Abstract
Objective: To investigate the therapeutic effect of nalmefene combined with noninvasive positive-pressure mechanical ventilation (NIPPV) on elderly patients with chronic obstructive pulmonary disease (COPD) complicated with type II respiratory failure and to explore its influence on TGF-β1/Smads signaling pathway. Methods: In this retrospective study, data of 106 COPD patients with type II respiratory failure were collected and divided into a research group and a control group based on different treatment, with 53 cases in each group. Both groups were given NIPPV. Besides, the control group was treated with conventional therapy and the research group was treated with nalmefene for 7 days. The changes of heart rate, respiratory rate, clinical efficacy, pulmonary arterial pressure (PAP), serum inflammatory parameters, levels of TGF-β1/Smads signaling pathway related molecules and the incidences of adverse reactions of both groups were compared. Results: After treatment, the heart rate, respiratory rate, PAP, IL-6 and TNF-α concentrations in both groups were lower than those before treatment (P<0.05). The levels of PaO2 and SaO2 were higher and the levels of PaCO2 were lower than those before treatment in both groups (P<0.05). The expression levels of TGF-β1 and Smad2 in the research group were significantly lower than those in the control group (P<0.05). And all the above indicators in the research group were better than those in the control group after treatment (P<0.05). Besides, the incidence of adverse reactions in the research group was lower than that in the control group (P<0.05). Conclusion: Nalmefene combined with NIPPV can significantly improve the level of PaO2 and reduce inflammatory response in elderly COPD patients with type II respiratory failure, and the mechanism may be related to the inhibition of TGF-β1 and Smads expressions.
Keywords: Nalmefene, positive-pressure ventilation, chronic obstructive pulmonary disease, type II respiratory failure, TGF-β1/Smads, clinical efficacy
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease, and its pathogenesis is largely unclarified, which may be associated with multiple factors such as genetics, air pollution and airway inflammatory response. The typical symptom is respiratory airflow obstruction. In recent years, with the environmental deterioration and the expansion of elderly population, the number of patients with COPD has gradually increased [1]. COPD has a higher mortality rate compared with other diseases, which mainly due to frequent development of acute respiratory failure [2]. Positive-pressure mechanical ventilation is a common means to help patients restore respiratory function and maintain normal oxygen metabolism [3]. However, mechanical ventilation can only relieve the respiratory symptoms of patients and improve their blood-gas parameters and oxygenation index, it has some limitations on the effect of treating COPD patients with type II respiratory failure. Therefore, the effective drug treatment is needed to relieve pulmonary inflammation and improve clinical efficacy [4,5].
Nalmefene is an opioid receptor antagonist. Previous studies have confirmed that opioid receptor antagonists can significantly improve respiratory function in patients with respiratory diseases such as COPD [6]. Chen et al. observed the effect of opioid receptor antagonist combined with positive-pressure mechanical ventilation in COPD patients with type II respiratory failure for 3 days [7], and the results showed that research group had significantly better blood-gas indicators, dyspnea score and other indicators than control group. However, it is not clear whether Nalmefene works through inhibition of airway inflammation. It has been found that the TGF-β1/Smads signaling pathway plays an important role in the development and exacerbation of COPD [8]. TGF-β1 is highly expressed in COPD patients, and TGF-β can induce increased Smad2 and Smad4 expressions in bronchial epithelial cells, promote their mediated inflammatory response, induce the remodeling of airway and lung tissue in COPD patients and accelerate the progression of COPD. In this retrospective study, clinical data of 106 patients diagnosed with COPD complicated with type II respiratory failure were selected from our hospital to verify the effectiveness of the combined therapy and whether it was effective on the expressions of TGF-β1 and Smads in COPD patients.
Materials and methods
General information
A total of 106 patients with COPD complicated with type II respiratory failure who received treatment in our hospital from January 2019 to June 2020 were selected in this study, and the clinical data of these patients were retrospectively analyzed. All patients met the diagnostic criteria for COPD with type II respiratory failure based on the Guidelines for the Diagnosis and Treatment of COPD [9]. Patients were divided into a research group and a control group, with 53 cases in each group. The control group was treated with positive-pressure ventilation and conventional therapy and the research group was treated with nalmefene combined with NIPPV. The study was approved by the Ethics Committee of our hospital (No. 20190103).
