Abstract
Recent explorations on mesenchymal stem/stromal cells (MSC) have reported a promising future for cell-based therapies. MSCs are widely sourced from various tissues and express unique properties of regenerative potential and immunomodulation. Currently, there is a growing interest in utilizing MSC for treatment of chronic diseases to overcome the drawbacks of chemical drugs. Metabolic Syndrome (MetS) is described as a cluster of metabolic abnormalities categorized as abdominal obesity, dyslipidaemia, hypertension, hypertriglyceridemia, and hyperglycaemia. Patients diagnosed with MetS have a high predisposition for developing cardiovascular complications, diabetes, non-alcoholic fatty liver diseases, bone loss, cancer, and mortality. Hence, research on MSC as therapy for MetS and related diseases, is greatly valued and are advantaged by the low immunogenicity with high regenerative capacity. However, there are many obstacles to be addressed such as the safety, efficacy, and consistency of different MSC sources. Additionally, factors such as effective dose level and delivery method are equally important to achieve uniform therapeutic outcomes. This systematic review discusses the potential roles of MSC in managing the multiple clusters of MetS. Research articles during the past 20 years were systematically searched and filtered to update the progress in the field of MSC therapy in managing various components of MetS. The different sources of MSC, dosage, method of delivery and outcome measures for the stem cell therapies were compiled from the systematically selected research articles. It can be concluded from the review of the selected articles that MSCs can improve the various disorders of MetS such as abdominal obesity, hyperglycaemia, hypertriglyceridemia and hypertension, and represent a promising alternative to conventional therapy of the MetS cluster.
Keywords: Stem cell, mesenchymal stem cell, metabolic syndrome, abdominal obesity, hyperglycaemia, hypertriglyceridemia, hypertension
Introduction
Metabolic syndrome, risk factors and current treatment
Diabetes and obesity have been regarded as worldwide epidemics in recent decades despite our medical advances. The exponential growth in reported cases was a medical phenomenon dubbed as Metabolic Syndrome (MetS). It was hypothesized that a quarter of adults in the world are diagnosed with MetS. These individuals have double the risk of mortality from cardiovascular complications like stroke. Similarly, they are also five times more likely to develop type 2 diabetes. A joint interim statement proposed MetS as a multiplex of conditions that include abdominal obesity, hypertension, hyperglycaemia or insulin resistance and dyslipidaemia (high serum triglyceride (TGL) and/or decreased low high-density lipoprotein (HDL) cholesterol levels) [1-4]. MetS is confirmed when three or more of these symptoms are present in an individual. The causes for MetS are abundant but each has varied impacts based on the demands and availability of these resources in our era. Higher socioeconomic status, sedentary lifestyle, poor diet choices (or high BMI) and poor lifestyle choices (such as smoking) have the greatest magnitude. Smaller contributions can also be observed from the genetic background, level of education and the heritability of traits prone to MetS development [7,8].
MetS is described as chronic damage from low-grade inflammation in the body. Physiological hallmarks of MetS are insulin resistance, abdominal adiposity, atherogenic dyslipidaemia, vascular dysfunction, elevated blood pressure, hypercoagulable state and chronic stress [9]. While insulin resistance has been commonly coined with MetS, the increased correlations of obesity, hyperglycemia and hypertension cannot be disregarded.
Obesity or enhanced adiposity contributes to the greater secretion of fat metabolites. These are made up of glycerol, free fatty acids (FFA) and pro-inflammatory cytokines (tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6)), plasminogen activator inhibitor-1 (PAI-1) and active C-reactive protein (CRP) [8,9]. The secreted FFAs were found to have disrupted the phosphoinositide 3-kinase (PI3K)-Akt pathway that oversees many intracellular regulatory functions such as apoptosis and proliferation of cells. Obesity-induced inflammation was found as a fundamental development of insulin resistance in type 2 diabetes (T2DM) [8]. Insulin resistance of T2DM was mainly represented by the highly active metabolic tissues such as muscles, fat and liver. The NOD-like receptor protein 3 (NLRP3) plays an active role in the inflammatory response and insulin-mediated functions. The inflammasome carries NLRP3 with apoptosis-associated speck-like protein (ASC) and caspase-1. NLRP3 functionally interacts with ASC to activate caspase-1. The action of caspase-1 is to promote the secretion of proinflammatory cytokine (IL-1β and IL-18) and cellular maturation [10]. The loss of insulin-mediated regulation not only triggers localized macrophage activity but also supports unregulated lipogenesis and release of reactive oxygen species (ROS).
In contrast, hyperglycaemia occurs when excess concentrations of glucose are present in the circulatory system. The damaged pancreatic β-islet cells no longer secrete insulin and/or cells begin to develop insulin resistance [7]. In similar machination, the PI3K-Akt pathway is equally responsible for regulating the cellular activity of β-islet cells and is affected by the excess glucose. In parallel with obesity-induced inflammation, this also led to endothelial dysfunction by reduced nitric oxide (NO) production and the inactivation of glucose transporter type 4 (GLUT4). Loss of GLUC4 function prevents glucose translocation and greatly affects glucose-dependent survival of many tissues especially skeletal muscles. Unlike the PI3K-Akt pathway, the MAP kinase pathway is unaffected and continuously produces endothelin-1 (ET-1), which expresses the vascular cell adhesion molecules and mitogenic stimulation of vascular smooth muscle cells.
