Abstract
To determine if 1,25(OH)2D deficiency can induce age-related sarcopenia, the skeletal muscular phenotype of male wild-type (WT) and Cyp27b1 knockout (KO) mice were compared at 3 and 6 months of age. We found that muscle mass, grip strength and muscle fiber size were significantly decreased in aging Cyp27b1 KO male mice. The expression levels of genes related to mitochondrial metabolic activity, and antioxidant enzymes including SOD1, catalase, Nqo1 and Gcs were significantly down-regulated in skeletal muscle tissue of Cyp27b1 KO male mice; in contrast, the percentage of p16+ and p21+ myofibers, and the expression of p16, p19, p21, p53, TNFα, IL6 and MMP3 at mRNA and/or protein levels were significantly increased. We then injected tibialis anterior muscle of WT and Cyp27b1+/- male mice with BaCl2, and analyzed the regenerative ability of skeletal muscle cells 7 days later. The results revealed that the numbers of newly formed regenerating central nucleated fibers (CNF), the percentage of BrdU+ cells and the expression of MyoD, MyHC and Myf5 at mRNA levels were significantly down-regulated in the injured skeletal muscle tissue of Cyp27b1+/- mice. In summary, our studies indicate that 1,25(OH)2D deficiency can result in the development of age-related sarcopenia by inducing oxidative stress, skeletal muscular cell senescence and SASP, and by inhibiting skeletal muscle regeneration. Cyp27b1 KO mice can therefore be used as an animal model of age-related sarcopenia in order to investigate the pathogenesis of age-related sarcopenia and potentially to test intervention measures for treatment of sarcopenia.
Keywords: Vitamin D deficiency, sarcopenia, oxidative stress, muscular cell senescence and regeneration
Introduction
Sarcopenia, recently recognized as a disease by WHO [1], is a progressive and degenerative systemic skeletal muscle disease, characterized by decreased muscle quantity, muscle strength and muscle function [2]. The risk of falls, fractures, weakness and disability in sarcopenia patients is significantly increased, and independent life span is decreased. The prevalence of sarcopenia has been reported to be 10% in the population over 60 years of age, and rises to 30% in the population over 80 years of age [3]. With increased aging of the population, skeletal muscle aging has become a major public health problem, and has caused a heavy burden on family and social health care.
Age, endocrine disorders, malnutrition, chronic inflammation and vitamin D deficiency all increase the risk of sarcopenia [4]. Because old age, sex hormone deficiency and malnutrition are closely related to vitamin D deficiency [5-7], and vitamin D deficiency can cause chronic inflammation [8], more attention has been paid to the action of vitamin D in the pathogenesis of sarcopenia and to its supplementation in the prevention and treatment of sarcopenia. It is estimated that there are 1 billion people with vitamin D deficiency world-wide [4], and it is especially prevalent in the elderly [9]. Decreased skeletal muscle mass and strength, and increased incidence rates of sarcopenia and risk of falls are closely related to a decrease of serum vitamin D levels [10,11]. Meta-analysis revealed that supplementation with vitamin D significantly improved muscle function and balance, reducing falls by 14-34% [12]. Preclinical studies have shown that muscle fibers express VDR, and the proliferation and differentiation of myoblasts are regulated by vitamin D/VDR signaling [13]. VDR deficient mice showed decreased cross-sectional area (CSA) of muscle fibers, accompanied by dysregulated expression of myogenic transcription factors and MyHC isomers [14]. 1,25(OH)2D3 can stimulate C2C12 mouse myoblasts to differentiate into mature myocytes by up regulating the expression of IGF-2 and follistatin, and down regulating the expression of IGF-1 and myostatin [15]. However, it has not been reported if mice with deficiency of the active form of vitamin D display sarcopenia.
Vitamin D may be synthesized from 7-dehydrocholesterol in the skin following irradiation with ultraviolet light; alternatively, vitamin D may be absorbed from the diet. Vitamin D is converted to 25(OH)D by liver 25 hydroxylase, and then to active 1,25(OH)2D, mainly by kidney 1α-hydroxylase [Cyp27b1]; 1,25(OH)2D exerts its biological function by binding to VDR [16]. In order to study the in vivo mechanism of action of 1,25(OH)2D, we deleted the Cyp27b1 gene to successfully generate a mouse model of 1,25(OH)2D deficiency [17]. We previously reported that this mouse model displayed not only rickets, but also female infertility, male infertility, hypertension, premature aging, a high incidence of multiple tumors and osteoporosis [17-27], however, it has not been reported if this mouse model displays sarcopenia.
