Abstract
Background: Acute respiratory distress syndrome (ARDS) is a severe form of respiratory failure characterized by altered lung mechanics and poor oxygenation. Bronchial hyperresponsiveness has been reported in ARDS survivors and animal models of acute lung injury. Whether this hyperreactivity occurs at the small airways or not is unknown. Objective: To determine ex-vivo small airway reactivity in a rat model of acute lung injury (ALI) by hydrochloric acid (HCl) instillation. Methods: Twelve anesthetized rats were connected to mechanical ventilation for 4-hour, and randomly allocated to either ALI group (HCl intratracheal instillation; n=6) or Sham (intratracheal instillation of 0.9% NaCl; n=6). Oxygenation was assessed by arterial blood gases. After euthanasia, tissue samples from the right lung were harvested for histologic analysis and wet-dry weight ratio assessment. Precision cut lung slice technique (100-200 μm diameter) was applied in the left lung to evaluate ex vivo small airway constriction in response to histamine and carbachol stimulation, using phase-contrast video microscopy. Results: Rats from the ALI group exhibited hypoxemia, worse histologic lung injury, and increased lung wet-dry weight ratio as compared with the sham group. The bronchoconstrictor responsiveness was significantly higher in the ALI group, both for carbachol (maximal contraction of 84.5±2.5% versus 61.4±4.2% in the Sham group, P<0.05), and for histamine (maximal contraction of 78.6±5.3% versus 49.6±5.3% in the Sham group, P<0.05). Conclusion: In an animal model of acute lung injury secondary to HCL instillation, small airway hyperresponsiveness to carbachol and histamine is present. These results may provide further insight into the pathophysiology of ARDS.
Keywords: ARDS (acute respiratory distress syndrome), ALI (acute lung injury), small airway hyperresponsiveness, acid aspiration
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a severe form of acute lung injury (ALI) characterized by increased permeability of the alveolo-capillary barrier, edema with high protein content, hyaline membrane formation, and surfactant inactivation, determining an inadequate ventilation perfusion (V/Q) ratio [1].
Small airway remodeling [2] and dysfunction have been observed in ARDS patients [3-5], which may potentially cause changes in small airway reactivity and resistance. In normal conditions, due to the large global cross-sectional area, small airways contribute to 20% of total airway resistance [6] and might play a key role in the adequate distribution of ventilation. Airway hyperresponsiveness to methacholine has been reported in patients who survived the ARDS [7], as well as in animal models of ALI [8,9]. Nevertheless, these studies were not able to differentiate whether increased reactivity occurred at large or small airways. Due to technical limitations, the contribution of small airways to general airway reactivity remains uncertain. In this context, precision cut lung slices (PCLS), a technique widely used to study intrapulmonary blood vessels [10], allows to isolate small airways, perform stimulation and ex vivo analysis maintaining the architectural relationship between tissues and cell types involved in immune responses and bronchoconstriction [11-14]. This technique has been used to evaluate small airways of models of chronic obstructive pulmonary disease (COPD) and asthma [12,15]. However, according to our knowledge, it has never been used in ALI models. In the present study, we aimed to evaluate the ex vivo reactivity of small airways, in an experimental model of ALI.
Materials and methods
This protocol was approved by the Scientific Ethics Committee for the Care of Animals and Environment of the Pontificia Universidad Católica de Chile (N° 170801004) and by the Institutional Animal Care and Use Committee (IACUC) of Facultad de Medicina, Universidad de Chile (CBA#0614 FMUCH).
Animal preparation
Twelve male Sprague Dawley rats (300-350 grams) were anesthetized with 10 mg/kg xylazine and 100 mg/kg ketamine intraperitoneally (IP). After anesthesia, a 14 G cannula connected to a pressure transducer was inserted into the trachea. Then animals were connected to a mechanical ventilator for small animals (series SAR-830, CWE Inc., PA, USA). Ventilation strategy included tidal volume of 7 ml/kg, positive end-expiratory pressure (PEEP) of 2 cmH2O, inspiratory to expiratory ratio of 1:2, respiratory rate of 95 breaths per minute, and inspired oxygen fraction of 100%. In addition, a 22 G catheter was inserted into the right carotid artery for blood extraction and blood pressure monitoring. Anesthesia was administered through intravenous boluses of ketamine (50 mg/kg every 30 minutes), and muscle paralysis was achieved by intravenous boluses of rocuronium bromide 0.7 mg/kg every 20 minutes. Animals were kept at 37°C throughout the whole experiment.