Inclusion criteria: patients whose age ≥65 years old; patients who met the diagnostic criteria for severe type II respiratory failure (PaCO2≥80 mmHg, disturbance of consciousness and acidosis) [10]; patients whose family members were informed of this study and who participated voluntarily. Exclusion criteria: patients with severe respiratory tract infection; patients with heart, lung, kidney and other organ damage; patients with severe allergy to nalmefene; patients with primary immune and coagulation dysfunction.
Methods
Two groups of patients were treated with positive-pressure mechanical ventilation. A ventilator was applied in this study (FLEXOST20/ST25/ST30-H, Curative (Beijing) Medical Technology Co., Ltd., China). The exhalation/inspiratory pressure was adjusted according to the condition of patients. The initial value of exhalation pressure was 4 cm H2O, then it was gradually increased to 8 cm H2O; the inspiratory value was first set at 8 cm H2O, and it was gradually elevated to 18 cm H2O; oxygen flow was 3 L/min and respiratory rate 14-20 breaths/min. The ventilation was performed for at least 6 h per day, during which the vital signs of patients were monitored to maintain the saturation (SaO2) over 90%. If the condition deteriorated, the ventilation time would be prolonged per day and patients were treated for 7 days. If the condition improved during treatment, the ventilator could be removed, and patients were given other symptomatic and supportive treatment.
The control group was given the conventional treatment such as fluid replacement and correction of water-electrolyte balance. While in the research group, 1 mg/d of nalmefene (Haisco (Liaoning) Pharmaceutical Co., Ltd., China) was given to patients by intravenous infusion based on the treatment conducted in the control group. If the condition improved, treatment with the same dose would be continued; otherwise, the dose would be increased to 2 mg/d for 7 days.
Outcome measures
Main outcome measures
Overall clinical efficacy (whether symptoms such as obnubilation and cyanosis were relieved; whether the secondary outcome measures such as blood-gas indicators and heart rate reach the normal range), serum interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1) and Smad2 levels were measured.
Secondary outcome measures
Clinical data of heart rate, respiratory rate, pulmonary arterial pressure (PAP), blood-gas indicators and adverse reaction rate were collected.
Clinical efficacy was evaluated and it was divided into three categories: Significantly effective: after treatment, symptoms were significantly relieved, blood-gas indicators and heart rate reached the normal range; Effective: after treatment, the main outcome measures such as obnubilation and cyanosis, were significantly improved, and the secondary outcome measures such as blood-gas indicators and heart rate tended to be improved; Ineffective: no improvement in symptoms and indicators mentioned above [10]. Overall clinical efficacy equals (total cases - ineffective cases)/total cases *100%.
The heart rate, respiratory rate and PAP of patients in both groups were recorded before and after treatment. PAP was measured by color Doppler ultrasound [11].
The arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2) and blood oxygen saturation (SaO2) of patients from peripheral arterial blood before (before using the ventilator) and after treatment (right at the moment after removing the ventilator and receiving 7 days of treatment with oxygen flow of 3 L/min) were all collected [12].
Clinical data concerning related factors of patients before treatment and immediately after the treatment were collected. Blood samples (5 mL) were collected immediately from fasting patients before and after the treatment. After centrifugation, serum was drawn and stored at -80°C for future use. The levels of IL-6, TNF-α, TGF-β1 and Smad2 were measured by ELISA. The ELISA kits of IL-6 (No. 07011), TNF-α (No. 09122), TGF-β1 (No. 061211) and Smad2 (No. 121130) were purchased from Shanghai Xitang Biotechnology Co., Ltd., China. All indicators were detected according to the instructions of the kits.
Data concerning the occurrence of adverse reactions such as nausea, myalgia and chest distress from admission to 1 day before discharge were collected.
Statistical analysis
SPSS 23.0 software was used for data processing. The measurement data were expressed as mean ± standard deviation (x̅±s). After determination, the measurement data were conformed to the normal distribution. The paired sample t-test was applied for the intragroup comparison of indicators before and after treatment. The independent t-test was utilized for the comparison between groups at the same time point. The count data was expressed as % and detected by chi-square test. P<0.05 was considered statistically significant.