To address this issue, we compared the skeletal muscular phenotype of 3- and 6-month-old WT and Cyp27b1 KO male mice and analyzed the alterations of muscle mass, grip strength and muscle fiber size, the expression levels of mitochondrial metabolic activity-related genes and antioxidant enzymes, muscle cell senescence and senescence-associated secretory phenotype (SASP). Furthermore, the tibialis anterior (TA) muscle of WT and Cyp27b1+/- male mice were injected with BaCl2, and the regeneration of skeletal muscle cells was analyzed after 7 days.
Materials and methods
Animals
Cyp27b1 KO mice were prepared and genotyped as previously described [17]. WT and Cyp27b1 KO male mice at 3 and/or 6 months of age were used for this study. The body weight, the weight of tibialis anterior (TA) and gastrocnemius (Gas) muscles were measured and the muscle weight/body weight ratio was calculated. The experimental muscle injury model was established in 6-month-old WT and Cyp27b1+/- male mice as previously described [28]. After 7 days, the injured TA muscles were obtained from the mice for histomorphometry and molecular analysis. All experimental steps involved in this study were carried out in strict accordance with the guidelines of the Institute of experimental animals of Nanjing Medical University.
Muscle strength testing
The mouse grip instrument (SANS, Jiangsu, China) was used to evaluate the forelimb grip. In the grip test, front paws of mice were placed on the gripping device, the mouse was lifted by the tail and hind limbs suspended in the air, and then slowly pulled until the mouse’s forelimb grip disappeared. Three trials were carried out on the grip measurement of the forelimbs. The recorded maximum value (in grams) obtained by the software analysis is considered to be the muscle grip strength and is used for all analyses.
Histology and immunofluorescence staining
After euthanasia, the TA muscle was removed from the lower limbs, frozen and embedded under liquid nitrogen using OCT, and then cut into 5 µm sections on a cryostat. After rewarming the muscle sections at room temperature, they were stained with hematoxylin and eosin (H&E). For immunofluorescence (IF) staining, the sections were fixed in acetone, incubated with 3% H2O2, and then the slides were incubated with antibodies against p16 and p21 (Abcam, USA), The SOD1 antibody, BrdU antibody (Santa Cruz, USA), and laminin antibody (Proteintech, China) were incubated overnight at 4°C. They were then incubated with the secondary antibodies for staining.
Western blot
RIPA lysate was used to extract total protein from mouse TA muscle tissue, and a BCA kit was then used to determine the protein concentration. Western blot analysis was performed with primary antibodies against SOD1 (Santa Cruz, USA), p16, p19, p53, p21, and β-actin (Abcam, USA), then developed with ECL developer solution, and quantified with ImageJ analysis as previously described [26].
RNA isolation and real-time qRT-PCR
Total RNAs were extracted from TA muscle tissue and real-time qRT-PCR was performed as previously described [19]. The primer sequences are shown in Table 1.
Table 1.
Primers used for quantitative real-time PCR
| Primers | Forward | Reverse |
|---|---|---|
| α-S9 | CCCGGGCCAGCTTACCT | GCTGCACTGCTTTCCTGATAGA |
| Fe-S | GCTGGGCGCACACTTTGT | CACTGGCCTTGCAGGAAGAA |
| COX7α | TCTTCCAGGCCGACAATGAC | GCCCAGCCCAAGCAGTATAA |
| PGC1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| CAT | GCAGATACCTGTGAACTGTCCCT | TTACAGGTTAGCTTTTCCCTTCG |
| SOD1 | ATTACAGGATTAACTGAAGG | CAATGATGGAATGCTCTC |
| Nqo1 | AGGATGGGAGGTACTCGAATC | AGGCGTCCTTCCTTATATGCTA |
| Gcs | GGGGTGACGAGGTGGAGTA | GTTGGGGTTTGTCCTCTCCC |
| P16INK4a | GAAAGAGTTCGGGGCGTTG | GAGAGCCATCTGGAGCAGCAT |
| P21CIP1 | CCTGGTGATGTCCGACCTG | CCATGAGCGCATCGCAATC |
| TNFα | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| MMP3 | ACATGGAGACTTTGTCCCTTTTG | TTGGCTGAGTGGTAGAGTCCC |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Myod | CCACTCCGGGACATAGACTTG | AAAAGCGCAGGTCTGGTGAG |
| MyHc | CTTCTACAGGCCTGGGCTTAC | CTCCTTCTCAGACTTCCGCAG |
| Myf5 | AAGGCTCCTGTATCCCCTCAC | TGACCTTCTTCAGGCGTCTAC |
| Gapdh | CCACCCAGAAGACTGTGGAT | GGATGCAGGGATGATGTTCT |
Computer-assisted image analysis
The IF and H&E stained sections were photographed under a microscope (Leica, Germany), and analyzed with ImageJ to measure the CSA of muscle fibers and the average number of IF cells, which were then expressed as a percentage of positive cells out of total cells. The numbers of CNF in each field of view were counted manually in images from 3 H&E stained sections per group.
