Abstract
BACKGROUND
Hepatorenal syndrome (HRS) is a life-threatening condition among patients with advanced liver disease. Data trends specific to hospital mortality and hospital admission resource utilization for HRS remain limited.
AIM
To assess the temporal trend in mortality and identify the predictors for mortality among hospital admissions for HRS in the United States.
METHODS
We used the National Inpatient Sample database to identify an unweighted sample of 4938 hospital admissions for HRS from 2005 to 2014 (weighted sample of 23973 admissions). The primary outcomes were temporal trends in mortality as well as predictors for hospital mortality. We estimated odds ratios from multi-level mixed effect logistic regression to identify patient characteristics and treatments associated with hospital mortality.
RESULTS
Overall hospital mortality was 32%. Hospital mortality decreased from 44% in 2005 to 24% in 2014 (P < 0.001), while there was an increase in the rate of liver transplantation (P = 0.02), renal replacement therapy (P < 0.001), length of hospital stay (P < 0.001), and hospitalization cost (P < 0.001). On multivariable analysis, older age, alcohol use, coagulopathy, neurological disorder, and need for mechanical ventilation predicted higher hospital mortality, whereas liver transplantation, transjugular intrahepatic portosystemic shunt, and abdominal paracentesis were associated with lower hospital mortality.
CONCLUSION
Although there was an increase in resource utilizations, hospital mortality among patients admitted for HRS significantly improved. Several predictors for hospital mortality were identified.
Keywords: Hepatorenal syndrome, Liver transplantation, Mortality, Nationwide, Big data, Hospitalization, Outcomes, Predictors
Core Tip: In this study, we utilized the national inpatient sample database to assess the temporal trend in mortality and identify predictors for mortality among hospital admissions for hepatorenal syndrome in the United States. We demonstrated that the overall hospital mortality was 32%. Hospital mortality decreased from 44% in 2005 to 24% in 2014. There was an increase in the rate of liver transplantation, renal replacement therapy, length of hospital stay, and hospitalization cost.
INTRODUCTION
Hepatorenal syndrome (HRS) is a serious complication of cirrhosis with an incidence as high as 32% among patients with advanced liver disease[1-7]. Previous studies have consistently demonstrated high morbidity, mortality, and resource utilizations[1,8-17]. Several factors have been associated with poor outcomes, including high model for end stage liver disease (MELD) score[18], degree of acute kidney injury (AKI)[11,19], extrahepatic organ failure[20], and sepsis[18,21].
In recent decades, there have been significant advances in knowledge, treatment, and optimal management of patients with HRS[1,7-17,22-24]. While terlipressin, a synthetic vasopressin analog with predominant vasopressin 1A receptor effect[25], has been used to treat HRS in many Asian and European countries, it is currently not yet available in the United States for the treatment of HRS[1,26]. Thus, currently available treatment options for HRS in the United States include albumin volume expansion, octreotide with or without midodrine, and intravenous cardiovascular medications like vasopressin and norepinephrine[1]. Nevertheless, there have been improvements in the overall care for patients with HRS, including liver transplantation and renal replacement therapy. In addition, several studies have suggested the use of transjugular intrahepatic portosystemic shunt (TIPS) for patients with HRS[27-29]. However, data specific to HRS, hospital mortality trends, and hospital admission resource utilization remain limited.
In this study, we aimed to evaluate patient characteristics, in-hospital treatments, mortality, and resource utilization during hospital admissions for HRS in the United States. We also assessed the temporal trend in mortality and identified the predictors for mortality.
MATERIALS AND METHODS
Study population
We conducted a retrospective cohort study of hospital admissions for HRS from 2005 to 2014 in the national inpatient sample (NIS) database. The detail of the NIS database was previously described[30]. We identified hospital admission with a primary discharge diagnosis using the international classification of disease-9 (ICD-9) diagnosis code of 572.4. The Mayo Clinic institutional review board approved this study (IRB number 21-007353 and date of approval; July 27, 2021) and exempted the need for informed consent because the data in NIS database was publicly available and de-identified.