Study protocol and experimental groups
Following a stabilization period of 30 minutes, rats were randomly allocated into 2 groups: (i) Sham (n=6), which received intratracheal instillation of 2 ml/kg of saline solution (0.9%); and (ii) ALI group (n=6), treated with intratracheal instillation of 2 ml/kg of HCl 0.1 N and pH 1.5, as previously described [16,17]. Thereafter, animals were mechanically ventilated for 4 hours according to the ventilation strategy described above. Arterial blood samples (100 μl) were obtained every 60 minutes for blood gas analysis (i-STAT, Abbott Laboratories, Abbott Park, IL, USA). At the end of the study period, animals were euthanized with an anesthetic overdose. Middle and accessory right lung lobes were extracted to determine the lung wet-dry weight ratio. The right lower lobe was used for histological analysis, while the left lung was excised for PCLS and ex vivo experiments. Figure 1 illustrates the study protocol and tissue sampling diagram.
Figure 1.
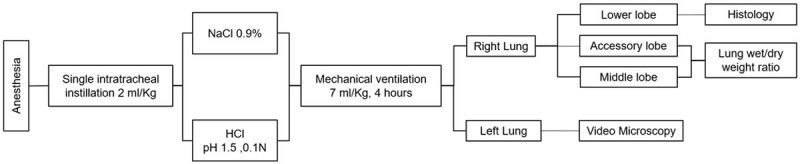
Diagram showing animal groups, intervention tissue sampling, and analysis.
Histology
Lung tissue was harvested and fixed with 10% buffered formaldehyde solution in cold formalin for histological analysis. Sections of 5 mm thick were obtained and stained with hematoxylin and eosin for analysis by light microscopy (0= not present and 4= severe) that included alveolar disruption, alveolar edema, neutrophil infiltration, and hemorrhage, as previously described [18]. Histological assessment was performed by an experienced investigator in lung pathology.
Precision cut lung slices (PCLS) preparation
The left lung was filled with 2% low-melting agarose solution at 37°C, with a volume of 2.25% of body weight. Then agarose was solidified at 4°C, and a vibratome (CompresstomeTM VF-300, Precisionary Instruments Inc.) was used to prepare 150 μm PCLS. Slices were incubated in 2 ml of Dulbecco’s modified Eagle medium and stored in an incubator (Incubator CO2 Precision Scientific 5410) at 37°C, 5% CO2, for 12 hours. Then, slices were washed and placed in a bath with Hanks’ Balanced Salt Solution (HBSS). Those with 100 to 200 µm of airway diameter and positive ciliary movement were selected for ex vivo experiments. At least 3 lung slices per animal were used for stimulation with carbachol (CCh) and another 3 for stimulation with histamine. Perfusion solution was changed every 3 minutes, starting with HBSS. For assessment of carbachol and histamine dose-dependent bronchoconstriction, a range from 0.001 to 100 μM was applied every 3 min at each concentration. A phase-contrast optical microscope connected to a camera (Digital Camera Type Color CMOS SXY-M90) was used, and pictures were recorded at 0.5 Hz (software S-VIEWER). Images were analyzed with the Image J software (version 1.52a). The initial lumen (baseline) was considered as the average of the first 90 images of each experiment. Then the percentage of airway lumen obtained at the end of each concentration of CCh or histamine was obtained in proportion to the baseline lumen (number of total pixels was considered as 100%).
Statistics
Statistical analysis was performed using GraphPad Prism 7. Mann-Whitney and Kruskal-Wallis tests were performed to determine the differences between groups. Friedman test was used to determine differences within groups, and Dunn’s test was used for post-hoc comparisons. For comparison of dose-response curves and exponential curves between groups, the F-test was used. The concentration-response curve is represented as mean ± SE and all other values as median and interquartile ranges (IQR). The sample size was calculated based on data from a previous study in a murine model of ozone-induced hyper-responsiveness in which healthy animals exhibited a maximal bronchoconstriction to carbachol of 60±15% with the PCLS technique, while animals exposed to ozone exhibited a maximal bronchoconstriction of 85±15% [12]. We calculated that 6 animals per group would be required in our study to demonstrate a similar difference with a power of 0.8 and a two-sided error of 0.05.