Consequently, these physiological changes contribute to essential hypertension which is a common symptom amongst obese and glucose-intolerant patients [9]. Homeostasis of blood is mainly supervised by the kidney through filtering of excess metabolites and regulation of blood pressure. However, the angiotensin released by adipocytes and other hormonal mediators of hypertension triggered by inflammation can disrupt normal renal functions. The steep gradient of electrolytes, excess glucose, macrophagic activity and vascular adhesion creates an opportunistic environment for hypertension.
The currently available clinical treatment for diabetes mainly involves oral antidiabetic drugs and regular insulin shots. These solutions have the potential to alleviate hyperglycaemia or temporarily fix insulin sensitivity in a specific tissue. However, long term recovery of insulin resistance and/or β-islet cell functions remains desirable [11]. While organ or tissue transplantation of the pancreas was considered promising, the procedures demand extensive donor screening, potential transplant rejection and the risk of undergoing invasive procedures [12]. In hypertension treatment, the currently used conventional drugs such as vasodilators, prostacyclin and anticoagulants have been reported unsuccessful in maintaining pulmonary artery blood pressure within the normal range [13].
Stem cells or mesenchymal stem cells
Recent evidence suggests that mesenchymal stem/stromal cells (MSC) as cell therapy could serve as solutions for the limitations of current therapeutics. This interest is mainly due to their secretion of bioactive metabolites involved in immunomodulation, chemotaxis, apoptotic and anti-fibrotic functions [14]. Hence, these properties render the MSC a potential candidate for treating degenerative and immunological diseases. The immunomodulatory properties and low immunogenic characteristics of MSC have also led to MSC becoming a promising solution for graft-versus-host diseases (GVHD) and autoimmune diseases [14].
The regenerative potential of MSC in repair and renewal has been investigated extensively. MSCs are multipotent progenitor cells able to differentiate into different cell types such as osteocyte, chondrocyte and adipocytes [15,16]. MSCs also exert power immunomodulatory effects that can inhibit T and B cell proliferation and function. Additionally, they also inhibit priming of the dendritic cells which would oversee the recruitment and activation of other immune cells such as natural killer (NK) cells and macrophages [15]. MSCs are classified to their respective sources, from the bone marrow [17], adipose tissue [20], umbilical cord [21], dental pulp, endometrium, skeletal muscles, liver and pancreas. MSCs can also be programmed to secrete immunosuppressive factors and cytoprotective factors as exemplified by the MSC derived from the Wharton’s Jelly of the umbilical cord (WJ-MSC). This subset of MSC has been recently regarded as promising candidates for stem cell-based therapy in treating several diseases. Contrary to most sources, WJ-MSCs are readily available from redundant tissues and require minimal to none of the invasive procedures. Therefore, the safety of parent and child is not subjected to danger or risk. Compared to developed or adult tissue sources, WJ-MSCs are collected from nascent tissues which have boasted greater proliferative capacity with immunosuppressive factors, and are therapeutically active with minimal genomic alterations from ageing and diseases [21].
The potential of MSCs and its exuded factors have displayed great therapeutic outcomes in managing various diseases and are now considered for the treatment of MetS and associated diseases. Here, we have conducted a systematic review to identify and analyse research that has utilizing stem cells or MSCs for the treatment of MetS.
Materials and methods
Search strategy
We conducted a systematic review for this topic to identify studies on stem cells or MSCs as a potential therapy for alleviating MetS. To conduct the comprehensive search, the search terms and keywords were selected based on the through Medical Subject Headings (MeSH) by PubMed. The search strategy involved a combination of the following three sets of keywords: (1) Potential OR Effect AND (2) Stem cell OR Mesenchymal stem cell NOT Induce pluripotent stem cell AND (3) Abdominal obesity OR Hyperglycaemia OR Hypertriglyceridemia OR Hypertension.
Selection of database and search results
We used PubMed, MEDLINE via Ovid MEDLINE and Scopus databases provided by Universiti Kebangsaan Malaysia to search for research articles. The search was limited to studies that had been published as journal articles, free full text, published in 2000-2020 and written in English. We excluded review articles, books, news, editorials or case studies. The downloaded bibliographies were uploaded through literature software (Mendeley). Files were downloaded separately, compiled into an individual folder and combined for duplicates. Duplicate removal occurred automatically but was also performed manually for closely matched research articles. The first screening of the title and abstract was performed according to the inclusion and exclusion criteria.
Data extraction and management
Two reviewers extracted all data from the selected articles. The selected papers underwent a thorough screening process before inclusion. First, all duplicates were removed. Then, the titles were screened for relevance to the topic of interest. Subsequently, the abstracts were screened, and unrelated studies were excluded. The following data were extracted: (1) First author and year of publication; (2) Type of metabolic syndrome cluster; (3) Animal model and sample size; (4) Type of stem cell, dosage and delivery method; (5) Parameters observed; (6) Results; (7) Comments and outcomes.
