Abstract
Objective: To compare complete neuroendoscopic and microscopic microvascular decompression (MVD) in primary trigeminal neuralgia (PTN) and their impacts on the microstructure of the trigeminal nerve. Methods: Eighty-seven PTN patients admitted in our hospital from July 2017 to December 2019 were selected for this prospective study and divided into the endoscopic group (n=45) (complete neuroendoscopic MVD) and the microscope group (n=42) (microscopic MVD) according to the treatment method each patient underwent. All the patients underwent MRI scanning, and the fractional anisotropy (FA) scores and the apparent diffusion coefficient (ADC) values of the neurovascular compression (NVC) sites were measured. The operation times, the treatment efficacy, the microstructural changes in the trigeminal nerve, the complications, and the recurrence and mortality rates at one year after the operations were compared. Results: The endoscopic group observed a superior therapeutic effect compared with the microscope group one year after the surgeries (P=0.046). After the surgeries, the endoscopic group observed a greater increase in their FA values and a larger decline in their ADC values than the microscope group did (P=0.014, 0.015, 0.011, 0.002). The complication rate in the endoscopic group was 11.11%, and the complication rate in the microscopic group was 30.95% (P=0.022). One year after the surgeries, we found a lower recurrence rate in the endoscopic group (P=0.001). The perforator vessels from the offending vessel to the outlet area of the durmedulla, the distances between the front edge of the bone window and the inner surfaces of the petrous part of the temporal bone ≥ the distance between the duration ≥ the duration of conventional MVD were independent risk factors for complications after MVD in the hemifacial spasm patients (P=0.001, 0.037, 0.023, 0.005). Conclusion: Complete neuroendoscopic MV yields better long-term treatment outcomes than microscopic MVD, and it is more effective at improving the microstructure of the trigeminal nerve and has fewer postoperative complications.
Keywords: Complete neuroendoscopy, microscopy, microvascular decompression, primary trigeminal neuralgia, microstructure
Introduction
Primary trigeminal neuralgia (PTN) is mostly diagnosed among adults, with an approximate incidence of 70%-80% in individuals over 40 years old and is more common in females [1]. Microvascular decompression (MVD), the preferred surgical treatment for PTN patients who are intolerant to drug side effects or whose conservative treatment is ineffective, not only relieves clinical symptoms but also preserves the neural function of the trigeminal nerve [2]. However, the visibility of the surgical field of microscope MVD is relatively poor, resulting in a poor long-term relief effect in some patients and more complications. Complete neuroendoscopic MVD enjoys a bright and clear panoramic visualization which can reduce the traction of the cranial and cerebellar nerves, lessen the occurrence of complications, and ensure better safety [3]. Studies have shown that neurovascular compression (NVC) is the main cause of PTN [4]. A diffusion tensor imaging (DTI) sequence can observe the microstructure of the trigeminal nerve and quantify the data to reflect the microstructure, which is of great significance for the further study of PTN’s etiology [5]. However, there are no studies on the microstructural changes after MVD for the treatment of PTN, and whether the efficacy of microscopic and complete neuroendoscopic MVD for the treatment of PTN is apparent or not is still poorly understood. In view of this, the efficacy of complete neuroendoscopic versus microscopic MVD for the treatment of PTN and their effects on the microstructure of the trigeminal nerve were analyzed and reported as follows.
Materials and methods
General information
This trial is a prospective study. Eighty-seven PTN patients, including 40 males and 47 fe-males, who were diagnosed in our hospital from July 2017 to December 2019, were recruited as the study cohort. Inclusion criteria: (1) Patients whose PTN was diagnosed using MRI and CT, and patients who met the Chinese expert consensus for the diagnosis and treatment of trigeminal neuralgia [6]. (2) Patients with paroxysmal pain in the forehead or face lasting from a few seconds to 2 min. (3) Patients with trigger points in the painful area with periodic recurrences. (4) Patients with a unilateral onset of the disease. Exclusion criteria: (1) Patients whose nerve fibers were damaged by surgery, drugs, or electrocoagulation. (2) Patients with malignant tumors in their other systems or organs. (3) Patients unable to tolerate MVD. (4) Patients who were lost to follow-up or who quit the study. The patients were divided into an endoscopic group (45 patients) and a microscopic group (42 patients), depending on the treatment method each patient underwent. All the enrolled patients signed an informed consent form. This study was certified by the ethics committee, and the ethics approval number is 2016-12-14.