Results
Lung injury markers
At the end of 4-hour study period the ALI group exhibited a significant alteration in gas exchange and increased peak airway pressure (Pawpeak) compared with the Sham group (PaO2/FiO2 of 117 [81-135] vs. 274 [255-396], P=0.02; PaCO2 of 62 [53-70] mmHg vs. 43 [38-47] mmHg, P=0.007; Pawpeak 17.8 [17.8-18.5] vs. 13.2 [12.0-14.7] cmH2O; P=0.004) (Figure 2). The lung wet-dry weight ratio was significantly higher in the ALI group in middle and accessory lobe (7.9 [5.8-8.7] vs. 5.4 [4.9-5.9]; P=0.03 and 7.15 [6.99-7.64] vs. 5.84 [4.96-6.25]; P=0.002, respectively) (Figure 3A). Histological analysis showed that the ALI group in comparison with the Sham group had higher hemorrhage (2 [1-2.75] vs. 0.25 [0-1.25]; P=0.0281), neutrophil infiltration (1.32 [1.19-1.63] vs. 0 [0-0.63]; P=0.0043), alveolar edema (3.75 [2.5-4] vs. 1.5 [0.75-2]; P=0.026) and a greater alveolar disruption trend (1.75 [1-2] vs. 1 [0-1.25]; P=0.0584) (Figure 3B and 3C). No differences were found in heart rate and blood pressure between the groups.
Figure 2.
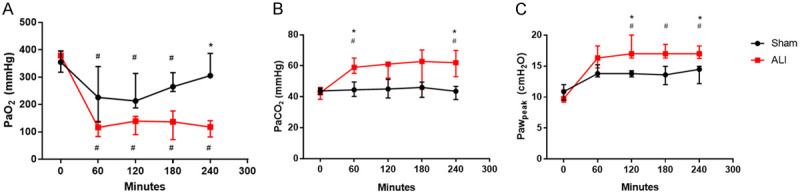
HCl instillation induces impaired gas exchange and pulmonary mechanics. (A) Partial pressure of O2 (PaO2), (B) Partial pressure of CO2 (PaCO2) and (C) Peak airway pressure (Pawpeak) in 4 hours of experimental period. #P<0.05, compared to baseline of their group; *P<0.05, ALI compared to Sham. ALI: Acute Lung Injury.
Figure 3.
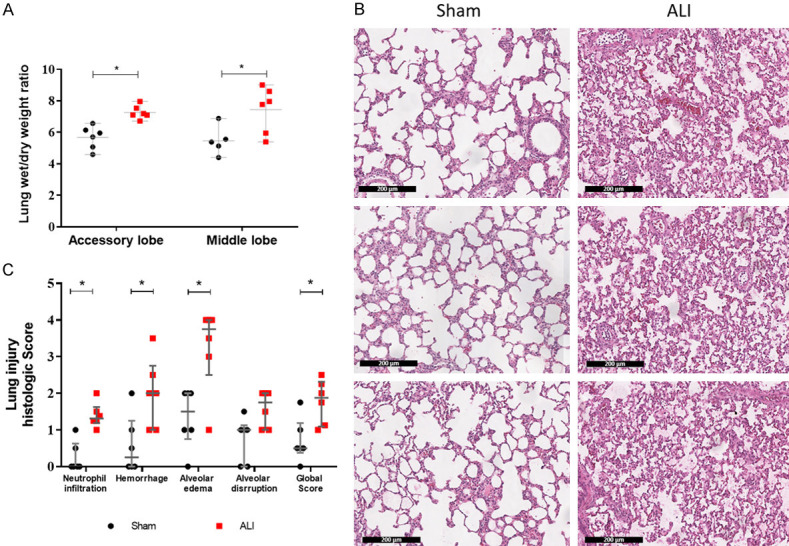
HCl instillation induces severe acute lung injury. A. Pulmonary edema represented by lung wet/dry weight ratio. B. Representative hematoxylin and eosin stain from 3 rats, left Sham and right ALI samples (scale 200 µm). C. Semiquantitative score for lung injury by a semiquantitative score based on severity (0= not present, 4= severe) in 5 regions of right lower lobe. Values are represented as median and interquartile ranges. #P<0.05, compared to baseline of their group; *P<0.05, ALI compared to Sham. ALI: Acute Lung Injury.