Results
Comparison of general information
There were no significant differences in gender, age, smoking history, course of COPD, course of respiratory failure, COPD severity classification, BMI and complications between two groups (P>0.05). See Table 1.
Table 1.
Comparison of general information
| Group | Control group (N=53) | Research group (N=53) | χ2 | P |
|---|---|---|---|---|
| Gender (Male/Female) | 32/21 | 30/23 | 0.155 | 0.693 |
| Age (years) | 70.7±5.2 | 70.9±5.3 | 0.196 | 0.845 |
| Smoking history | 26 | 24 | 0.151 | 0.697 |
| Course of COPD | 7.96±1.33 | 7.89±1.25 | 0.279 | 0.781 |
| Course of respiratory failure | 3.26±0.92 | 3.57±0.73 | 1.922 | 0.057 |
| COPD severity classification (II/III/IV, cases) | 15/28/10 | 12/29/12 | 0.533 | 0.766 |
| BMI (kg/m2) | 25.31±3.52 | 25.63±3.61 | 0.462 | 0.645 |
| Complications (cases) | ||||
| Hypertension | 38 | 32 | 1.514 | 0.218 |
| Coronary heart disease | 29 | 27 | 0.151 | 0.697 |
| Diabetes | 11 | 12 | 0.056 | 0.814 |
| Cerebral infarction | 10 | 9 | 0.064 | 0.800 |
| Other complications | 7 | 10 | 0.631 | 0.427 |
Note: COPD: chronic obstructive pulmonary disease.
Comparison of the clinical efficacy
The effective rate of treatment in the research group was higher than that of the control group (P<0.05). See Table 2.
Table 2.
Comparison of the clinical efficacy
| Groups | Number of cases | Ineffective | Effective | Significantly effective | Overall effective rate |
|---|---|---|---|---|---|
| Control group | 53 | 13 (24.53) | 16 (30.19) | 24 (45.28) | 40 (75.47) |
| Research group | 53 | 5 (9.43) | 18 (33.96) | 30 (56.60) | 48 (90.57) |
| χ2 | 4.340 | 4.283 | |||
| P | 0.114 | 0.038 | |||
Comparison of heart rate, respiratory rate and PAP
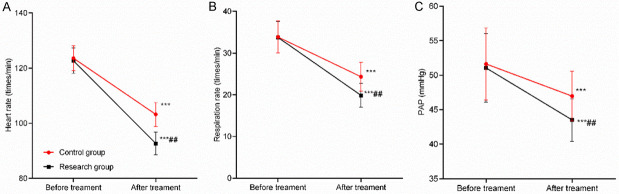
No significant difference was found in heart rate, respiratory rate and PAP before treatment (P>0.05). After treatment, those indicators were significantly reduced in both groups. While the research group showed better improvement in those indicators compared with the control group (P<0.05). See Table 3 and Figure 1.
Table 3.
Comparison of heart rate, respiratory rate and PAP (x̅±s)
| Index | Control group (n=53) | Research group (n=53) | t | P |
|---|---|---|---|---|
| Heart rate (times/min) | ||||
| Before treatment | 123.61±4.52 | 122.74±4.57 | 0.985 | 0.327 |
| After treatment | 103.21±4.34*** | 92.67±4.17*** | 12.749 | 0.001 |
| Respiratory rate (times/min) | ||||
| Before treatment | 33.85±3.87 | 33.76±3.79 | 0.121 | 0.904 |
| After treatment | 24.33±3.41*** | 19.87±2.87*** | 7.285 | 0.001 |
| PAP (mmHg) | ||||
| Before treatment | 51.63±5.24 | 51.05±4.97 | 0.585 | 0.560 |
| After treatment | 46.97±3.61*** | 43.52±3.09*** | 5.286 | 0.001 |
Note: Compared in the same group before and after treatment;
P<0.001.
PAP: pulmonary arterial pressure.
Figure 1.
Comparison of heart rate, respiratory rate and PAP before and after treatment. A: Comparison of heart rate before and after treatment; B: Comparison of respiratory rate before and after treatment; C: Comparison of PAP before and after treatment. Compared with that of the same group before treatment, ***P<0.001; compared with that of the control group after treatment, ##P<0.01.