Results
Search results
The two reviewers independently screened the articles according to the defined inclusion and exclusion criteria. To minimize bias while selecting articles, a joint discussion was conducted to achieve a consensus when differences emerged after the final screening. The primary searches using the keyword combinations (Section 2.1) identified only 28 articles: 11 from PubMed, 0 from Scopus and 17 from MEDLINE. After title screening, one article was rejected based on the inclusion criteria; it was not related to MetS. Finally, a total of 18 studies were selected for data extraction. Figure 1 shows the PRISMA flow diagram for the systematic review process.
Figure 1.
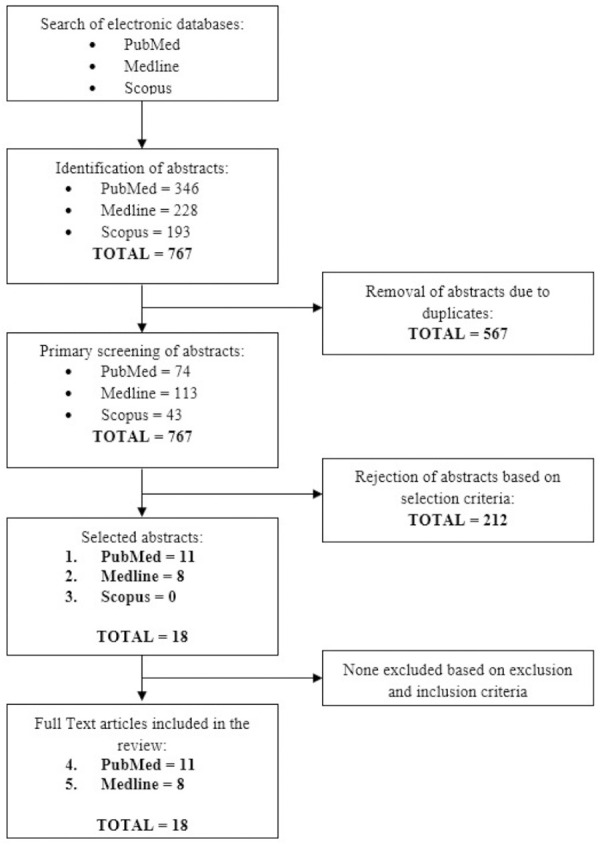
Flow chart of the article selection process from PubMed, Medline and Scopus and databases.
Study characteristics
The literature search returned 18 articles related to the potential of MSCs for treating MetS or diseases in this cluster. The studies used various sources of MSCs, such as tonsil-derived MSCs (TMSCs), human first-trimester umbilical cord perivascular layer cells (hFTM-PVs), amniotic fluid stem cells (AFSCs), umbilical cord-derived MSCs (UC-MSCs), cord blood mononuclear cells (CB-MNCs), bone marrow MSCs (BM-MSCs), human placenta-derived MSCs (hPD-MSC), adipose-derived stem cells (ASCs) and human menstrual blood progenitor cells (MBPCs). The articles mainly discussed hyperglycaemia in T1DM and T2DM, and only two papers [18,21] discussed pulmonary arterial hypertension (PAH). Most of the studies were conducted in in-vivo models: 11 in mice and 7 in rats. The animals were injected with MSCs either intravenously (tail vein or jugular vein) or intraperitoneally. One study reported the administration of MSCs through the spleen [16], left cardiac ventricle [17] and under the kidney capsule [10]. All of the studies concluded that the administration of MSCs potentially improves the condition of patients with diabetes and hypertension. Table 1 summarizes the included studies.
Table 1.
Characteristics, results, comments and outcomes of studies included in this review
| No. | First author & Year of publication | Type of Metabolic Syndrome Cluster | Animal model & sample size (per group) | Type of Stem Cell, Dosage & Delivery Method | Parameters Observed | Results | Comments and Outcomes |
|---|---|---|---|---|---|---|---|
| 1. | Lee Y. (2019) [1] | T2DM | BALB/c mice: | TMSCs | BW, IPGTT, Histology, Immunofluorescence, RT-PCR and ELISA (INS, CHOL, TGL). | After 10 weeks, treated mice reported, | TMCS successfully improved HFD-induced GLUC intolerance by enhancing INS secretion. |
| ▪ Male | ▪ 2×106 cells/mouse | ▪ BW (↓) | |||||
| ▪ 8 weeks old | ▪ Once every 2 weeks for 10 weeks | ▪ IPGTT (↑) | |||||
| ▪ n=10 | ▪ IP injection | ▪ INS (-) | |||||
| 2. | Cao M. (2015) [2] | T1DM | Nude mice: | Undifferentiated hFTM-PV or differentiated (EB-LCA and ILC) | BW, GLUC, INS and Mortality rate. | After 9 weeks, treated mice reported, | Transplantation of undifferentiated and differentiated (EB-LCA and ILCS) cells of hFTM-PV alleviated STZ-induced diabetes in mice. |
| ▪ Male | ▪ Dosage not reported | ▪ INS (↑) | |||||
| ▪ 4-6 weeks old | ▪ IP injection | ||||||
| ▪ n=8-16 | |||||||
| 3. | Villani V. (2014) [5] | T1DM | NOD/SCID mice: | AFSC | GLUC, INS, IHC, H&E, RT-PCR. | After 4 weeks, treated mice reported, | Transplantation of AFSC can treat insulin-dependent diabetes by protection and stimulation of endogenous β-cell regeneration. |
| ▪ Male | ▪ 1×106 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 4-6 weeks old | ▪ Intracardiac injection | ▪ INS (↑) | |||||
| ▪ n=5-13 | |||||||
| 4. | Xiao N. (2013) [6] | T1DM | C57/BL6 mice: | UC-MSC and CB-MNCs | GLUC, H&E, IHC, PCR (human Alu). | After 5 weeks, treated mice with ratio 1:4 reported, | Co-transplantation of UC-MSC and CB-MNCs successfully reversed hyperglycaemia and effectively recovered pancreatic function in diabetic mice. |
| ▪ Female | ▪ three groups (ratio of 1:1, 1:4 & 1:10) | ▪ GLUC (↓) | |||||
| ▪ 6-8 weeks old | ▪ 1×106 cells/mouse | ▪ Pancreatic islets (↑) | |||||
| ▪ n=8-10 | ▪ IV injection | ||||||