Data collection
We abstracted patient and hospital characteristics, procedures, outcomes, and resource utilization from the database (Supplementary Table 1). Patient characteristics included age, sex, race, etiology of liver disease, medical comorbidity based on Elixhauser index[31], and admission day. Hospital characteristics included hospital size, ownership, location, teaching status, and region. Procedures included renal replacement therapy, liver transplantation, TIPS, abdominal paracentesis, and mechanical ventilation. Outcomes included hospital mortality, resource utilization, including length of hospital stay, and hospitalization cost. Since this study used data over 10 different calendar years, we adjusted hospitalization costs for inflation using the consumer price index and converted them to 2014 United States dollar equivalents.
Statistical analysis
The NIS database contains hospitalization data from a stratified sample of 20% of hospitals in the United States. As such, we used discharge weight provided by the Healthcare Cost and Utilization (HCUP) to estimate the total number of hospital admissions for HRS. We used descriptive statistics to summarize patient and hospital characteristics, procedures, outcomes, and resource use of HRS admission. We fitted logistic regression model for hospital mortality and liver transplantation, and standard least square linear regression for length of hospital stay, and hospitalization cost, using calendar years as the independent variable to assess the annual trend from 2005 through 2014. We estimated adjusted odds ratio (OR) for hospital mortality from multivariable multi-level mixed effect logistic regression, employing hospital identification number as random effect with patients-level characteristics clustered within hospital-level characteristics. We performed all statistical analyses using STATA, version 15 (StataCorp LP, College Station, TX, United States).
RESULTS
Patient characteristics, in-hospital treatments, outcomes, and resource use in hospital admission for HRS
There were 4938 hospital admissions with HRS as the primary diagnosis in the unweighted sample and 23973 admissions in the weighted sample. Table 1 shows patient and hospital characteristics of hospital admissions for HRS. The mean age was 58.8 ± 12.3 years, and the majority of patients were males (63%). Alcohol-related liver disease (46%) and viral hepatitis (25%) were the most common liver disease etiologies. Most patients were admitted to large urban teaching hospitals. Of those patients admitted for HRS, 21% received renal replacement therapy and 2% underwent liver transplant during their hospitalization. During this 10-year period, there was a 32% mortality observed for HRS admissions. The mean length of hospital stay was 8.8 d and the mean hospitalization cost was 73731 United States dollars.
Table 1.