Comparison of PaO2, SaO2 and PaCO2
There was no significant difference in PaO2, SaO2 and PaCO2 levels between two groups before treatment (P>0.05). After treatment, PaO2 and SaO2 levels were significantly increased in both groups and they were higher in the research group than those in the control group. PaCO2 level was enormously decreased after treatment in both groups and it was lower in the research group than that in the control group (all P<0.05). See Table 4.
Table 4.
Comparison of PaO2, SaO2 and PaCO2 (x̅±s)
| Index | Control group (n=53) | Research group (n=53) | t | P |
|---|---|---|---|---|
| PaO2 (mmHg) | ||||
| Before treatment | 54.52±5.31 | 54.94±5.29 | 0.408 | 0.684 |
| After treatment | 60.82±6.71*** | 64.97±7.52*** | 2.998 | 0.003 |
| SaO2 (%) | ||||
| Before treatment | 81.21±3.22 | 81.03±3.31 | 0.284 | 0.777 |
| After treatment | 91.52±2.85*** | 95.79±3.02*** | 7.486 | 0.001 |
| PaCO2 (mmHg) | ||||
| Before treatment | 85.82±4.64 | 86.43±4.71 | 0.672 | 0.504 |
| After treatment | 45.75±4.27*** | 39.45±3.82*** | 8.005 | 0.001 |
Note: Compared in the same group before and after treatment;
P<0.001.
Comparison of serum IL-6 and TNF-α
There was no significant difference in serum IL-6 and TNF-α levels between patients of two groups before treatment (P>0.05). The levels of serum IL-6 and TNF-α were significantly decreased after treatment in both groups and they were lower in the research group than those in the control group (P<0.05). See Table 5.
Table 5.
Comparison of serum IL-6 and TNF-α (x̅±s, pg/mL)
| Index | Control group (n=53) | Research group (n=53) | t | P |
|---|---|---|---|---|
| IL-6 | ||||
| Before treatment | 30.25±3.82 | 30.51±3.91 | 0.346 | 0.730 |
| After treatment | 23.49±3.25*** | 16.52±2.57*** | 12.247 | 0.001 |
| TNF-α | ||||
| Before treatment | 97.82±10.22 | 97.11±10.07 | 0.361 | 0.719 |
| After treatment | 63.94±5.93*** | 52.16±5.12*** | 10.946 | 0.001 |
Note: Compared in the same group before and after treatment;
P<0.001.
IL-6: interleukin-6; TNF-α: tumor necrosis factor-α.
Comparison of serum TGF-β1 and Smad2 expression levels
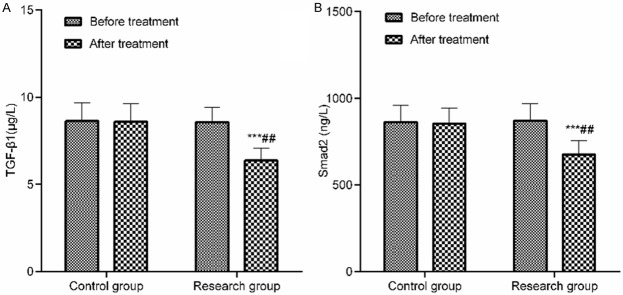
There was no statistically significant difference in the expression levels of serum TGF-β1 and Smad2 between two groups before treatment (P>0.05). The TGF-β1 and Smad2 expression levels in the research group decreased significantly after treatment and they were lower than those in the control group (P<0.05). See Table 6 and Figure 2.
Table 6.
Comparison of serum TGF-β1 and Smad2 expression levels
| Index | Control group (n=53) | Research group (n=53) | t | P |
|---|---|---|---|---|
| TGF-β1 (ug/L) | ||||
| Before treatment | 8.63±1.06 | 8.59±1.03 | 0.197 | 0.844 |
| After treatment | 8.57±0.85 | 6.37±0.71*** | 14.461 | 0.001 |
| Smad2 (ng/L) | ||||
| Before treatment | 863.64±95.22 | 871.58±96.57 | 0.426 | 0.671 |
| After treatment | 854.16±90.63 | 675.64±81.22*** | 10.679 | 0.001 |
Note: Compared in the same group before and after treatment;
P<0.001.
