W, Wang L, Chen S, Tian B, Huang K, Corrigan CJ, Ying S, Wang W, Wang C. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1α and VEGF expression in vascular endothelial cells. EBioMedicine. 2018;33:196–210. doi: 10.1016/j.ebiom.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, He C, Wu W, Zhou G, Liu F, Cong Y, Liu Z. Hypoxia inducible factor-1α-induced interleukin-33 expression in intestinal epithelia contributes to mucosal homeostasis in inflammatory bowel disease. Clin Exp Immunol. 2017;187:428–440. doi: 10.1111/cei.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu F, Shi L, Mu R, Zhu J, Li Y, Ma X, Li C, Jia R, Yang D, Li Y, Li Z. Hypoxia-inducible factor-1α and interleukin 33 form a regulatory circuit to perpetuate the inflammation in rheumatoid arthritis. PLoS One. 2013;8:e72650. doi: 10.1371/journal.pone.0072650. [DOI] [PMC free article] [PubMed] [Google Scholar]