Clinical trial registration: https://clinicaltrials.gov/, ClinicalTrials.gov Identifier: NCT03131465.
Methods
The endoscopy group was administered complete neuroendoscopic MVD (Model: PE184A, AESCULAP). The patients were generally anesthetized in a prone position, with their heads and necks parallel to the ground and their bodies and heads fixed. After routine disinfection, the retrosigmoid keyhole approach was performed. An internal oblique incision of 6 to 7 cm was made near the hairline behind the ear. The incision was done layer by layer until the transverse sinus and sigmoid sinus were exposed. The dura mater was incised in a “Y” shape to release the cerebrospinal fluid, and then the cerebellopontine cistern was opened for the exposure of the corresponding cranial nerves. The neuroendoscopy was employed to explore the trigeminal nerve roots to identify the compressed location and direction of the offending vessel. The operations were performed lightly and gently to avoid damaging the blood vessels and nerves. Teflon cotton was placed in the space between the nerve and the offending vessel, then the dead angles such as the pontine and the front of the nerve were explored under the neuroendoscope, and the incision was sutured after hemostasis.
The microscope group was administered microscopic MVD (model: M400E, LEICA). General anesthesia was administered to the patients after they were placed in a supine position. After routine disinfection, the craniotomy was performed, the cerebrospinal fluid was released to reduce the intracranial pressure and to fully expose the corresponding cranial nerves, and the root area of the trigeminal nerve was surveyed. The arachnoid membrane around the nerve roots was opened to expose and target the offending vessel. Teflon spacers were used for separation. If only the local arachnoid bundle around the nerve root was found and no offending vessel was targeted, neurolysis was performed and the trigeminal nerve was given decompression to promote the recovery of the trigeminal nerve. The intraoperative blood loss volume was measured: the apparent blood loss during the operation = the difference in gauze weight before and after the operation + the amount of fluid in the drainage tube-the amount of saline flushing during the operation.
Outcome measures
The duration of the surgery, the clinical treatment at two weeks and one year after the surgery, the microstructural changes in the trigeminal nerve, and the occurrences of complications were compared between the two groups.
Clinical outcome measures [7]: Complete relief: the pain symptoms disappeared completely and there was no need to take medication. Partial relief: the pain symptoms were alleviated with an occasional need for medication. No relief: no improvement or even worse clinical symptoms appeared.
Microstructural measurements: A signal pioneer 3.0 T magnetic resonance scanner (GE, USA) with an 8-channel head quadrature coil was applied. The scan plane was set parallel to the cisternal segment of the trigeminal nerve, the scan range was the entire pons, and the acquisition of the microstructural images was performed using a DTI sequence with the following scanning parameters set: a TR of 7100 MS, a TE of 94 MS, and a 20 cm ×20 cm field of view, 160×160 matrices, 2.0-mm slice thicknesses, we selected 1000 s/mm2 as the B value, and the gradient field direction was set to 30. The DTI images were uploaded to the AW4.4 workstation, and the microstructure was analyzed using functool post-processing software. The regions of interest (ROI) were outlined to measure the fractional anisotropy scores (FA), the apparent diffusion coefficient (ADC), the ROI, and the colony-stimulating factor (CSF) while avoiding the cerebrospinal fluid surrounding the trigeminal nerve as much as possible, with consistent areas. The trigeminal nerve was segmented and measured in triplicate from the brain touch to the Meckel’s cavity, and the average value was taken as the outcome of the measurements, with three repeated measurements as the final outcome.
The complications include nausea and vomiting, hearing impairment, facial numbness, and cerebrospinal fluid leakage. One year after the operations, the two groups’ recurrence rates were counted and compared.
Statistical methods
All the data were expressed as the means ± standard deviation. The calculations were performed using SPSS 26.0 statistical software (SPSS, Chicago, IL, USA). The single-factor analyses were performed using ANOVA. Multivariate logistic regression was used to analyze the complications after the microvascular decompression. P values <0.05 were considered statistically significant. GraphPad Prism 8 was used to plot the graphics.
Results
Comparison of the clinical data
There were no significant differences between the two groups in terms of gender, age, disease duration, or site of onset (P>0.05, see Table 1).
Table 1.