Small airway responsiveness to carbachol and histamine
An increase in small airway responsiveness to CCh was observed in ALI compared to the Sham group (Figure 4 and Supplementary Material). Airway contraction was detected at concentrations above 0.1 µM of CCh in both groups, and reached a maximal response at 10 µM, with higher contraction in the ALI group (84.5±2.5% for Ali group vs. 61.4%±4.2% for Sham group) (P<0.05) (Figure 4B). See the additional video file for more details.
Figure 4.
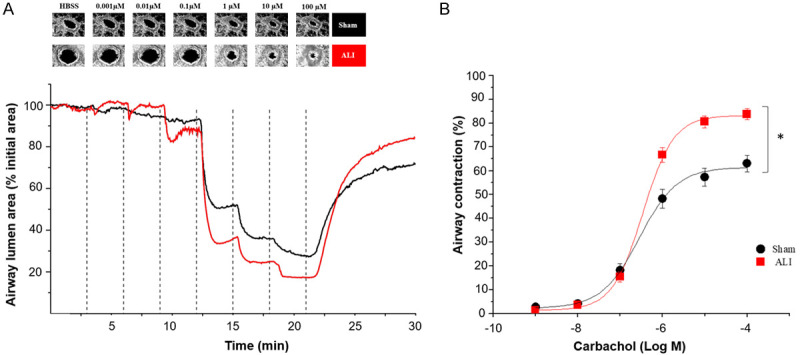
Acute lung injury induces small airway hyperresponsiveness to carbachol in precision cut lung slices (PCLS). A. Representative microscopic images of a lung slice showing contraction of a single airway treated with increasing concentrations of carbachol (from 0.001 to 100 micromolar) and graphical representation of the airway area (percentage relative to initial area). B. Concentration-response curve calculated by percentage of airway contraction with respect to the baseline. 37 lung slices from sham group and 36 from ALI group were used. Data are represented in mean ± SE. *P<0.05. Black line: Sham group, red line: ALI group. ALI: Acute Lung Injury; HBSS: Hanks’ Balanced Salt Solution.
A similar pattern was observed with Histamine, in which airway contraction started at 10 µM, and the maximal response was observed at 100 µM. However, a plateau was not reached even with maximal histamine concentration applied (Figure 5). The maximal contraction was 78.6±5.3% in the ALI group compared to 49.6±5.3% in the Sham group (P<0.05) (Figure 5B).
Figure 5.
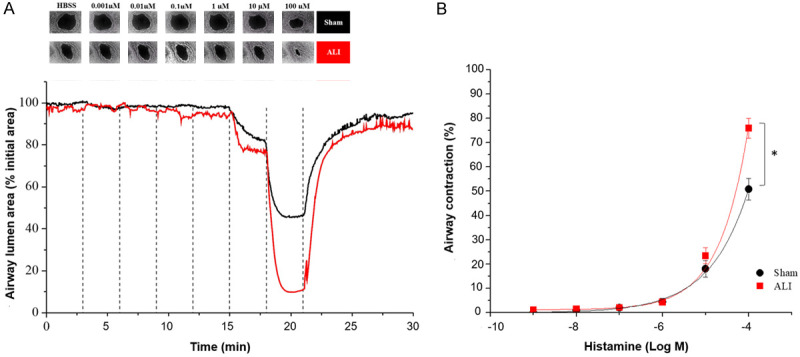
Acute lung injury induces small airway hyperresponsiveness to histamine in precision cut lung slices (PCLS). A. Representative microscopic images of a lung slice showing contraction of a single airway treated with increasing concentrations of histamine and graphical representation of the airway area (percentage relative to initial area). B. Concentration-response curve calculated by percentages of airway contraction with respect to the baseline. 30 lung slices from sham group and 29 from ALI group were used. Data are represented in mean ± SE. *P<0.05. Black line: Sham group, red line: ALI group. ALI: Acute Lung Injury; HBSS: Hanks’ Balanced Salt Solution.