| 5. | Bhansali S. (2015) [9] | T1DM | Wistar rats: | BM-MSC | BW, GLUC, IPGTT, H&E and IHC. | After 6 weeks, BrdU-labelled BM-MSCs confirmed localization in pancreas and treated rats reported, | Transplantation of BM-MSC corrected hyperglycaemia and stimulated pancreatic β-cell neogenesis. |
| ▪ Male | ▪ 4.8×106 cells/rat | ▪ GLUC (↓) | |||||
| ▪ n=6 | ▪ IV injection | ▪ INS (↑) | |||||
| ▪ Pancreatic islets (↑) | |||||||
| 6. | Kadam S. (2010) [10] | T1DM | BALB/C mice: | PD-MSC | BW, GLUC, IPGTT, INS and H&E. | After 15 days, treated mice showed, | Transplantation of undifferentiated PD-MSCs reversed STZ-induced hyperglycaemia and increased secretion of insulin by islets. |
| ▪ Male | ▪ 1.5×105 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 6-8 weeks old | ▪ Intrapancreatic injection | ||||||
| ▪ n=5 | |||||||
| 7. | Zhou Y. (2015) [11] | T1DM | SD rat: | UC-MSC | BW, GLUC, INS, C-peptide, H&E, IHC, PCR, Western Blot. | After 42 days, hyperglycaemic progression halted by day 6 and treated rats reported, | Transplantation of UC-MSC exert trophic effects on β-cell survival by activating pancreatic P13K pathway and suspected to have been mediated by secreted IGF1. |
| ▪ Male | ▪ 3×106 cells/rat | ▪ GLUC (↓) | |||||
| ▪ 8 weeks old | ▪ IV injection | ▪ pAKT (↓) | |||||
| ▪ n=8 | ▪ Pancreatic islets (↑) | ||||||
| 8. | Maldonado M. (2017)[12] | T1DM | Kunming mice: | UCWJC | GLUC, C-peptide, IHC, Immunofluorescence, RT-PCR, Urine Assay. | After 11 weeks, UCWJC migrated to damaged organs and treated mice reported, | Transplantation of UCWJC normalized hyperglycaemia levels, promoted secretion of insulin from extrapancreatic cells and improved renal function. |
| ▪ Male | ▪ 1×107 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 10 weeks old | ▪ IP injection | ▪ C-peptide (↑) | |||||
| ▪ n=10 | |||||||
| 9. | Sun X. (2017) [13] | T2DM | SD rats: | UC-MSC | GLUC, INS, IPGTT, C-peptide, RT-PCR, Western Blot. | After 35 days, treated rats reported, | Transplantation of UC-MSC in diabetic rats inhibited NLRP3 inflammasome activation and decreased inflammatory cytokines, thus relieving insulin resistance of T2DM. |
| ▪ Male | ▪ 3×106 cells/rat | ▪ GLUC (↓) | |||||
| ▪ 8 weeks old | ▪ IV injection | ▪ INS (↓) | |||||
| ▪ n=8 | ▪ IPGTT (↑) | ||||||
| 10. | Murai N. (2017) [14] | T1DM | C57/BL6 mice: | BM-MSC | BW, GLUC, INS, Immunofluorescence, H&E, IHC. | After 28 days, intrapancreatic injection treated mice reported, | Transplantation of BM-MSC through intrapancreatic route reversed hyperglycaemia and restored BW. BM-MSC was absent in pancreas after 1 month, but effectively improved plasma INS levels and islet morphohistology. |
| ▪ Male | ▪ 1×106 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 7-9 weeks old | ▪ Intrapancreatic or IV injection | ▪ BW (↑) | |||||
| ▪ n=11-14 | Meanwhile, IV injection did not present any significant changes. | ||||||
| 11. | El-Hossary N. (2016) [15] | T1DM | Albino rats: | UC-MSC | BW, GLUC, H&E, Pancreatic Islet Measurement. | After 8 weeks, IV injection treated rats reported, | Transplantation of UC-MSC by IV route supported β-cell differentiation and proliferation in the pancreas, which alleviated hyperglycaemia and reduced further deterioration of the β-cell islets pathology. |
| ▪ Male | ▪ 2×106 cells/rat | ▪ GLUC (↓) | |||||
| ▪ 6-8 weeks old | ▪ IP or IV injection | ▪ BW (↑) | |||||
| ▪ n=5-8 | ▪ Pancreatic islets (↑) | ||||||
| These changes were not observed in the IP injected rats. | |||||||
| 12. | Yaochite JNU. (2016) [16] | T1DM | C57/BL6 mice: | T1DM derived BM-MSC and healthy BM-MSC | GLUC, INS, IPGTT, H&E, IHC, Cytokine level. | After 35 days, T1DM derived BM-MSC treated mice reported, | Transplantation of T1DM derived BM-MSC successfully reversed hyperglycaemia, improved β-cell mass, increased INS production and modulated pancreatic cytokine production. |
| ▪ Male | ▪ 1×106 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 10 weeks old | ▪ intrasplenic injection | However, both groups | |||||
| ▪ n=24 | ▪ INS (↑) | ||||||
| ▪ Pancreatic islets (↑) | |||||||