Comparison of the general data between the two groups (x̅±s; n, %)
| group | Number of cases | Sex | Years (years) | Disease duration (months) | Site of onset | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| male | female | left | right | ||||
| Endoscopic group | 45 | 20 (44.44) | 25 (55.56) | 48.96±6.46 | 6.51±1.81 | 25 (55.56) | 20 (44.44) |
| Microscopy group | 42 | 20 (47.62) | 22 (52.38) | 49.56±6.22 | 6.44±1.67 | 24 (57.14) | 18 (42.86) |
| t/χ2 value | 0.088 | 0.441 | 0.187 | 0.022 | |||
| P value | 0.767 | 0.661 | 0.852 | 0.881 | |||
Comparison of the operation durations and the clinical treatment efficacy
The operation duration times in the endoscopic group were (165.89±25.38) min, which was not statistically different from that the times of (156.57±24.27) min in the microscopic group (P>0.05). The MRI images of two groups of patients before and after surgery are presented in Figure 1. No evidence of differences was found between the two groups at two weeks after surgery (P>0.05) (Table 2), yet the endoscopic group had better clinical outcomes than the microscopic group at one year after the surgery (P<0.05) (Table 3).
Figure 1.
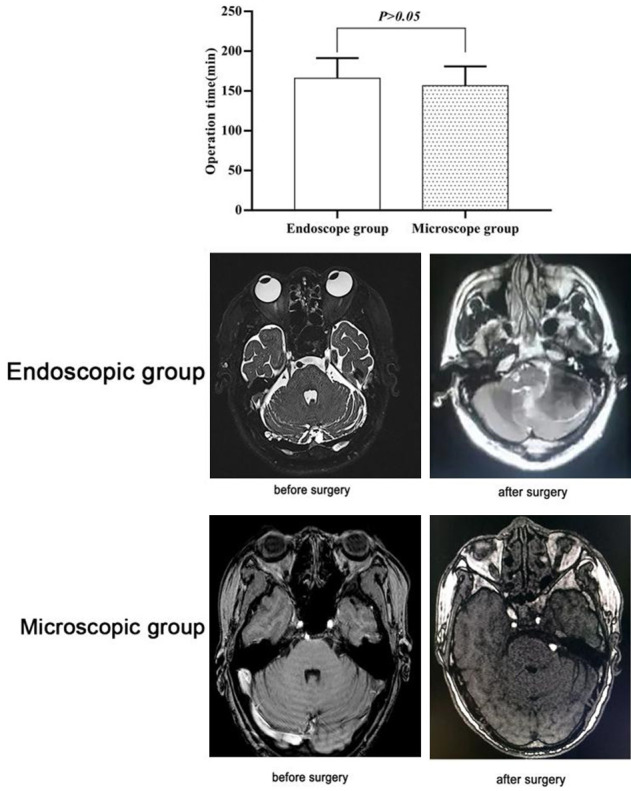
A comparison of operation times between the two groups and MRI images of the two groups before and after the surgeries.
Table 2.
Comparison of clinical treatment efficacy between the two groups at two weeks after surgery
| group | Number of cases | complete remission | Partial remission | No remission |
|---|---|---|---|---|
| Endoscopic group | 45 | 38 (84.44) | 6 (13.33) | 1 (2.22) |
| Microscopy group | 42 | 30 (71.43) | 10 (23.81) | 2 (4.76) |
| Z-scores | 1.112 | |||
| P value | 0.292 | |||
Table 3.
Comparison of the clinical treatment efficacy between the two groups at one year after surgery
| group | Number of cases | complete remission | Partial remission | No remission |
|---|---|---|---|---|
| Endoscopic group | 45 | 42 (93.33) | 3 (6.67) | 0 (0.00) |
| Microscopy group | 42 | 31 (73.81) | 8 (19.05) | 3 (7.14) |
| Z-scores | 3.990 | |||
| P value | 0.046 | |||
Comparison of the microstructural changes in the trigeminal structure
Before the surgery, the two groups showed no significant differences in their FA and ADC values (P>0.05). The two groups’ FA values witnessed a surge one year after the operations (P<0.05), with a higher result in the endoscopic group than the microscopic group (P<0.05, Figure 2). The two groups’ ADC values saw a slump one year after the operations (P<0.05), and the endoscopic group was significantly lower than the microscopic group (P<0.05) (Table 4, Figure 3).
Figure 2.