Discussion
In the present study, we observed the presence of small airway hyperresponsiveness in an ALI rat model secondary to intratracheal instillation of HCl. Although previous studies had shown evidence of small airway dysfunction in ALI models and in ARDS patients [7-9], to our knowledge, this is the first study showing small airway hyperresponsiveness in ALI model assessed by PCLS.
Since gastric content aspiration constitutes an important cause of ARDS [19], the acid aspiration model by HCl instillation can be considered a representative clinical model to study this condition in a controlled and reproducible way [20]. The refractory hypoxemia and worse histologic lung injury observed in the ALI group were expected and explained by several reasons. The pH of HCl would result in the loss of microvascular integrity, followed by an acute neutrophilic inflammatory response with extravasation of fluid and proteins into the lungs, which finally results in pulmonary edema. All these characteristics were verified in our study by histology, wet-dry weight ratio, and gas exchange [21,22].
In terms of airway dysfunction, previous studies have shown airway hyperresponsiveness in a mice model of HCl aspiration by measuring global changes in respiratory system resistance in response to methacholine [9]. Our results confirm these findings and, by applying PCLS, we found that hyperresponsiveness occurs at the small airways.
Chronic obstructive pulmonary disease (COPD) is characterized by airway remodeling and hyperresponsiveness with limited expiratory flow and the consequent gas trapping. Maarsingh and colleagues using a PCLS technique in a guinea pig model of COPD and COPD patients demonstrated small airway hyperresponsiveness. They observed that the maximal contraction in the small airway from COPD patients was 22% greater than that of controls; a similar difference was found in the animal model [15]. Interestingly, we observed that the maximum contraction of the small airways of the rats with ALI was 23% greater than that of the sham group, a similar difference to what was found by Maarsingh et al. Likewise, the results found in our model are very similar to those of Cooper et al., who used PCLS from mice exposed to ozone, a classic model of airway hyperreactivity, observing similar dose-response curves from HCL rat model, with a maximum contraction close to 80% with 100 µM CCh [12].
Pathology of acute lung injury has been usually focused on alveolar damage with scarce information about airway involvement. Thus, small airways have been proposed to be a “silent zone” as their contribution to respiratory system resistance is rather low [4,6,23]. Nevertheless, structural analysis of small airways by histology has revealed epithelial denudation, inflammation, wall thickening, and extracellular matrix remodeling, both in ALI animal models and in ARDS patients [2,4,24]. The structural and functional alterations described in small airways may be implicated in the hyperresponsiveness observed in the present study.
Expiratory flow limitation with the presence of intrinsic positive end-expiratory pressure (PEEPi) and airway closure has been consistently reported in a high proportion of ARDS patients [3,5,25-27] and ALI models [28]. Although this phenomenon has been attributed to edema (29) and instability of airway walls [29-31], the possible relation with hyperresponsiveness has not been explored. Farrow et al. studied a group of asthmatic patients with single-photon emission computed tomography before and after a methacholine test and observed that airway hyperresponsiveness is directly implicated in airway closure (39). Whether small airway hyperresponsiveness is involved in the airway closure phenomenon of ARDS patients is still unknown.
Our study presents limitations. Unfortunately, our ventilator setting did not allow us to calculate airway or lung resistance or to detect airway closure, which could have contributed to better interpretation of the mechanical relevance of our findings. In addition, we only studied one specific model of ALI. Although HCl induces diffuse alveolar damage, it may also cause direct injury to the airways. However, small airway inflammation and epithelial injury are consistently observed in most ALI models and in ARDS patients, which suggests that hyperreactivity may also be present.
Conclusions
We report for the first time using an animal model for acute lung injury by HCl exposure, small airway hyperresponsiveness to carbachol and histamine. Nevertheless, further studies using PCLS in different ARDS models, as well as in lung tissues obtained from ARDS patients, are needed to determine the clinical relevance of the small airway hyperresponsiveness.