Statistical analysis
All measurement data are displayed as average ± SEM. The differences between the 2 groups were analyzed using two-tailed non paired Student’s t test, and the differences among more than 2 groups were analyzed using one-way ANOVA. P<0.05 is considered statistically significant.
Results
1,25(OH)2D deficiency leads to sarcopenia
To determine if 1,25(OH)2D deficiency leads to the occurrence of sarcopenia, the changes in body weight, the weight of the tibialis anterior (TA) and gastrocnemius (Gas) muscles, the muscle weight/body weight ratio and the grip strength were examined in WT and Cyp27b1 KO male mice at 3 and 6 months of age. The results showed that the body weight and the weight of TA muscle and Gas muscle in 6-month-old WT mice were higher than those in 3-month-old WT mice, while they were lower in Cyp27b1 KO mice than those in age-matched WT mice, and did not significantly increase in 6-month-old Cyp27b1 KO mice compared with 3-month-old Cyp27b1 KO mice (Figure 1A, 1B). The muscle weight/body weight ratio and grip strength were not altered between 6- and 3-month-old WT mice, while they were significantly reduced in Cyp27b1 KO mice compared with age-matched WT mice, and especially reduced in 6-month-old Cyp27b1 KO mice (Figure 1C, 1D). These results imply that 1,25(OH)2D deficiency can lead to a decline of both muscle mass and performance resulting in sarcopenia.
Figure 1.
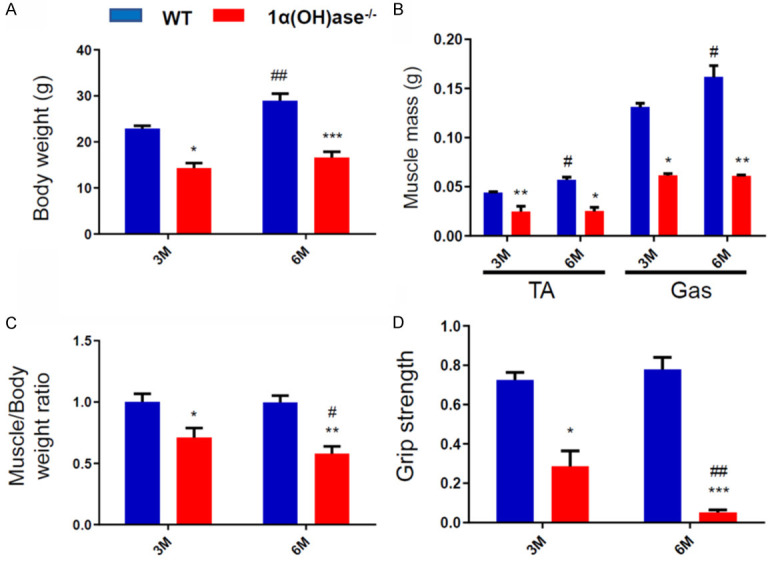
1,25(OH)2D deficiency leads to sarcopenia. (A) Body weight, (B) weight of tibialis anterior (TA) muscle and gastrocnemius (Gas) muscle, (C) muscle weight/body weight ratio, and (D) grip strength in 3- and 6-month-old WT and Cyp27b1 KO male mice. Values are means ± SEM of 6 determinations per group. *P<0.05; **P<0.01; ***P<0.001, compared with WT mice. #P<0.05; ##P<0.01, compared with 3-month-old genotype matched mice.