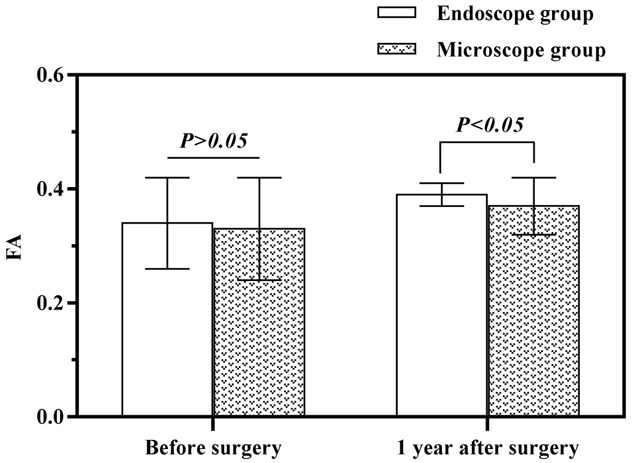
Comparison of FA values between the two groups before and at one year after the surgeries.
Table 4.
Comparison of the microstructural changes in the trigeminal structures between the two groups before the operations and at one year after the operations (x̅±s)
| group | point of time | FA values | ADC (×10-9 mm2/s) |
|---|---|---|---|
| Endoscopy Group (45) | Preoperative | 0.34±0.08 | 2.02±0.25 |
| 1 year after surgery | 0.39±0.02 | 1.67±0.17 | |
| ta | 4.067 | 7.766 | |
| Pa | <0.001 | <0.001 | |
| Microscopy group (42) | Preoperative | 0.33±0.09 | 1.97±0.26 |
| 1 year after surgery | 0.37±0.05 | 1.82±0.27 | |
| ta | 2.518 | 2.593 | |
| Pa | 0.014 | 0.011 | |
| tb | 0.549 | 0.914 | |
| Pb | 0.585 | 0.363 | |
| tc | 2.480 | 3.123 | |
| Pc | 0.015 | 0.002 |
Figure 3.
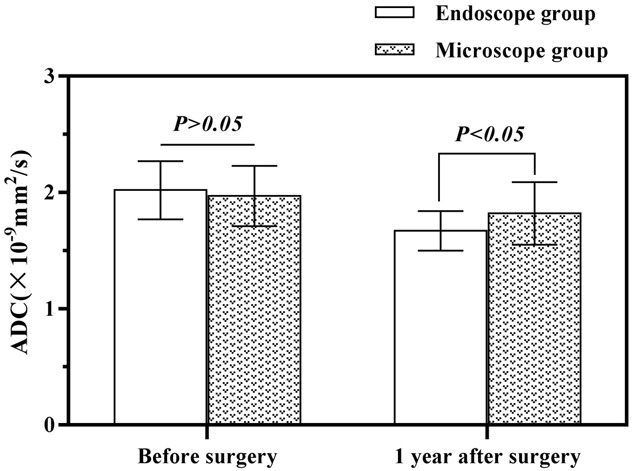
The preoperative and one-year postoperative ADC values in the two groups.
Comparison of incidences of complications
The patients in the endoscopic group had a lower incidence of complications compared to the microscopic group (11.11% vs 30.95%, P<0.05) (Table 5).
Table 5.
Comparison of postoperative complications between the two groups
| group | Number of cases | Nausea & Vomiting | Hearing impairment | Facial numbness | CSF leak | Total complications |
|---|---|---|---|---|---|---|
| Endoscopic group | 45 | 2 (4.44) | 1 (2.22) | 2 (4.44) | 0 (0.00) | 5 (11.11) |
| Microscopy group | 42 | 6 (14.29) | 2 (4.76) | 4 (9.52) | 1 (2.38) | 13 (30.95) |
| χ2 value | 2.52 | 0.421 | 0.873 | 1.084 | 5.212 | |
| P value | 0.112 | 0.517 | 0.35 | 0.298 | 0.022 |
Comparison of the recurrence rates
One year after the surgery, the endoscopic group had a notably lower recurrence rate compared with the microscopic group, which recorded two deaths (P<0.05, Table 6).
Table 6.