| 13. | Lee RH. (2006) [17] | T1DM | NOD/SCID mice: | BM-MSC | GLUC, INS, H&E, IHC, RT-PCR, Urine assay. | After 32 days, treated mice reported, | Transplantation of BM-MSC selectively home to β-cell islets and renal glomeruli of diabetic mice and had repairs tissues to restore physiological conditions and functions. |
| ▪ Male | ▪ 2.5×106 cells/mouse | ▪ GLUC (↓) | |||||
| ▪ 7-8 weeks old | ▪ intracardial injection | ▪ Urine volume (↓) | |||||
| ▪ n=6-9 | ▪ BW (--) | ||||||
| ▪ INS (↑) | |||||||
| ▪ Pancreatic islets (↑) | |||||||
| 14. | Lee H. (2017) [18] | PAH | SD rats: | UCB-MSC | Haemodynamics, BW, Organ weight, H&E, Wall thickness, Western Blot. | After 4 weeks, low-dose UC-MSCs was as competent as the high-dose in treated rats and reported, | Transplantation of low-dose and earlier treatment was as effective as high-dose UC-MSC in improving symptoms of PAH. Despite that, the dual or reversal treatment remains more effective as treatment for PAH. |
| ▪ Male | ▪ High-dose: 3×106 cells/rat | ▪ Mean RV pressure (↓) | |||||
| ▪ 6 weeks old | ▪ Mid-dose: 1.5×106 cells/rat | ▪ Pulmonary pathology (↑) | |||||
| ▪ n=5-6 | ▪ Low-dose: 3×105 cells/rat | ▪ Heart collagen-3 protein (↓) | |||||
| ▪ IV injection | |||||||
| 15. | Van Linthout S. (2017) [19] | Diabetic cardiomyopathy | SD rats: | PD-MSC | GLUC, IHC, Gene expression, Western Blot, Vascularization & Endothelial function. | After 13 days, treated rats reported high concentration of PD-MSC that was found in the LV pressure, lungs, kidney, pancreas and partially found in spleen. PD-MSC improved, | Transplantation of PD-MSC alleviated early symptoms of diabetic cardiomyopathy as seen by improved LV diastolic relaxation and relieved cardiomyocyte stiffness by increasing titin phosphorylation. |
| ▪ Male | ▪ 1×106 cells/rat | ▪ vascularization (↑) | |||||
| ▪ 8-9 weeks old | ▪ IV injection | ▪ cardiac inflammation (↓) | |||||
| ▪ n=5-7 | ▪ endothelial function (↑) | ||||||
| ▪ regulatory T-cells (↑) | |||||||
| 16. | Zhang L. (2013) [20] | DN | SD rats: | ASC | BW, GLUC, CHOL, ALB, TGL, Urine analysis, H&E, IHC, Western Blot, RT-PCR. | After 32 weeks, treated rats reported, | Transplantation of repeated, systemic administration of ASC attenuated hyperglycaemia. Secretion of GDNF by ASCs may play an important role in amelioration of diabetic podocyte injury. |
| ▪ Male | ▪ 5×106 cells/rat | ▪ ALB (↑) | |||||
| ▪ 2 months old | ▪ Once every 4 weeks for 32 weeks | ▪ TGL (↑) | |||||
| ▪ n=8-11 | ▪ IV injection | ▪ CHOL (↓) | |||||
| ▪ urinary protein excretion (↓) | |||||||
| ▪ Renal hypertrophy (↓) | |||||||
| 17. | Lee H. (2015) [21] | PAH | SD rats: | UC-MSC | Organ weight, RV Pressure, Immunofluorescence, H&E, Western Blot, IHC, Cytokine and Gene expression. | After 3 weeks, treated rats reported, | Transplantation of UC-MSC alleviated symptoms of PAH as seen by the decreased pulmonary arteriole thickening and gene expression. This success may prove advantageous over chemical drugs for treatment of PAH. |
| ▪ Male | ▪ 3×106 cells/rat | ▪ RV pressure (↑) | |||||
| ▪ 6 weeks old | ▪ IV injection | ▪ Cardiac hypertrophy (↓) | |||||
| ▪ n=16-24 | ▪ Expression of proteins and immunomodulatory metabolites (↓) | ||||||
| 18. | Wu X. (2014) [22] | T1DM | BALB/c mice: | MBPC | BW, GLUC, INS, C-peptide, IPGTT, H&E, IHC, Immunofluorescence, RT-PCR. | After 42 days, treated mice reported, | Transplantation of MBPC efficiently reversed hyperglycaemia and restored islet structures. The MBPCs could stimulate β-cell regeneration and promote endogenous progenitor cell differentiation into β-cells. MBPC is easily isolated in a non-invasive manner. |
| ▪ Male | ▪ 3×105 cells/mouse | ▪ BW (↑) | |||||
| ▪ 8 weeks old | ▪ IV injection | ▪ GLUC (↓) | |||||
| ▪ Sample size not reported | ▪ Pancreatic Islets (↑) | ||||||
| ▪ β-cell numbers (↑) |