TGF-β1: transforming growth factor-β1.
Figure 2.
Comparison of serum TGF-β1 and Smad2 expression levels before and after treatment. A: Comparison of serum TGF-β1 expression level before and after treatment; B: Comparison of serum Smad2 expression level before and after treatment. Compared with that of the same group before treatment, ***P<0.001; compared with that of the control group after treatment, ##P<0.01.
Comparison of incidence of adverse reactions
The incidence rate of adverse reactions in the research group was significantly lower than that in the control group (P<0.05). See Table 7.
Table 7.
Comparison of incidence of adverse reactions
| Group | Number of cases | Nausea | Chest distress | Myalgia | Incidence of adverse reactions |
|---|---|---|---|---|---|
| Control group | 53 | 3 (5.66) | 4 (7.55) | 3 (5.66) | 10 (18.87) |
| Research group | 53 | 0 (0.00) | 1 (1.89) | 1 (1.89) | 2 (3.77) |
| t | 3.087 | 1.889 | 1.039 | 6.014 | |
| P | 0.079 | 0.169 | 0.308 | 0.014 |
Discussion
The number of patients with COPD has increased in recent years, and COPD has become one of the major respiratory diseases threatening the health of the elderly [13]. During treatment of the elderly COPD patients with severe type II respiratory failure, it is essential to restore respiratory function as soon as possible, maintain normal metabolism, and avoid multiple organ dysfunction caused by acidosis [14]. Mechanical ventilation is currently one of the commonly used means to treat such patients. In previous clinical treatment, the invasive ventilation with endotracheal intubation is easy to cause pulmonary infection in patients and it has poor compliance. On the contrary, noninvasive ventilation, which is easily accepted, can reduce ventilator-related complications in patients and has positive effect on the clinical treatment [15,16].
Nalmefene is one of the common opioid receptor blockers, which can down-regulate opioid peptide levels, inhibit free radical generation and improve respiratory function in patients [17]. In this study, relevant indicators including blood gas indicators, showed a trend of improvement compared with those before treatment. It confirmed the effectiveness of Nalmefene combined with NIPPV treatment on elderly COPD patients with type II respiratory failure. Guo et al. has pointed out that nalmefene combined with positive-pressure ventilation can improve the blood-gas indicators of COPD patients with respiratory failure, which is similar to the results of this study [18].
In addition, inflammatory responses are prevalent in COPD patients. IL-6 and TNF-α are regarded as common indicators for the temporary detection of the body’s inflammatory response [19,20]. It has been reported that the serum levels of IL-6 and TNF-α in COPD patients were increased [21]. They were higher in COPD patients during the acute phase than those of the stable phase and were negatively correlated with the pulmonary function of patients. In this study, the serum IL-6 and TNF-α levels were significantly decreased in both groups after treatment, and the levels in the research group were lower than those in the control group, indicating that nalmefene combined with NIPPV can significantly improve the inflammatory response of patients. Chen et al. have shown that nalmefene can promote the secretion of proteins by Clara cells, then the proteins would be stimulated by macrophage so as to reduce the inflammatory response of patients [22], which is similar to the results of this study.
Nalmefene can inhibit Th1- and Th2-mediated immune response and improve the patient’s immunity. Meanwhile, the inhibition of immune response can inhibit airway remodeling to some extent [23]. TGF-β1/Smads are associated with the occurrence and development of many diseases. TGF-β1 can accelerate angiogenesis, and interact with other cytokines, resulting in intense expression of Smad2 and Smad4 in bronchial epithelial cells. It is an important signaling pathway for the development of pulmonary inflammation and airway remodeling in COPD patients [24,25]. TGF-β1 and Smad2 are important signaling molecules of this pathway. There were few previous studies on the effect of nalmefene combined with NIPPV on TGF-β1 and Smads factors in elderly COPD patients. In this study, the expression levels of TGF-β1 and Smad2 in the research group were significantly decreased after treatment, which were lower than those in the control group, indicating that the combined treatment showed better therapeutic effect by inhibiting the expressions of TGF-β1 and Smads. Wu et al. has demonstrated that the combination of opioid antagonists and NIPPV can significantly improve the respiratory function of COPD patients, which shows some similarity with the conclusion of this study [26].