Comparison of the one-year postoperative recurrence rates and the mortality between the two groups
| group | Number of cases | Recurrence | No recurrence | Death |
|---|---|---|---|---|
| Endoscopic group | 45 | 3 (6.67) | 42 (93.33) | 0 (0) |
| Microscopy group | 42 | 9 (21.43) | 33 (78.57) | 2 (4.76) |
| X2 | 1.365 | 5.362 | ||
| P value | 0.001 | 0.998 | ||
Multivariate logistic regression was used to analyze the complications after the microvascular decompression
The results of our multivariate logistic regression analysis demonstrated that the perforator vessels from the offending vessel to the outlet area of the medulla, the distance between the front edge of the bone window and the inner surface of the petrous part of the temporal bone ≥ edulla, subdural operation time ≥ edulla, and conventional microvascular decompression were independent risk factors for complications after microvascular decompression in patients with hemifacial spasms (P<0.05). See Tables 7, 8.
Table 7.
Single-factor analysis of the complications after the microvascular decompression
| Index | Complications (n=18) | No complication (n=69) | X2 | P |
|---|---|---|---|---|
| the perforator vessels from the offending vessel to the outlet area of the medulla | 18 | 1 | 2.365 | 0.002 |
| Distance between the front edge of the bone window and the inner surface of the petrous part of the temporal bone ≥ istanc | 17 | 0 | 2.77 | 0.001 |
| Subdural operation time ≥ ubdura | 18 | 2 | 5.365 | 0.003 |
| Conventional microvascular decompression | 16 | 2 | 4.654 | 0.005 |
Table 8.
A multivariate logistic regression analysis of the complications after microvascular decompression
| Index | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| the perforator vessels from the offending vessel to the outlet area of the medulla | 0.865 | 0.364 | 6.345 | 0.001 | 2.37 | 1.32, 4.26 |
| Distance between the front edge of the bone window and the inner surface of the petrous part of the temporal bone ≥ istanc | 0.82 | 0.366 | 3.41 | 0.037 | 2.13 | 1.36, 3.99 |
| Subdural operation time ≥ ubdura | 0.768 | 0.322 | 4.82 | 0.023 | 2.06 | 1.32, 3.68 |
| Conventional microvascular decompression | 0.748 | 0.268 | 8.362 | 0.005 | 2.11 | 1.16, 3.99 |
Discussion
The trigeminal nerve starts from the anterolateral side of the pontine, travels forward, downward, and outward in the subarachnoid space of the prepontic cistern, and enters Meckel’s cave from below the free edge of the tentorium to form the trigeminal ganglion. There are currently two theories for the pathogenesis of PTN [8]: 1. Peripheral theory: it is believed that the sensory root of the trigeminal nerve is compressed by the capillary to cause PTN, and local lesions and hyperplasia of the sheath of the trigeminal nerve root cause axon short circuits and cause pain. 2. Central theory. This theory believes that the blood vessels compress the trigeminal nerve and cause excessive excitement and pain. At present, despite the feasibility of both surgery and drugs for the treatment of PTN, surgical treatment is more preferred, as the drug treatment is undermined by its side effects and poor therapeutic efficacy.
The MVD treatment is minimally invasive, safe, and has a high cure rate, and it can cure PTN and retain nerve function and vascular integrity. The microscope is the earliest application in the MVD, but its operational space is rather small, and the trigeminal nerve root is deep in the brain stem. Moreover, during the operation, the peripheral blood vessels, auditory nerves, cerebellum, etc. need to be stretched to fully expose the surgical field of vision, which can easily lead to the omission of the responsible blood vessels, cause traction damage, etc., reducing the surgical effect [9]. Later, neuroendoscopy is also gradually applied to MVD. Complete neuroendoscopic MVD uses artificial dura mater to suspend the compressed trigeminal nerve vessel, thereby separating the trigeminal nerve root from the responsible blood vessel and eliminating the oppressive stimulation, thereby alleviating the pain [10]. In this study, there was no difference between the operation durations and clinical treatment efficacy at two weeks after the operations between the two groups. The reason may be that the repeated flushing and wiping of the endoscope in neuroendoscopic MVD increased the operation time. However, this is related to the proficiency of the surgeon, which means the time can be shortened when the surgeon masters the techniques. Therefore, there was no significant difference between the two groups [11]. Neuroendoscopic MVD can expose the three-dimensional structure of the cerebellopontine angle from different angles, observe the junctions of nerves and blood vessels at close range, and can more clearly observe the blind spots that cannot be seen in the microscopic MVD operation. During the treatment process, the trigeminal nerve can be more fully exposed to view abnormal blood vessels, so that the trigeminal nerve and the offending vessel can be more completely separated, and the oppressive stimulation can be eliminated, which therefore results in a better long-term treatment effect [12].