Acknowledgements
Mauricio Henriquez and Alejandro Bruhn acknowledge support from CONICYT through grants, URG-035/18, FONDECYT # 1140468, and FONDECYT # 1161556. Roque Basoalto and Yorschua Jalil acknowledge partial support from CONICYT-PFCHA/Doctorado Nacional 2020-folio 2120175 and 2019-folio 21191025, respectively.
Disclosure of conflict of interest
None.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ALI
Acute lung injury
- PCLS
Precision cut lung slices
- V/Q
Ventilation perfusion ratio
- PEEPi
Intrinsic positive end expiratory pressure
Supplementary Material
References
- 1.Radermacher P, Maggiore SM, Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- 2.Morales MM, Pires-Neto RC, Inforsato N, Lancas T, da Silva LF, Saldiva PH, Mauad T, Carvalho CR, Amato MB, Dolhnikoff M. Small airway remodeling in acute respiratory distress syndrome: a study in autopsy lung tissue. Crit Care. 2011;15:R4. doi: 10.1186/cc9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieillard-Baron A, Prin S, Schmitt JM, Augarde R, Page B, Beauchet A, Jardin F. Pressure-volume curves in acute respiratory distress syndrome: clinical demonstration of the influence of expiratory flow limitation on the initial slope. Am J Respir Crit Care Med. 2002;165:1107–1112. doi: 10.1164/ajrccm.165.8.2106104. [DOI] [PubMed] [Google Scholar]
- 4.Jain M, Sznajder JI. Peripheral airways injury in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:37–43. doi: 10.1097/MCC.0b013e3282f37976. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Del Sorbo L, Grieco DL, Shklar O, Junhasavasdikul D, Telias I, Fan E, Brochard L. Airway closure in acute respiratory distress syndrome: an underestimated and misinterpreted phenomenon. Am J Respir Crit Care Med. 2018;197:132–136. doi: 10.1164/rccm.201702-0388LE. [DOI] [PubMed] [Google Scholar]
- 6.Jain M, Sznajder JI. Bench-to-bedside review: distal airways in acute respiratory distress syndrome. Crit Care. 2007;11:206. doi: 10.1186/cc5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson DL, Goodman M, Spector SL, Petty TL. Long-term follow-up and bronchial reactivity testing in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis. 1978;117:449–454. doi: 10.1164/arrd.1978.117.3.449. [DOI] [PubMed] [Google Scholar]
- 8.Held HD, Uhlig S. Mechanisms of endotoxin-induced airway and pulmonary vascular hyperreactivity in mice. Am J Respir Crit Care Med. 2000;162:1547–1552. doi: 10.1164/ajrccm.162.4.9912079. [DOI] [PubMed] [Google Scholar]
- 9.Allen GB, Leclair TR, von Reyn J, Larrabee YC, Cloutier ME, Irvin CG, Bates JH. Acid aspiration-induced airways hyperresponsiveness in mice. J Appl Physiol (1985) 2009;107:1763–1770. doi: 10.1152/japplphysiol.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriquez M, Fonseca M, Perez-Zoghbi JF. Purinergic receptor stimulation induces calcium oscillations and smooth muscle contraction in small pulmonary veins. J Physiol. 2018;596:2491–2506. doi: 10.1113/JP274731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henjakovic M, Martin C, Hoymann HG, Sewald K, Ressmeyer AR, Dassow C, Pohlmann G, Krug N, Uhlig S, Braun A. Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen-sensitized mice represent a suitable alternative to in vivo studies. Toxicol Sci. 2008;106:444–453. doi: 10.1093/toxsci/kfn178. [DOI] [PubMed] [Google Scholar]