Sarcopenia induced by 1,25(OH)2D deficiency is related to muscle atrophy
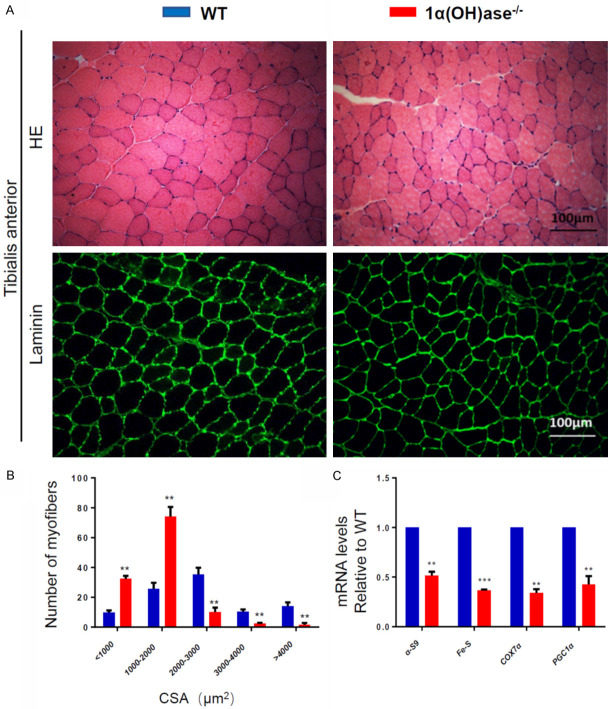
To assess if sarcopenia caused by 1,25(OH)2D deficiency is related to muscle atrophy, we compared the differences of CSA of myofibers between 6-month-old WT and Cyp27b1 KO male mice. The results showed that the number of myofibers with CSA<2000 μm2 in Cyp27b1 KO mice was significantly more than that in WT mice, while the number of myofibers with CSA>2000 μm2 in Cyp27b1 KO mice was significantly less than that in WT mice (Figure 2A, 2B). We also used real-time qRT-PCR to examine the changes of mitochondrial related gene expression levels in 6-month-old WT and Cyp27b1 KO mouse skeletal muscle tissue. We found that the expression of α-S9, Fe-S, Cox7α and PGC1α were significantly downregulated in skeletal muscle tissue of Cyp27b1 KO mice (Figure 2C). These results imply that sarcopenia induced by 1,25(OH)2D deficiency is related to muscle atrophy and decreased mitochondrial metabolic activity.
Figure 2.
Sarcopenia caused by 1,25(OH)2D deficiency is related to muscle atrophy. A. Representative micrographs of tibialis anterior muscle sections from 6-month-old WT and Cyp27b1 KO male mice stained with H&E (upper panel) and IF for laminin (bottom panel). B. CSA of myofibers (μm2). C. Mitochondrial-related gene expression levels in 6-month-old WT and Cyp27b1 KO mouse skeletal muscle tissue, including α-S9, Fe-S, COX7α and PGC1α. Values are means ± SEM of 6 determinations per group. **P<0.01; ***P<0.001, compared with WT mice.
1,25(OH)2D deficiency inhibits antioxidant capacity of skeletal muscle tissue
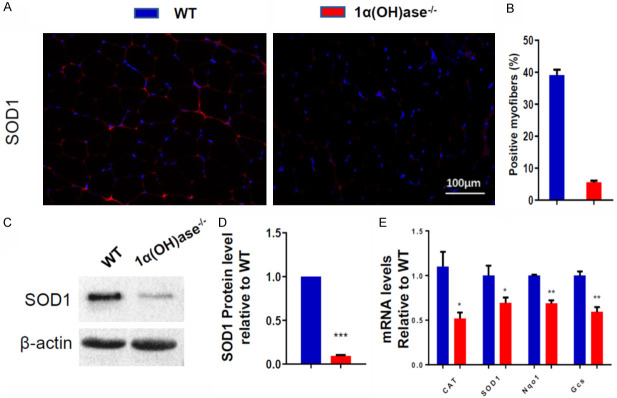
In order to determine if sarcopenia induced by 1,25(OH)2D deficiency is related to the decrease of antioxidant capacity of skeletal muscle tissue, the changes of antioxidant enzyme protein or mRNA expression levels in skeletal muscle tissue of 6-month-old Cyp27b1 KO and WT mice were examined. We found that the percentage of SOD1+ myofibers, the protein expression levels of SOD1 (Figure 3A-D) and mRNA expression levels of catalase, SOD1, Nqo1 and Gcs in skeletal muscle tissue (Figure 3E) were significantly decreased in Cyp27b1 KO mice compared with WT mice. These results suggest that sarcopenia induced by 1,25(OH)2D deficiency may be related to the decrease of antioxidant capacity of skeletal muscle tissue.
Figure 3.
1,25(OH)2D deficiency inhibits antioxidant capacity of skeletal muscle tissue. (A) Representative micrographs of tibialis anterior muscle sections from 6-month-old WT and Cyp27b1 KO male mice stained with IF for SOD1 and (B) SOD1 positive myofibers (%). (C, D) Western blots for SOD1protein expression in skeletal muscle tissue. (E) mRNA expression levels in skeletal muscle tissue of the antioxidant enzymes CAT, SOD1, Nqo1 and Gcs. Values are means ± SEM of 6 determinations per group. *P<0.05; **P<0.01; ***P<0.001, compared with WT mice.