ROI is an image area selected from the image, which is the focus of the image analysis, and the DTI sequence can non-invasively evaluate the integrity of the myelin sheath in vivo. By measuring FA, ADC, and other parameters, it is possible to quantitatively analyze the changes in the microstructure such as the demyelination of fibers caused by neurovascular compression and the breakage of the shaft. The FA value reflects the direction and integrity of the white matter of the brain. The decrease of the FA value in patients with PTN indicates that vascular compression leads to the loss of nerve fiber axonal or demyelination [13]. The ADC value reflects the degree of diffusion of the tissue water. The long-term compression of blood vessels causes chronic hypoperfusion of nerve roots and increases the permeability of cell membranes. The diffusion of local water molecules also accelerates, which ultimately leads to an increase in the ADC value [14]. The FA values of the two groups witnessed a surge one year after the operation, with higher values in the endoscopic group than in the microscope group. The results of this study also showed a lower incidence of complications (11.11%) in the endoscopic group compared with 30.95% in the microscopic group. The reason may be that the neuroendoscope has a small cross-section, but the endoscope is elongated, so it is easy to operate in a long and narrow cavity, with better illumination. It also enjoys a positive panoramic field of vision and can detect hidden areas and abnormal blood vessels outside the nerve root. The neuroendoscope has a small cross-section, but the endoscope is elongated, easy to operate in the long and narrow space, and the endoscope has a strong deep illumination and a good panoramic view. It can detect the hidden area and abnormal blood vessels outside the nerve root and can rotate at small angles, so it can clearly view the vessels and nerves of the pontine cerebellum and reduce traction. It can also reduce the release of cerebrospinal fluid and prevent complications such as subdural hematoma and intracerebellar hemorrhage. The safety is relatively high [15-17]. At the same time, the endoscope can be rotated at a small angle, which can clearly view the blood vessels and nerves of the cerebellopontine and reduce traction. It can also reduce the release of cerebrospinal fluid and prevent complications such as subdural hematoma and intracerebellar hemorrhage, and its safety is relatively higher [18-20]. One year after the surgery, a notably lower recurrence rate in the endoscopic group was obtained in comparison with the microscopic group which recorded two deaths (P<0.05). The results of our multivariate logistic regression analysis demonstrated that the perforator vessels from the offending vessel surrounding the outlet area of the medulla, the distance between the front edge of the bone window and the inner surface of the petrous part of the temporal bone ≥ edulla, subdural operation time ≥ edulla, and conventional microvascular decompression were independent risk factors for complications after microvascular decompression in patients with hemifacial spasms.
In conclusion, complete neuroendoscopic MVD and microscopic MVD have comparable surgical durations and short-term treatment outcomes for PTN, but long-term treatment with complete neuroendoscopic MV is better than microscopic MVD, and it is more effective at improving the microstructure of the trigeminal nerve and has fewer postoperative complications. However, this study is limited by the absence of a large cohort, so this will be addressed in future studies. Furthermore, future studies will be conducted to investigate the preoperative and postoperative imaging changes, and the degree of PTN nerve myelin damage at the different stages, to reflect the patients’ postoperative recoveries through the microstructure.
Disclosure of conflict of interest
None.