Symbols: (↑) = increase; (↓) = decrease; (--) = no changes. Abbreviations: T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus; PAH, Pulmonary artery hypertension; DN, Diabetic nephropathy; TMSC, Tonsil-derived mesenchymal stromal cells; EB, Embryoid body; ILC, Islet-like clusters; UC-MSC, Umbilical cord-derived mesenchymal stromal cells; UC-WJC, Umbilical cord Wharton’s Jelly Cells; UCB-MSC, umbilical cord blood-derived mesenchymal stromal cells; PD-MSC, placenta-derived mesenchymal stromal cells; CB-MNC, Cord blood mononuclear cells; MBPC, Menstrual blood progenitor cells; BM-MSC, Bone marrow mesenchymal stromal cells; ASC, Adipose-derived stem cells; BrdU, bromodeoxyuridine; SD, Sprague Dawley; STZ, streptozocin; MCT, Monocrotaline; HFD, high fat diet; NX, nephrectomy; NPH, neutral protamine Hagedorn; IP, intraperitoneal; IV, intravenous; BW, Body weight; GLUC, Glucose; INS, insulin; CHOL, cholesterol; TGL, triglyceride; ALB, Albumin; IPGTT, intraperitoneal glucose tolerance test; RT-PCR, Reverse transcription polymerase chain reaction; ELISA, Enzyme-linked immunosorbent assay; H&E, haemotoxylin-eosin; IHC, immunohistochemistry; RV, right ventricular; LV, left ventricular; GDNF, glial cell line-derived neurotrophic factor.
Discussion
MSCs have been in the spotlight because they have been widely used as therapeutic candidates for various diseases due to their low immunogenicity and homing mechanism. MSCs have the potential to interact with and modulate resident cells and influence the stem cell niche through differentiation or a paracrine signalling mechanism. MSCs have a unique identity from their expression of surface markers CD73, CD90 and CD105 while absent of MHC class II molecules, CD11b, CD14 and CD34 [1-4]. Furthermore, the MHC class I cell surface receptor HLA-1 is downregulated in MSCs, which indicates that MSCs have immunomodulatory properties [3,4]. MSCs have multilineage differentiation potential to form adipocytes, osteocytes and chondrocytes. Some studies have shown promising results in differentiating MSCs into islet-like clusters [6,9-11,14-17,20,22] and type II alveolar epithelial cells [19].
MSCs can be obtained from the umbilical cord, cord blood, bone marrow, adipose, the tonsil, placenta, amniotic fluid and menstrual blood. In the present study, we found that UC-MSCs and BM-MSCs are widely used. The analysis showed that five studies used UC-MSCs [6,11-13,15], four used BM-MSCs [9,14,16,17], two used PD-MSCs [10,19], two used UCB-MSCs [18,21] and only one study each used TMSCs [1], hFTM-PV [2], AFSCs [3], CB-MNCS [5], ASCs [20] and MBPCs [22]. UC-MSCs were discovered to have a greater affinity for differentiation potential, proliferative and immunomodulatory properties. Having derived in an early developmental origin, these cells have greater therapeutic potential compared to cells isolated from developed adult tissue sources like the bone marrow or adipose tissue.
We compared the therapeutic effect of MSCs introduced to in-vivo models of T1DM, T2DM, PAH, diabetic cardiomyopathy (DC) and diabetic nephropathy (DN). Twelve studies targeted treatment for T1DM resulting from destruction of the insulin-producing β-cells [2-4,9-12,13-16,21]. Of the remaining studies, two targeted T2DM resulting from insulin resistance [1,13], two targeted PAH resulting from endothelial dysfunction [18,21], one targeted DC, where coronary artery disease is absent [19], and one targeted DN resulting from high glucose levels leading to kidney damage [20].
The studies used either a high-fat diet (HFD), streptozotocin (STZ)-induced diabetic animal models or monocrotaline (MCT)-induced animal models of hypertension. The studies used either mice or rats as the test animals. Among the studies using mice, four used C54/BL6 mice [4,14,16,19], three used BALB/c mice [1,10,22], two used NOD/SCID mice [5,17] and one used nude mice [2]. Among the studies using rats, five used Sprague-Dawley rats [11,13,18,20,21] and one study each used Wistar [9], Kunming [12] and albino rats [15]. Most of the studies used male animals, and only one study used female mice [7]. Furthermore, the majority of the studies (n=13) induced the diabetic animal model using intraperitoneal injection of STZ [2,6,9-12,14-17,19,20,22]. The other diabetic models were induced by HFD [1], intracardial injection of STZ [5], or a combination of HFD with STZ injection [13]; another two studies on a PAH animal model used MCT injection [18,21].