Patient characteristics, in-hospital treatments, outcomes, and resource use in hospital admission for hepatorenal syndrome (mean ± SD)
|
|
Unweighted, n (%)
|
Unweighted % ± SE
|
Weighted, n (%)
|
Weighted % ± SE
|
| Total, n (%) | 4938 | 23973 | ||
| Sex | ||||
| Male | 3130 | 63.39 ± 0.68 | 15183 | 63.33 ± 0.31 |
| Female | 1808 | 36.61 ± 0.68 | 8790 | 36.67 ± 0.31 |
| Age (yr) | 58.8 ± 12.3 | 58.8 ± 12.3 | ||
| 18-39 | 266 | 5.39 ± 0.32 | 1299 | 5.42 ± 0.15 |
| 40-59 | 2461 | 49.84 ± 0.71 | 11933 | 49.77 ± 0.32 |
| 60-79 | 1927 | 39.02 ± 0.69 | 9365 | 39.06 ± 0.31 |
| ≥ 80 | 284 | 5.75 ± 0.33 | 1376 | 5.74 ± 0.15 |
| Race | ||||
| White | 3098 | 72.23 ± 0.68 | 15050 | 72.12 ± 0.31 |
| Black | 421 | 9.81 ± 0.45 | 2055 | 9.85 ± 0.21 |
| Hispanic | 511 | 11.91 ± 0.49 | 2495 | 11.95 ± 0.22 |
| Asian/Pacific islander | 81 | 1.89 ± 0.21 | 395 | 1.89 ± 0.09 |
| Native American | 57 | 1.33 ± 0.17 | 280 | 1.34 ± 0.07 |
| Other | 121 | 2.82 ± 0.25 | 593 | 2.84 ± 0.11 |
| Admission day | ||||
| Weekday | 3955 | 80.09 ± 0.57 | 19223 | 80.18 ± 0.26 |
| Weekend | 983 | 19.91 ± 0.57 | 4751 | 19.82 ± 0.26 |
| Liver disease etiology | ||||
| Alcoholic liver disease | 2249 | 45.54 ± 0.71 | 10935 | 45.61 ± 0.32 |
| Viral hepatitis | 1218 | 24.66 ± 0.61 | 5915 | 24.67 ± 0.28 |
| Comorbidities | ||||
| Diabetes Mellitus | 1260 | 25.52 ± 0.62 | 6132 | 25.58 ± 0.28 |
| Hypertension | 1937 | 39.23 ± 0.69 | 9437 | 39.36 ± 0.32 |
| Fluid/electrolyte disorders | 3548 | 71.85 ± 0.64 | 17233 | 71.88 ± 0.29 |
| Coagulopathy | 2115 | 42.83 ± 0.70 | 10286 | 42.90 ± 0.32 |
| Anemia | 1937 | 39.23 ± 0.69 | 9422 | 39.30 ± 0.31 |
| Weight loss | 872 | 17.66 ± 0.54 | 4255 | 17.75 ± 0.25 |
| Cancer | 658 | 13.32 ± 0.48 | 3198 | 13.34 ± 0.22 |
| Congestive heart failure | 630 | 12.76 ± 0.47 | 3042 | 12.69 ± 0.21 |
| Chronic pulmonary disease | 613 | 12.41 ± 0.47 | 2973 | 12.40 ± 0.21 |
| Obesity | 456 | 9.23 ± 0.41 | 2218 | 9.25 ± 0.19 |
| Neurological disorders | 234 | 4.74 ± 0.30 | 1147 | 4.78 ± 0.14 |
| Pulmonary circulation disorders | 176 | 3.56 ± 0.26 | 847 | 3.53 ± 0.12 |
| Valvular disease | 164 | 3.32 ± 0.25 | 795 | 3.32 ± 0.12 |
| Peripheral vascular disorders | 119 | 2.41 ± 0.22 | 587 | 2.45 ± 0.10 |
| Depression | 413 | 8.36 ± 0.39 | 2002 | 8.35 ± 0.18 |
| HIV/AIDS | 36 | 0.73 ± 0.12 | 173 | 0.72 ± 0.05 |
| Substance use | ||||
| Smoking | 583 | 11.81 ± 0.46 | 2825 | 11.78 ± 0.21 |
| Alcohol | 1930 | 39.08 ± 0.69 | 9394 | 39.19 ± 0.31 |
| Drug use | 209 | 4.23 ± 0.29 | 1023 | 4.27 ± 0.13 |
| Bed size | ||||
| Small | 611 | 12.30 ± 0.50 | 2898 | 12.09 ± 0.21 |
| Medium | 1210 | 24.41 ± 0.66 | 5905 | 24.63 ± 0.28 |
| Large | 3117 | 63.28 ± 0.74 | 15171 | 63.28 ± 0.31 |
| Location/Teaching status | ||||
| Rural | 651 | 13.89 ± 0.53 | 3167 | 13.21 ± 0.22 |
| Urban, non-teaching | 1723 | 36.76 ± 0.74 | 8320 | 34.70 ± 0.31 |
| Urban, teaching | 2564 | 49.35 ± 0.77 | 12487 | 52.08 ± 0.32 |
| Hospital region | ||||
| Northeast | 984 | 20.24 ± 0.62 | 4817 | 20.09 ± 0.26 |