References
- 1.Hu H, Chen L, Ma R, Gao H, Fang J. Acupuncture for primary trigeminal neuralgia: a systematic review and PRISMA-compliant meta-analysis. Complement Ther Clin Pract. 2019;34:254–267. doi: 10.1016/j.ctcp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Sharma R, Katiyar V, Gurjar H. Letter: primary modality for medically refractory trigeminal neuralgia: microvascular decompression or gamma knife therapy? Oper Neurosurg (Hagerstown) 2018;14:E31–E32. doi: 10.1093/ons/opx243. [DOI] [PubMed] [Google Scholar]
- 3.Arrese I, Sarabia R. Microvascular decompression for trigeminal neuralgia secondary to vertebrobasilar dolichoectasia. Case report, literature review, and pooled case analysis. Neurocirugia (Astur) 2016;27:304–309. doi: 10.1016/j.neucir.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Yoshino N, Shimada M, Tetsumura A, Matsumura T, Fukayama H, Kurabayashi T. Trigeminal neuralgia: differences in magnetic resonance imaging characteristics of neurovascular compression between symptomatic and asymptomatic nerves. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:113–118. doi: 10.1016/j.oooo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Fukuoka T, Nishimura Y, Hara M, Nomura K, Ryu H, Yoshikawa S, Wakabayashi T. Flat posterior cranial fossa affects outcomes of microvascular decompression for trigeminal neuralgia. World Neurosurg. 2018;111:e519–e526. doi: 10.1016/j.wneu.2017.12.114. [DOI] [PubMed] [Google Scholar]
- 6.Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia-diagnosis and treatment. Cephalalgia. 2017;37:648–657. doi: 10.1177/0333102416687280. [DOI] [PubMed] [Google Scholar]
- 7.Maarbjerg S, Heinskou TB, Wolfram F, Rochat P, Brennum J, Bendtsen L. Diagnostics and treatment of trigeminal neuralgia. Ugeskr Laeger. 2016;178:V02160146. [PubMed] [Google Scholar]
- 8.Hussain MA, Konteas A, Sunderland G, Franceschini P, Byrne P, Osman-Farah J, Eldridge P. Re-exploration of microvascular decompression in recurrent trigeminal neuralgia and intraoperative management options. World Neurosurg. 2018;117:e67–e74. doi: 10.1016/j.wneu.2018.05.147. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Wang XH, Zhang Y, Zhang X, Tang YD, Zhou P, Zhu J, Li ST. Management of primary bilateral trigeminal neuralgia with microvascular decompression: 13-case series. World Neurosurg. 2018;109:e724–e730. doi: 10.1016/j.wneu.2017.10.072. [DOI] [PubMed] [Google Scholar]
- 10.Jones MR, Urits I, Ehrhardt KP, Cefalu JN, Kendrick JB, Park DJ, Cornett EM, Kaye AD, Viswanath O. A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep. 2019;23:74. doi: 10.1007/s11916-019-0810-0. [DOI] [PubMed] [Google Scholar]
- 11.Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences (Riyadh) 2015;20:107–114. doi: 10.17712/nsj.2015.2.20140501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Stefano G, Maarbjerg S, Truini A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J Headache Pain. 2019;20:20. doi: 10.1186/s10194-019-0969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J, Dong X, Zhao X. Treatment of trigeminal neuralgia by radiofrequency of the Gasserian ganglion. Rev Neurosci. 2016;27:739–743. doi: 10.1515/revneuro-2015-0065. [DOI] [PubMed] [Google Scholar]
- 14.Liu JK, Apfelbaum RI. Treatment of trigeminal neuralgia. Neurosurg Clin N Am. 2004;15:319–334. doi: 10.1016/j.nec.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Fernández Rodríguez B, Simonet C, Cerdán DM, Morollón N, Guerrero P, Tabernero C, Duarte J. Familial classic trigeminal neuralgia. Neurologia (Engl Ed) 2019;34:229–233. doi: 10.1016/j.nrl.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Pu B, Li F, Liu K, Hua C, Li C, Zhao C, Li J, Li X. Analysis of failed microvascular decompression in patients with trigeminal neuralgia. J Neurol Surg B Skull Base. 2020;81:567–571. doi: 10.1055/s-0039-1692683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Zhang X, Yao J, Li H, Jiang Y. Microvascular decompression for trigeminal neuralgia due to venous compression alone. J Craniofac Surg. 2018;29:178–181. doi: 10.1097/SCS.0000000000004174. [DOI] [PubMed] [Google Scholar]
- 18.Abdulrauf SI, Urquiaga JF, Patel R, Albers JA, Sampat VB, Baumer M, Marvin E, Pierson M, Kragel R, Walsh J. Awake microvascular decompression for trigeminal neuralgia: concept and initial results. World Neurosurg. 2018;113:e309–e313. doi: 10.1016/j.wneu.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Holste K, Chan AY, Rolston JD, Englot DJ. Pain outcomes following microvascular decompression for drug-resistant trigeminal neuralgia: a systematic review and meta-analysis. Neurosurgery. 2020;86:182–190. doi: 10.1093/neuros/nyz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang V, Gomez-Marroquin E, Enciso R, Padilla M. Trigeminal neuralgia management after microvascular decompression surgery: two case reports. J Dent Anesth Pain Med. 2020;20:403–408. doi: 10.17245/jdapm.2020.20.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]