1,25(OH)2D deficiency induces skeletal muscle cell senescence and SASP
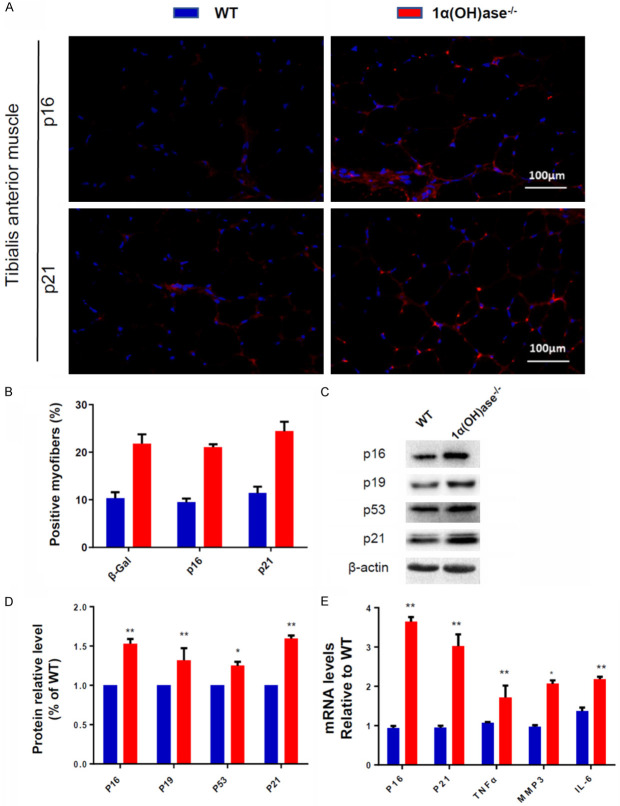
To determine if sarcopenia induced by 1,25(OH)2D deficiency is related to skeletal muscle cell senescence and SASP, we examined the changes of skeletal muscle cell senescence and SASP related parameters in 6-month-old male WT and Cyp27b1 KO male mice. We found that the percentage of p16 and p21 positive myofibers (Figure 4A, 4B), the expression of p16, p19, p21 and p53 protein levels (Figure 4C, 4D) and the expression of p16, p21, TNFα, IL-6 and MMP3 mRNA levels (Figure 4E) in Cyp27b1 KO mice were significantly higher than in WT mice. These results imply that sarcopenia induced by 1,25(OH)2D deficiency may be related to increased skeletal muscle cell senescence and SASP.
Figure 4.
1,25(OH)2D deficiency induces senescence and SASP in skeletal muscle tissue. (A) Representative micrographs of tibialis anterior muscle sections from 6-month-old WT and Cyp27b1 KO male mice stained with IF for p16 and p21 (B) p16 and p21 positive myofibers (%). (C, D) Western blots for p16, P19, p21 and p53 protein expression in skeletal muscle tissue. (E) mRNA expression levels of p16, p21, TNFα, IL-6 and MMP3 in skeletal muscle tissue. Values are means ± SEM of 6 determinations per group. *P<0.05; **P<0.01, compared with WT mice.
1,25(OH)2D deficiency inhibits skeletal muscle cell regeneration
To determine if 1,25(OH)2D deficiency inhibits the regeneration of skeletal muscle cells, we used a standard muscle injury model, and injected BaCl2 into TA muscle of WT and Cyp27b1+/- male mice. Seven days later, the regeneration ability of skeletal muscle cells was analyzed. We found that the numbers of newly formed regenerating centrally nucleated fibers (CNF) and the percentage of BrdU+ cells in Cyp27b1+/- mice were significantly lower than those in wild-type mice (Figure 5A-D). We also used real-time qRT-PCR to examined the changes of skeletal muscle differentiation-related gene expression levels in 6-month-old WT and Cyp27b1+/- mouse skeletal muscle tissue. The results showed that the gene expression levels of MyoD, MyHC and Myf5 were significantly downregulated in skeletal muscle tissue of Cyp27b1+/- mice (Figure 5E). These results suggest that 1,25(OH)2D deficiency inhibits the regeneration of skeletal muscle cells by inhibiting myogenesis.
Figure 5.