| Midwest | 1122 | 23.03 ± 0.65 | 5406 | 22.55 ± 0.27 |
| South | 1699 | 34.03 ± 0.73 | 8261 | 34.46 ± 0.31 |
| West | 1133 | 22.70 ± 0.64 | 5489 | 22.90 ± 0.27 |
| Medical procedures/interventions | ||||
| Renal replacement therapy | 1018 | 20.61 ± 0.58 | 4929 | 20.56 ± 0.26 |
| Paracentesis | 2226 | 45.08 ± 0.71 | 10843 | 45.23 ± 0.32 |
| Mechanical ventilation | 499 | 10.10 ± 0.43 | 2412 | 10.06 ± 0.19 |
| TIPS | 46 | 0.93 ± 0.14 | 218 | 0.91 ± 0.06 |
| Liver transplantation | 85 | 1.68 ± 0.18 | 404 | 1.68 ± 0.08 |
| LTA | 66 | 1.34 ± 0.16 | 321 | 1.34 ± 0.07 |
| SLKT | 19 | 0.38 ± 0.09 | 93 | 0.39 ± 0.04 |
| Outcomes | ||||
| Mortality | 1573 | 31.90 ± 0.66 | 7616 | 31.81 ± 0.30 |
| Length of hospital stay (d) | 8.8 ± 10.9 | 8.8 ± 11.0 | ||
| Hospitalization cost (United States $) | 735701 ± 135526 | 73731 ± 135876 |
SE: Standard error; HIV/AIDS: Human immunodeficiency virus/acquired immunodeficiency syndrome; TIPS: Transjugular intrahepatic portosystemic shunt; LTA: Liver transplant alone; SLKT: Simultaneous liver-kidney transplantation.
Trends in hospital mortality, liver transplantation, length of stay, hospitalization cost in hospital admission for HRS
Table 2 showed the annual trend in hospital mortality, liver transplantation, length of hospital stay, and hospitalization cost in HRS admissions from 2005 to 2014.
Table 2.
The annual trend in hospital mortality, liver transplantation, renal replacement therapy, length of hospital stay, hospitalization cost in hepatorenal syndrome admission from 2005 to 2014 (mean ± SD)
|
Year
|
Unweighted sample1
|
Weighted sample1
|
Hospital mortality weighted % ± SE
|
Liver transplantation weighted % ± SE
|
Renal replacement therapy weighted % ± SE
|
Length of stay (d)
|
Hospital cost (United States $)
|
| Total | 4931 | 23941 | 31.8 ± 0.7 | 1.7 ± 0.2 | 20.6 ± 0.6 | 8.8 ± 11.0 | 73731 ± 135876 |
| 2005 | 312 | 1471 | 43.8 ± 2.8 | 0.7 ± 0.5 | 13.8 ± 2.0 | 8.2 ± 8.6 | 42857 ± 67978 |
| 2006 | 330 | 1551 | 40.8 ± 2.7 | 0.6 ± 0.4 | 13.6 ± 1.9 | 7.2 ± 7.6 | 41841 ± 67254 |
| 2007 | 287 | 1358 | 36.7 ± 2.9 | 1.1 ± 0.6 | 17.0 ± 2.2 | 8.0 ± 8.9 | 49879 ± 77833 |
| 2008 | 367 | 1737 | 37.1 ± 2.5 | 1.9 ± 0.7 | 21.7 ± 2.2 | 8.6 ± 9.4 | 65419 ± 109901 |
| 2009 | 486 | 2363 | 31.1 ± 2.1 | 2.2 ± 0.7 | 20.8 ± 1.8 | 8.9 ± 11.3 | 71737 ± 123006 |
| 2010 | 610 | 2973 | 31.1 ± 1.9 | 0.9 ± 0.4 | 21.3 ± 1.7 | 9.3 ± 13.7 | 69778 ± 106971 |
| 2011 | 628 | 2934 | 30.3 ± 1.9 | 1.9 ± 0.5 | 23.3 ± 1.7 | 8.6 ± 9.6 | 82917 ± 154746 |
| 2012 | 603 | 3015 | 31.2 ± 1.9 | 2.0 ± 0.6 | 21.9 ± 1.7 | 9.1 ± 13.5 | 74951 ± 113671 |
| 2013 | 622 | 3110 | 28.3 ± 1.8 | 2.7 ± 0.7 | 21.4 ± 1.6 | 9.4 ± 12.1 | 95671 ± 210352 |
| 2014 | 686 | 3430 | 24.1 ± 1.6 | 1.7 ± 0.5 | 22.4 ± 1.6 | 9.0 ± 9.0 | 90829 ± 149495 |
| P value | < 0.001 | 0.02 | < 0.001 | < 0.001 | < 0.001 | ||
Sample of hepatorenal syndrome patients having complete data on mortality status.