- 12.Cooper PR, Mesaros AC, Zhang J, Christmas P, Stark CM, Douaidy K, Mittelman MA, Soberman RJ, Blair IA, Panettieri RA. 20-HETE mediates ozone-induced, neutrophil-independent airway hyper-responsiveness in mice. PLoS One. 2010;5:e10235. doi: 10.1371/journal.pone.0010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Cooper PR, Damera G, Mukhopadhyay I, Cho H, Kehrl JH, Panettieri RA, Druey KM. β-agonist-associated reduction in RGS5 expression promotes airway smooth muscle hyper-responsiveness. J Biol Chem. 2011;286:11444–11455. doi: 10.1074/jbc.M110.212480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Kim Y, Park SJ, Bae B, Kang HR, Cho SH, Yoo HY, Nam JH, Kim WK, Kim SJ. Airway smooth muscle sensitivity to methacholine in precision-cut lung slices (PCLS) from ovalbumin-induced asthmatic mice. Korean J Physiol Pharmacol. 2015;19:65–71. doi: 10.4196/kjpp.2015.19.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maarsingh H, Bidan CM, Brook BS, Zuidhof AB, Elzinga CRS, Smit M, Oldenburger A, Gosens R, Timens W, Meurs H. Small airway hyperresponsiveness in COPD: relationship between structure and function in lung slices. Am J Physiol Lung Cell Mol Physiol. 2019;316:L537–L546. doi: 10.1152/ajplung.00325.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto S, Amaya F, Oh-Hashi K, Kiuchi K, Hashimoto S. Expression of neutral endopeptidase activity during clinical and experimental acute lung injury. Respir Res. 2010;11:164. doi: 10.1186/1465-9921-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safdar Z, Yiming M, Grunig G, Bhattacharya J. Inhibition of acid-induced lung injury by hyperosmolar sucrose in rats. Am J Respir Crit Care Med. 2005;172:1002–1007. doi: 10.1164/rccm.200501-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, Larsson A, Hedenstierna G, Bugedo G, Bruhn A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care. 2014;18:505. doi: 10.1186/s13054-014-0505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39:818–826. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocco PR, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730–740. [PubMed] [Google Scholar]
- 24.Gonzalez-Lopez A, Astudillo A, Garcia-Prieto E, Fernandez-Garcia MS, Lopez-Vazquez A, Batalla-Solis E, Taboada F, Fueyo A, Albaiceta GM. Inflammation and matrix remodeling during repair of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;301:L500–509. doi: 10.1152/ajplung.00010.2011. [DOI] [PubMed] [Google Scholar]
- 25.Koutsoukou A, Armaganidis A, Stavrakaki-Kallergi C, Vassilakopoulos T, Lymberis A, Roussos C, Milic-Emili J. Expiratory flow limitation and intrinsic positive end-expiratory pressure at zero positive end-expiratory pressure in patients with adult respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:1590–1596. doi: 10.1164/ajrccm.161.5.9904109. [DOI] [PubMed] [Google Scholar]
- 26.Koutsoukou A, Bekos B, Sotiropoulou C, Koulouris NG, Roussos C, Milic-Emili J. Effects of positive end-expiratory pressure on gas exchange and expiratory flow limitation in adult respiratory distress syndrome. Crit Care Med. 2002;30:1941–1949. doi: 10.1097/00003246-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Yonis H, Mortaza S, Baboi L, Mercat A, Guérin C. Expiratory flow limitation assessment in patients with acute respiratory distress syndrome. a reappraisal. Am J Respir Crit Care Med. 2018;198:131–134. doi: 10.1164/rccm.201711-2326LE. [DOI] [PubMed] [Google Scholar]
- 28.Broche L, Pisa P, Porra L, Degrugilliers L, Bravin A, Pellegrini M, Borges JB, Perchiazzi G, Larsson A, Hedenstierna G, Bayat S. Individual airway closure characterized in vivo by phase-contrast CT imaging in injured rabbit lung. Crit Care Med. 2019;47:e774–e781. doi: 10.1097/CCM.0000000000003838. [DOI] [PubMed] [Google Scholar]
- 29.Halpern D, Grotberg JB. Surfactant effects on fluid-elastic instabilities of liquid-lined flexible tubes: a model of airway closure. J Biomech Eng. 1993;115:271–277. doi: 10.1115/1.2895486. [DOI] [PubMed] [Google Scholar]
- 30.Heil M, Hazel AL, Smith JA. The mechanics of airway closure. Respir Physiol Neurobiol. 2008;163:214–221. doi: 10.1016/j.resp.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Coudroy R, Lu C, Chen L, Demoule A, Brochard L. Mechanism of airway closure in acute respiratory distress syndrome: a possible role of surfactant depletion. Intensive Care Med. 2019;45:290–291. doi: 10.1007/s00134-018-5501-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