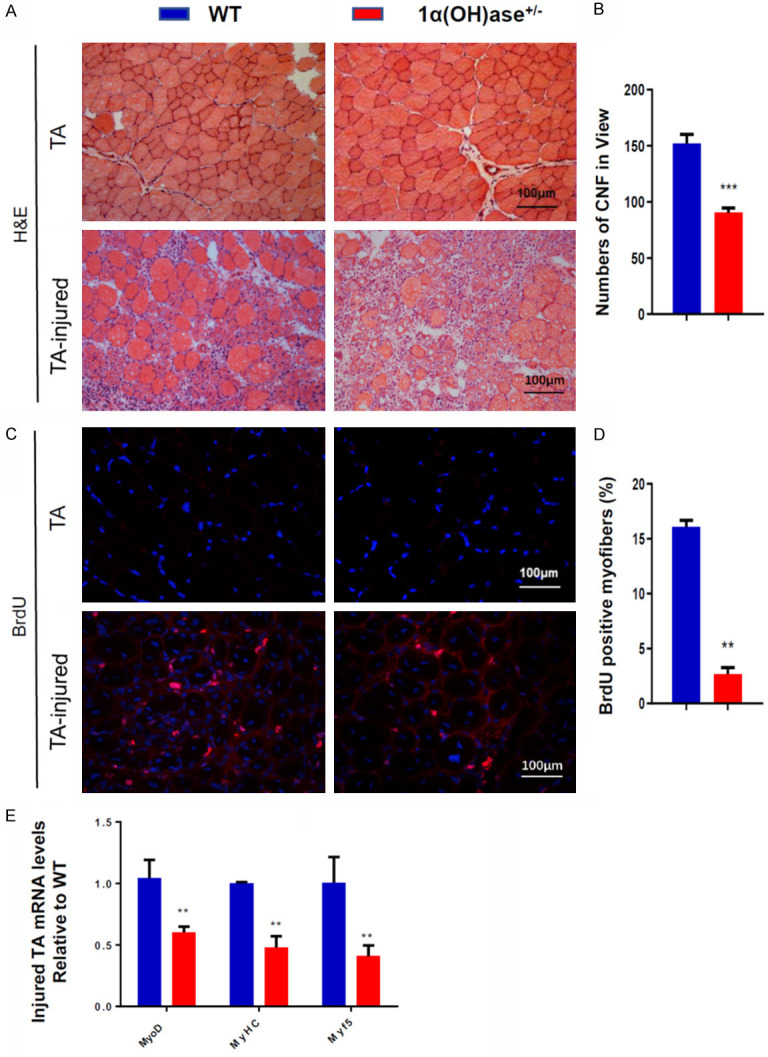
1,25(OH)2D deficiency inhibits skeletal muscle cell regeneration. A. Representative micrographs of tibialis anterior muscle sections without or with injected BaCl2 from 6-month-old WT and Cyp27b1+/- male mice stained with H&E. B. The numbers of newly formed regenerating CNF per view (n>3). C. Representative micrographs of tibialis anterior muscle sections without or with injected BaCl2 from 6-month-old WT and Cyp27b1+/- male mice immunostained for BrdU. D. BrdU positive myofibers. E. mRNA expression levels of MyoD, MyHC and Myf5 in skeletal muscle tissue at 7 days after injected BaCl2. Values are means ± SEM of 6 determinations per group. **P<0.01, compared with WT mice.
Discussion
Our recent studies demonstrated that 1,25(OH)2D deficiency accelerates age-related osteoporosis [19,21,22,26]. To investigate if 1,25(OH)2D deficiency also induced age-related sarcopenia, we compared the skeletal muscular phenotypes of WT and Cyp27b1 KO male mice at 3 and 6 months of age. We found that 1,25(OH)2D deficiency resulted in the reduction of muscle mass, grip strength and muscle fiber size with decreased mitochondrial metabolic activity, indicating that 1,25(OH)2D deficiency can indeed lead to the occurrence of sarcopenia. Studies on the pathogenetic mechanisms demonstrated that 1,25(OH)2D deficiency reduced antioxidant capacity of skeletal muscle tissue, increased skeletal muscle cell senescence and SASP and inhibited skeletal muscle cell regeneration. 1,25(OH)2D deficiency therefore accelerated muscle aging, resulting in muscle fiber atrophy and subsequent muscle loss, and consequently Cyp27b1 deficient mice appears to be an appropriate animal model of age-related sarcopenia.
Previous preclinical studies with VDR ablation [29] and our current study with Cyp27b1 KO mice have both demonstrated decreased muscle mass, grip strength and smaller fibers, and clinical studies have also indicated that vitamin D deficiency is associated with muscle fiber atrophy, increased risk of muscle loss and falls [30]. However, results from clinical trials on the role of vitamin D in sarcopenia remain uncertain and conflicting. In a meta-analysis of elderly people with vitamin D deficiency involving 13 randomized controlled trials, it was found that daily supplementation of 800-1000 IU could improve lower limb strength and balance [31]. On the other hand, meta-analysis evaluation of 17 randomized controlled trials with 5072 participants showed that vitamin D supplementation in adults with a baseline 25(OH)D level >10 ng/ml had no significant effect on grip strength [32]. The conflicting results from clinical trials may be explained by experiments using Cyp27b1 knockout mice. Serum 25(OH)D levels are not decreased in Cyp27b1 KO mice relative to WT mice [17], however, Cyp27b1 KO mice display female infertility, male infertility, hypertension, premature aging, high incidence of multiple tumors and osteoporosis [17-27]. These results suggest that sufficient 1,25(OH)2D rather than 25(OH)D levels are necessary for the prevention of vitamin D deficiency-induced diseases. In human studies, as a result of declining renal function with age, a decrease in 1,25(OH)2D production by approximately 50% has been reported [33]. In a recent study, we found that the protein expression levels of Cyp27b1 in kidney, intestine and bone of 3-, 9- and 18-month-old WT mice were progressively down-regulated [21]. These results support the thesis that Cyp27b1 expression levels may be too low to synthesize sufficient 1,25(OH)2D and supplementation of vitamin D alone in the elderly cannot prevent the occurrence and development of aging related diseases, including age-related sarcopenia.