SE: Standard error.
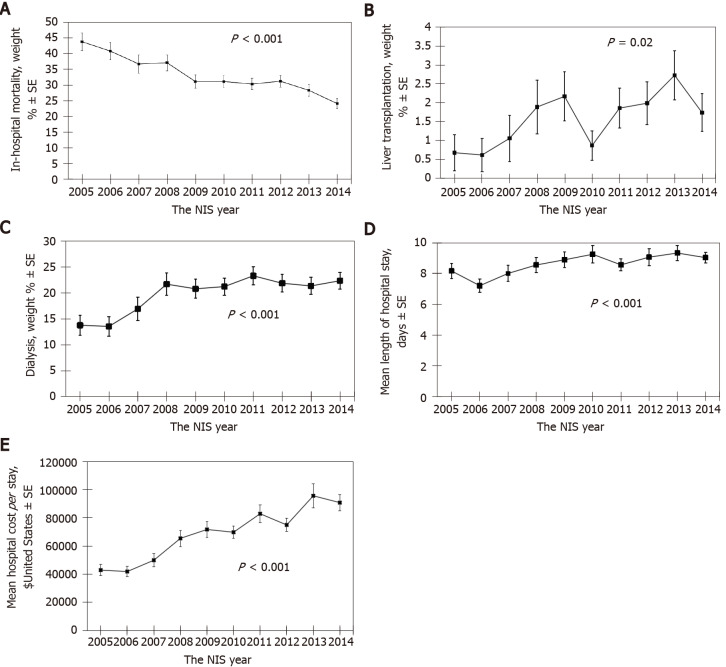
There was a decreasing trend in hospital mortality from 44% in 2005 to 24% in 2014 among hospital admissions for HRS in the United States (OR: 0.92, 95%CI: 0.90-0.94 per year; P < 0.001) (Figure 1A).
Figure 1.
Data on admissions in the United States due to hepatorenal syndrome. A: Decreasing trend in hospital mortality among hospital admissions; B: Increase in the rate of liver transplantation among hospital admissions; C: Trend of renal replacement therapy among hospital admissions; D: Trend of mean length of hospital stay among hospital admissions; E: Trend of hospitalization cost among hospital admissions. NIS: National inpatient sample.
Meanwhile, there was an increase in the rate of liver transplantation (OR: 1.11, 95%CI: 1.02-1.20 per year; P = 0.02) (Figure 1B) and renal replacement therapy (OR: 1.05, 95%CI: 1.02-1.08 per year; P < 0.001) (Figure 1C) performed in hospitalization for HRS.
There was an increasing trend in mean length of hospital stay (coefficient estimate 0.2 d per year; P < 0.001) (Figure 1D) and hospitalization cost (coefficient estimate 5778 United States dollars per year; P < 0.001) (Figure 1E) among hospitalization for HRS during 10-year period from 2005 to 2014.
Predictors for hospital mortality
In multivariable analysis (Table 3), older age (OR: 1.45 for 40-59 years, 1.77 for 60-79 years, 2.12 for ≥ 80 years, compared to 18-39 years; all P < 0.001), alcohol use (OR: 1.35; P < 0.001), coagulopathy (OR: 1.15; P = 0.001), and presence of a neurological disorder (OR: 1.38; P < 0.001) predicted higher hospital mortality.
Table 3.