Besides Although we have demonstrated that the combined therapy might work through inhibition of TGF-β1 and Smads expressions, there are some limitations of this study. First, WB or quantitative fluorescence PCR was not used to detect the differential expression of signaling pathway related factors. In addition, no animal model has been established in this study to further investigate the molecular mechanism. Therefore, large improvements are needed in subsequent studies to avoid some bias in the experiment data. The study doesn’t include any cases of death, which may be related to the short observation period or few grade IV patients. Therefore, we need to implement a multi-centered study with a longer period and larger sample.
In summary, nalmefene combined with NIPPV has a significant positive effect on elderly COPD patients with type II respiratory failure, which is possibly related to inhibition of TGF-β1/Smads signaling pathway activation.
Disclosure of conflict of interest
None.
References
- 1.Lambert AA, Bhatt SP. Respiratory symptoms in smokers with normal spirometry: clinical significance and management considerations. Curr Opin Pulm Med. 2019;25:138–143. doi: 10.1097/MCP.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 2.Hill NS. No place like home: initiation of non-invasive ventilation for stable severe COPD. Thorax. 2020;75:196–197. doi: 10.1136/thoraxjnl-2019-213787. [DOI] [PubMed] [Google Scholar]
- 3.Wiles SP, Aboussouan LS, Mireles-Cabodevila E. Noninvasive positive pressure ventilation in stable patients with COPD. Curr Opin Pulm Med. 2020;26:175–185. doi: 10.1097/MCP.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 4.Jünger C, Reimann M, Krabbe L, Gaede KI, Lange C, Herzmann C, Rüller S. Non-invasive ventilation with pursed lips breathing mode for patients with COPD and hypercapnic respiratory failure: a retrospective analysis. PLoS One. 2020;15:e0238619. doi: 10.1371/journal.pone.0238619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zannin E, Milesi I, Porta R, Cacciatore S, Barbano L, Trentin R, Fanfulla F, Vitacca M, Dellacà RL. Effect of nocturnal EPAP titration to abolish tidal expiratory flow limitation in COPD patients with chronic hypercapnia: a randomized, cross-over pilot study. Respir Res. 2020;21:301. doi: 10.1186/s12931-020-01567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng YB, Liu YH. Analysis of the efficacy of nasal mask ventilator combined with naloxone in the treatment of chronic obstructive pulmonary disease and pulmonary encephalopathy. Chin J Emerg Resusc Disaster Med. 2019;014:398–400. [Google Scholar]
- 7.Chen F, Du JH. Effect of mechanical ventilation combined with naloxone on operation related indexes and therapeutic effect in COPD patients with respiratory failure. Shaanxi Med J. 2020;4:466–469. [Google Scholar]
- 8.Cai DL, Huang ZW, Bao YH, Jiang DS, Wang YY. Expression of TGF-β1/Smads signaling pathway in patients with chronic obstructive pulmonary disease and pulmonary infection. Chin J Nosocomiol. 2020;30:3253–3257. [Google Scholar]
- 9.Chronic Obstructive Pulmonary Disease Group, Chinese Society of Respiratory Medicine. COPD diagnosis and treatment guidelines (2007 revised edition) Contin Med Educ. 2007;21:31–42. [Google Scholar]
- 10.Chen WB, Cheng DY. Respiratory system disease diagnosis and treatment technology. In: Chen WB, Cheng DY, editors. Beijing: People’s Med Publishing House; 2000. [Google Scholar]
- 11.Ren AH, Qiao-Hong JI, Zhi-Hong HU, Yan JB, Fan LL, Yong-Mei HU, Physiology DO, Medicine SO. Establishment of predicted value equation of pulmonary ventilation function in middle-aged and elderly people. Chin Gen Pract. 2016 [Google Scholar]