Our recent studies imply that oxidative stress plays a crucial role in aging and age-related osteoporosis induced by 1,25(OH)2D deficiency [19,21], and mitochondrial dysfunction and oxidative stress have been reported to play important roles in age-related muscle atrophy [34,35]. Indeed, elderly SOD1 knockout mice show increased mitochondrial H2O2 production, resulting in muscle atrophy [36]. We therefore asked if sarcopenia induced by 1,25(OH)2D deficiency is related to decreased antioxidant capacity of skeletal muscle tissue. We found that expression levels of SOD1 mRNA and protein, and of Cat, Nqo1 and Gcs mRNA were significantly down-regulated in 1,25(OH)2D deficient skeletal muscle tissue. These studies therefore support our observation that that oxidative stress plays a critical role in the pathogenesis of 1,25(OH)2D deficiency-induced sarcopenia.
Cell cycle arrest can be induced in a variety of ways, many of which activate 2 main regulatory axes, p16-Rb and p19-p53-p21 [37]. Our recent studies demonstrated that 1,25(OH)2D deficiency induced aging and age-related bone loss by increasing senescent cells and SASP [19,26], and previous reports have linked senescence and SASP with muscle aging [38]. Sarcopenia occurred in rapidly aging BubR1H/H mice, and after genetically removing senescent cells, an increase in muscle fiber diameter was found [39], clinically, a cross sectional study showed that the serum TNF-α and IL-6 were higher in elderly sarcopenia patients [40]. In the present studies we confirmed that the expression levels of p16, p19, p53 and p21 and TNFα, IL-6 and MMP3 were increased in 1,25(OH)2D deficient muscular tissue. Therefore, our results are consistent with the thesis that 1,25(OH)2D deficiency can induce sarcopenia by increasing skeletal muscle cell senescence and SASP.
Skeletal muscle has excellent regenerative ability, which depends on muscle stem cells, but this regenerative ability decreases with age after injury [41]. We therefore employed an injury model to examine the effect of 1,25(OH)2D deficiency on skeletal muscle regeneration, and found that the numbers of newly formed regenerating CNF and the percentage of BrdU+ cells were significantly reduced. The durability of muscle regeneration requires effective satellite cell expansion after injury. Their differentiation produces myoblasts that can rebuild damaged fibers and their self-renewal to supplement the muscle stem cell bank for subsequent injury and repair [6]. Satellite cell activation is partly mediated by the induced expression of MyoD and Myf5 [42,43], and in our regenerating CNF we found down-regulated expression levels of MyoD, Myf5 and MyHC in 1,25(OH)2D deficient mice Therefore, our results indicate that 1,25(OH)2D deficiency inhibits the regeneration of skeletal muscle cells by inhibiting the proliferation and differentiation of skeletal muscle cells.
In summary, results from this study indicate that 1,25(OH)2D deficiency can induce the occurrence of age-related sarcopenia by initiating oxidative stress, skeletal muscular cell senescence and SASP, and inhibiting skeletal muscle regeneration. Cyp27b1 KO mice can be used as an animal model of age-related sarcopenia and may be considered as a potential tool to study the pathogenesis of age-related sarcopenia and to test intervention measures for sarcopenia.
Acknowledgements
This work was supported by grants from National Key R&D Program of China (2018YFA0800800 to DM) and from the National Natural Science Foundation of China (81730066 to DM, 31972901, 31571430 to JY) and by a grant to DG from the Canadian Institutes of Health Research.
Disclosure of conflict of interest
None.