In the included studies, the diverse dosages, treatment frequencies, delivery vehicles and delivery routes contributed to improving the success rates of treatment. We determined from these studies that three dosages can be used: low dose (1.0-9.9×105 cells/animal), intermediate dose (1-4.9×106 cells/animal) and high dose (5-10×106 cells/animal). Thirteen studies injected the intermediate dose [1,2,6,7,10,11,13-17,19,21], three studies tested the low dose [9,18,22] and only two studies tested the high dose [12,20]. All studies administered single-dose injections, where the two exceptions administered injections every 2 weeks for a total of 10 weeks [4] and every 4 weeks for 32 weeks [19]. Most of the studies used intravenous (9 studies) [7,9-11,13-15,19,20,22] and intraperitoneal (3 studies) [1,2,12] injection as the delivery route. A few studies injected the cells into the jugular vein (2 studies) [18,21], left cardiac ventricle (2 studies) [6,17] or spleen [16] and kidney capsule [10].
In the diabetic models, MSC transplantation ameliorated diabetic symptoms, such as reversing hyperglycaemia, maintaining body weight and improving the survival rate, insulin levels and glucose tolerance [5]. In the hypertension models, MSC transplantation improved ventricular hypertrophy and pressure [18,21]. The administered MSCs were found distributed in the regions of cardiac, pulmonary, thymus, hepatic, splenic and renal functions [19]. In most cases, the MSCs migrated and successfully functioned at the site of tissue damage and affected areas.
The common comorbidities and cause of MetS are abdominal obesity. Eating habits, level of physical activity, smoking and family history are the known factors that lead to obesity in humans. The pathological changes of individuals diagnosed with MetS are observed through weight gain, hyperglycaemia, hyperinsulinemia, loss of pancreatic β-cell, insulin resistance, dyslipidemia, inflammation and fibrosis [1-4]. The two main mechanisms of MSCs are the reversal of the inflammatory destruction and the recovery of β-cell function. MSCs home in on inflammatory and injury sites before secreting bioactive factors to establish a suitable microenvironment that would stimulate β-cell regeneration and inhibit the T cell-mediated immune responses [19]. Some studies claim that MSCs increase islet regeneration, but MSCs themselves do not differentiate into β-cells after transplantation, as there is no evidence of the presence of MSCs in the islets [20].
The injection of MSCs also had effects on the pulmonary vasculature, blood pressure and right ventricular structure and function [21]. MSCs are believed to influence cardiovascular disease and acute lung injury. Early treatment with MSCs improves pulmonary pathology and suggests that MSCs secreted paracrine factors that help regulate collagen position in the cardiopulmonary system [18]. MSCs have a protective effect against GVHDs through immunosuppressive action via T cell regulation. This finding shows that MSCs have the immunoregulatory ability to foster the recovery of damaged alveolar and vascular cells. The advantages of MSCs include the reduction of pulmonary arteriole thickening and the expression of several genes, as compared with chemical drugs that cannot prevent disease progression or reduce mortality. Moreover, the expected effectiveness of MSCs lies in increasing the anti-inflammatory cytokines, evoking a positive sympathetic response and cardiopulmonary remodelling.
This review reveals that MSC treatment in animal models ameliorates the disorders that are a part of the MetS cluster. Although MSCs isolated from various sources can be used in MSC therapy, UC-MSCs were the most studied MSC type. The advantages of using UC-MSCs is that they can be obtained conveniently and less invasively [6,11-13,15], and they exhibit multilineage differentiation potential and higher proliferative and immuno-suppressive properties [22]. The various MSC dosages studied showed a dose-dependent effect when administered to the animal models. The studies included in the present review widely used intermediate-dose of MSCs. Some of the studies showed a similar level of improvement regardless of whether the low or intermediate dose was used. The findings demonstrate that low-dose stem cell therapy may also yield good effects in managing MetS, depending on the targeted disease model.
Conclusions
MetS is a complicated clinical condition and has emerged as a threatening global epidemic. The current state of medical practices cannot offer solutions for patients diagnosed with this syndrome. It is likely due to the facts that pathophysiology of MetS is complex, and the precise mechanisms linking each condition remain unclear, therefore limiting the progress and development of alternative medicine. Thus, we propose the potential of MSC as therapy for MetS. MSCs have been credited as a good choice that has a potential effect on the management of multiple MetS clusters, supported by the favourable outcomes in safety and efficacy studies. MSCs can improve the various disorders of MetS such as abdominal obesity, hyperglycaemia, hypertriglyceridemia and hypertension, and represent a promising alternative to conventional therapy of the MetS cluster. From the analysis and review of recent works, the superior source of MSCs is from the UC-MSCs, where the cells can be obtained conveniently, less invasively, and have excellent regenerative and immunosuppressive properties.