- 12.Luo JY, Wang XY, Cai TB, Jiang WF. Study of setting of ventilator volume tidal and airway pressure alarm threshold with continuous extra-sternum heart compression in cardiopulmonary resuscitation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:102–105. doi: 10.3760/cma.j.issn.2095-4352.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet J, Grouse L, Zhong N. The fight against chronic respiratory diseases in the elderly: the European Innovation Partnership on Active and Healthy Aging and beyond. J Thorac Dis. 2015;7:108–110. doi: 10.3978/j.issn.2072-1439.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu TD, Ejike CO, Wise RA, McCormack MC, Brigham EP. Investigation of the obesity paradox in chronic obstructive pulmonary disease, according to smoking status, in the United States. Am J Epidemiol. 2019;188:1977–1983. doi: 10.1093/aje/kwz185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ennouri E, Meddeb K, Toumi R, Boussarsar M. Letter on “Early prediction of noninvasive ventilation failure in COPD patients: derivation, internal validation, and external validation of a simple risk score”. Ann Intensive Care. 2019;9:139. doi: 10.1186/s13613-019-0613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maloney LM, Alptunaer T, Coleman G, Ismael S, McKenna PJ, Marshall RT, Hernandez C, Williams DW. Prehospital naloxone and emergency department adverse events: a dose-dependent relationship. J Emerg Med. 2020;59:872–883. doi: 10.1016/j.jemermed.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Franchitto N, De Haro L, Pélissier F. Focusing solely on the effect of the medication without taking a holistic view of the patient does not seem very constructive. Clin Toxicol (Phila) 2018;56:309. doi: 10.1080/15563650.2017.1373781. [DOI] [PubMed] [Google Scholar]
- 18.Guo Q, Li JJ, Tian Q, He W, Ke ZH. Comparative study and health economic evaluation of the clinical efficacy of nalmefene and naloxone combined with non-invasive positive pressure ventilation in the treatment of chronic obstructive pulmonary disease patients with type II respiratory failure. Pract J Card Cereb Pneumal Vasc Dis. 2019;27:71–74. [Google Scholar]
- 19.Yalcin AD, Bisgin A, Gorczynski RM. IL-8, IL-10, TGF-β, and GCSF levels were increased in severe persistent allergic asthma patients with the anti-IgE treatment. Mediators Inflamm. 2012;2012:720976. doi: 10.1155/2012/720976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MQ, Wan Y, Jin Y, Xin JB, Zhang JC, Xiong XZ, Chen L, Chen G. Cigarette smoking promotes inflammation in patients with COPD by affecting the polarization and survival of Th/Tregs through up-regulation of muscarinic receptor 3 and 5 expression. PLoS One. 2014;9:e112350. doi: 10.1371/journal.pone.0112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng M, Wu Y, Chang Q. Study on the correlation of serum StRem-1 with TNF-α and IL-6 in patients with chronic obstructive pulmonary disease. Acta Medica Mediterranea. 2020;36:483–487. [Google Scholar]
- 22.Chen R, Liu HF. Efficacy of nalmefene combined with NIPPV in the treatment of elderly COPD with severe type II respiratory failure. Chin Med Record. 2020;21:98–102. [Google Scholar]
- 23.Sun YL, Lin S, Deng WX, Qiu YX, Yang Y, Ji JC, Guan WT, Gong BQ. Effects of nalmefene combined with non-invasive positive pressure ventilation on Th1, Th2 and sTREM-1 in patients with AECOPD complicated with respiratory failure. Chin J Gerontology. 2018;38:2634–2637. [Google Scholar]
- 24.Wang W, Zha G, Zou JJ, Wang X, Li CN, Wu XJ. Berberine attenuates cigarette smoke extract-induced airway inflammation in mice: involvement of TGF-β1/Smads signaling pathway. Curr Med Sci. 2019;39:748–753. doi: 10.1007/s11596-019-2101-8. [DOI] [PubMed] [Google Scholar]
- 25.Fan T, Ge M, Guo Z, He H, Zhang N, Li Y, Song D. Discovery of 9O-substituted palmatine derivatives as a new class of anti-COL1A1 agents via repressing TGF-β1/Smads and JAK1/STAT3 pathways. Molecules. 2020;25:773. doi: 10.3390/molecules25040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Zhu XD. Analysis of noninvasive positive pressure ventilation combined with naloxone injection in the treatment of chronic obstructive pulmonary disease combined with respiratory failure. Chin Rem Clin. 2019;19:113–115. [Google Scholar]