References
- 1.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez-Rodriguez D, Marco E, Cruz-Jentoft AJ. Defining sarcopenia: some caveats and challenges. Curr Opin Clin Nutr Metab Care. 2020;23:127–132. doi: 10.1097/MCO.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 3.Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. 2017;13:340–347. doi: 10.1038/nrrheum.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han A, Bokshan SL, Marcaccio SE, DePasse JM, Daniels AH. Diagnostic criteria and clinical outcomes in sarcopenia research: a literature review. J Clin Med. 2018;7:70. doi: 10.3390/jcm7040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Gupta SK, Sukumar R. Hyperparathyroidism and malnutrition with severe vitamin D deficiency. World J Surg. 2009;33:2303–2313. doi: 10.1007/s00268-009-0044-0. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Laughlin GA, Li H, Nielson CM, Wang PY, Dam TT, Cauley JA, Ensrud KE, Stefanick ML, Lau E, Hoffman AR, Orwoll ES Osteoporotic Fractures in Men (MrOS) Research Group. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2012;27:2306–2313. doi: 10.1002/jbmr.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kweder H, Eidi H. Vitamin D deficiency in elderly: risk factors and drugs impact on vitamin D status. Avicenna J Med. 2018;8:139–146. doi: 10.4103/ajm.AJM_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Gutierrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr. 2011;21:295–302. doi: 10.1053/j.jrn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 10.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66–69. doi: 10.1016/j.nut.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 13.Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int. 2014;2014:121254. doi: 10.1155/2014/121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 15.Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2 vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology. 2011;152:2976–2986. doi: 10.1210/en.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 17.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Yang R, Qiao W, Yuan X, Wang S, Goltzman D, Miao D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int J Cancer. 2018;143:368–382. doi: 10.1002/ijc.31317. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell. 2019;18:e12951. doi: 10.1111/acel.12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 21.Qiao W, Yu S, Sun H, Chen L, Wang R, Wu X, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D insufficiency accelerates age-related bone loss by increasing oxidative stress and cell senescence. Am J Transl Res. 2020;12:507–518. [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Qiao W, Cui M, Yang C, Wang R, Goltzman D, Jin J, Miao D. The polycomb protein Bmi1 plays a crucial role in the prevention of 1,25(OH)2 D deficiency-induced bone loss. J Bone Miner Res. 2020;35:583–595. doi: 10.1002/jbmr.3921. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Chen L, Zhang W, Wang R, Goltzman D, Miao D. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015;308:E51–62. doi: 10.1152/ajpendo.00076.2014. [DOI] [PubMed] [Google Scholar]
- 24.Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1,25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol Endocrinol Metab. 2010;299:E928–935. doi: 10.1152/ajpendo.00378.2010. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D. Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improves mineral ion homeostasis in mice that are homozygous for both the 1alpha-hydroxylase and parathyroid hormone null alleles. Endocrinology. 2006;147:4801–4810. doi: 10.1210/en.2006-0403. [DOI] [PubMed] [Google Scholar]
- 26.Yang R, Chen J, Zhang J, Qin R, Wang R, Qiu Y, Mao Z, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis. Aging Cell. 2020;19:e13095. doi: 10.1111/acel.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Yi X, Yao Z, Chakkalakal JV, Xing L, Boyce BF. TNF receptor-associated factor 6 mediates TNFalpha-induced skeletal muscle atrophy in mice during aging. J Bone Miner Res. 2020;35:1535–1548. doi: 10.1002/jbmr.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girgis CM, Cha KM, Houweling PJ, Rao R, Mokbel N, Lin M, Clifton-Bligh RJ, Gunton JE. Vitamin D receptor ablation and vitamin D deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int. 2015;97:602–610. doi: 10.1007/s00223-015-0054-x. [DOI] [PubMed] [Google Scholar]
- 30.Abiri B, Vafa M. Vitamin D and muscle sarcopenia in aging. Methods Mol Biol. 2020;2138:29–47. doi: 10.1007/978-1-0716-0471-7_2. [DOI] [PubMed] [Google Scholar]
- 31.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 32.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–871. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42:319–332. doi: 10.1016/j.ecl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coen PM, Musci RV, Hinkley JM, Miller BF. Mitochondria as a target for mitigating sarcopenia. Front Physiol. 2018;9:1883. doi: 10.3389/fphys.2018.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lourenco Dos Santos S, Baraibar MA, Lundberg S, Eeg-Olofsson O, Larsson L, Friguet B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015;5:267–274. doi: 10.1016/j.redox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qaisar R, Bhaskaran S, Premkumar P, Ranjit R, Natarajan KS, Ahn B, Riddle K, Claflin DR, Richardson A, Brooks SV, Van Remmen H. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle. 2018;9:1003–1017. doi: 10.1002/jcsm.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 38.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 39.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur J Med Res. 2017;22:25. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol. 2002;27:19–41. doi: 10.1139/h02-002. [DOI] [PubMed] [Google Scholar]
- 42.Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112:2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- 43.Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]