Acknowledgements
The study was approved by the Universiti Kebangsaan Malaysia Research Ethics Committee (approval code: UKM FPR.4/244/AP-2017-009/2). This work was supported by grants provided by Universiti Kebangsaan Malaysia (AP-2017-009/2 and FF-2020-469/1) and Ming Medical Sdn. Bhd. (FF-2020-469). All authors have read and agreed to the published version of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Lee Y, Shin SH, Cho KA, Kim YH, Woo SY, Kim HS, Jung SC, Jo I, Jun HS, Park WJ. Administration of tonsil-derived mesenchymal stem cells improves glucose tolerance in high fat diet-induced diabetic mice via insulin-like growth factor-binding protein 5-mediated endoplasmic reticulum stress modulation. Cell. 2019;8:368. doi: 10.3390/cells8040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao M, Zhang JB, Dong DD, Mou Y, Li K, Fang J, Wang ZY, Chen C, Zhao J, Yie SM. Alleviation of streptozotocin-induced diabetes in nude mice by stem cells derived from human first trimester umbilical cord. Genet Mol Res. 2015;14:12505–12519. doi: 10.4238/2015.October.16.18. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Goldenberg R, Punthakee Z. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2013;37(Suppl 1):S8–11. doi: 10.1016/j.jcjd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhao L, Yu D, Wang Z, Ding G. Metabolic syndrome prevalence and its risk factors among adults in China: a nationally representative cross-sectional study. PLoS One. 2018;13:e0199293. doi: 10.1371/journal.pone.0199293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villani V, Milanesi A, Sedrakyan S, Da Sacco S, Angelow S, Conconi MT, Di Liddo R, De Filippo R, Perin L. Amniotic fluid stem cells prevent β-cell injury. Cytotherapy. 2014;16:41–55. doi: 10.1016/j.jcyt.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao N, Zhao X, Luo P, Guo J, Zhao Q, Lu G, Cheng L. Co-transplantation of mesenchymal stromal cells and cord blood cells in treatment of diabetes. Cytotherapy. 2013;15:1374–1384. doi: 10.1016/j.jcyt.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Chan AML, Ng AMH, Mohd Yunus MH, Idrus RBH, Law JX, Yazid MD, Chin KY, Shamsuddin SA, Lokanathan Y. Recent developments in rodent models of high-fructose diet-induced metabolic syndrome: a systematic review. Nutrients. 2021;13:2497. doi: 10.3390/nu13082497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhansali S, Kumar V, Saikia UN, Medhi B, Jha V, Bhansali A, Dutta P. Effect of mesenchymal stem cells transplantation on glycaemic profile & their localization in streptozotocin induced diabetic Wistar rats. Indian J Med Res. 2015;142:63–71. doi: 10.4103/0971-5916.162116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Curr Diabetes Rev. 2010;7:168–82. doi: 10.1900/RDS.2010.7.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Hu Q, Chen F, Zhang J, Guo J, Wang H, Gu J, Ma L, Ho G. Human umbilical cord matrix-derived stem cells exert trophic effects on β-cell survival in diabetic rats and isolated islets. Dis Model Mech. 2015;8:1625–1633. doi: 10.1242/dmm.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maldonado M, Huang T, Yang L, Xu L, Ma L. Human umbilical cord Wharton jelly cells promote extra-pancreatic insulin formation and repair of renal damage in STZ-induced diabetic mice. Cell Commun Signal. 2017;15:43. doi: 10.1186/s12964-017-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Hao H, Han Q, Song X, Liu J, Dong L, Han W, Mu Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8:241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai N, Ohtaki H, Watanabe J, Xu Z, Sasaki S, Yagura K, Shioda S, Nagasaka S, Honda K, Izumizaki M. Intrapancreatic injection of human bone marrow-derived mesenchymal stem/stromal cells alleviates hyperglycemia and modulates the macrophage state in streptozotocin-induced type 1 diabetic mice. PLoS One. 2017;12:e0186637. doi: 10.1371/journal.pone.0186637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Hossary N, Hassanein H, El-Ghareeb AW, Issa H. Intravenous vs intraperitoneal transplantation of umbilical cord mesenchymal stem cells from Wharton’s jelly in the treatment of streptozotocin-induced diabetic rats. Diabetes Res Clin PR. 2016;121:102–111. doi: 10.1016/j.diabres.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Yaochite JN, de Lima KW, Caliari-Oliveira C, Palma PV, Couri CE, Simões BP, Covas DT, Voltarelli JC, Oliveira MC, Donadi EA, Malmegrim KC. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in-vitro and in-vivo immunomodulatory properties. Stem Cell Res Ther. 2016;7:14. doi: 10.1186/s13287-015-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Kim KC, Choi SJ, Hong YM. Optimal dose and timing of umbilical stem cells treatment in pulmonary arterial hypertensive rats. Yonsei Med J. 2017;58:570–580. doi: 10.3349/ymj.2017.58.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Linthout S, Hamdani N, Miteva K, Koschel A, Müller I, Pinzur L, Aberman Z, Pappritz K, Linke WA, Tschöpe C. Placenta-derived adherent stromal cells improve diabetes mellitus-associated left ventricular diastolic performance. Stem Cell Transl Med. 2017;6:2135–2145. doi: 10.1002/sctm.17-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Li K, Liu X, Li D, Luo C, Fu B, Cui S, Zhu F, Zhao RC, Chen X. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22:3074–3086. doi: 10.1089/scd.2013.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Lee JC, Kwon JH, Kim KC, Cho MS, Yang YS, Oh W, Choi SJ, Seo ES, Lee SJ. The effect of umbilical cord blood derived mesenchymal stem cells in monocrotaline-induced pulmonary artery hypertension rats. J Korean Med Sci. 2015;30:576–585. doi: 10.3346/jkms.2015.30.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23:1245–1257. doi: 10.1089/scd.2013.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]