Clinical characteristics associated with in-hospital mortality
| Characteristics |
Univariable analysis
|
Multivariable analysis
|
||
|
Unadjusted OR (95%CI)
|
P
value
|
Adjusted OR (95%CI)
|
P
value
|
|
| Female sex | 0.96 (0.85-1.09) | 0.52 | 0.91 (0.78-1.07) | 0.27 |
| Age (yr) | ||||
| 18-39 | 1 (ref) | - | 1 (ref) | - |
| 40-59 | 1.21 (0.91-1.60) | 0.19 | 1.45 (1.28-1.64) | < 0.001 |
| 60-79 | 1.24 (0.93-1.65) | 0.14 | 1.77 (1.68-1.87) | < 0.001 |
| ≥ 80 | 1.68 (1.17-2.42) | 0.005 | 2.12 (1.51-3.00) | < 0.001 |
| Race | ||||
| White | 1 (ref) | - | 1 (ref) | - |
| Black | 1.38 (1.11-1.71) | 0.003 | 1.26 (0.91-1.75) | 0.16 |
| Hispanic | 1.05 (0.86-1.29) | 0.61 | 1.12 (0.78-1.61) | 0.53 |
| Asian/Pacific Islander | 1.44 (0.91-2.27) | 0.12 | 1.30 (0.98-1.73) | 0.07 |
| Native American | 1.04 (0.59-1.84) | 0.88 | 1.11 (0.86-1.43) | 0.43 |
| Other | 0.88 (0.59-1.32) | 0.55 | 0.93 (0.48-1.81) | 0.84 |
| Weekend admission | 1.14 (0.98-1.32) | 0.08 | 1.05 (0.82-1.34) | 0.69 |
| Liver disease etiology | ||||
| Alcohol-related | 0.90 (0.80-1.01) | 0.08 | 0.85 (0.71-1.01) | 0.06 |
| Viral hepatitis | 0.98 (0.86-1.13) | 0.83 | 1.00 (0.81-1.24) | 1.00 |
| Comorbidities | ||||
| Smoking | 0.96 (0.79-1.16) | 0.66 | 1.15 (0.83-1.60) | 0.40 |
| Alcohol use | 0.98 (0.87-1.11) | 0.79 | 1.35 (1.26-1.45) | < 0.001 |
| Drug use | 0.84 (0.62-1.14) | 0.26 | 0.77 (0.55-1.08) | 0.13 |
| HIV/AIDS | 1.02 (0.51-2.07) | 0.95 | 0.81 (0.58-1.13) | 0.22 |
| Autoimmune arthritis | 1.10 (0.64-1.91) | 0.73 | 1.14 (0.54-2.41) | 0.72 |
| Congestive heart failure | 1.05 (0.88-1.26) | 0.59 | 0.99 (0.87-1.12) | 0.84 |
| Chronic pulmonary disease | 1.00 (0.84-1.21) | 0.96 | 0.95 (0.78-1.16) | 0.63 |
| Coagulopathy | 1.01 (0.90-1.15) | 0.82 | 1.15 (1.16-1.25) | 0.001 |
| Diabetes mellitus | 0.78 (0.67-0.89) | < 0.001 | 0.87 (0.73-1.04) | 0.12 |
| Hypertension | 0.76 (0.67-0.86) | < 0.001 | 0.83 (0.70-1.01) | 0.06 |
| Lymphoma | 1.42 (0.68-2.96) | 0.35 | 1.53 (0.42-5.60) | 0.52 |
| Fluid/electrolyte disorders | 0.85 (0.74-0.97) | 0.02 | 0.87 (0.75-1.01) | 0.07 |
| Cancer | 1.34 (1.13-1.59) | 0.001 | 1.40 (0.88-2.23) | 0.15 |
| Neurological disorders | 1.29 (0.98-1.70) | 0.07 | 1.38 (1.21-1.58) | < 0.001 |
| Obesity | 0.87 (0.70-1.07) | 0.20 | 0.92 (0.62-1.38) | 0.70 |
| Peripheral vascular disorders | 0.78 (0.51-1.18) | 0.23 | 0.78 (0.42-1.46) | 0.44 |
| Psychoses | 0.80 (0.55-1.16) | 0.24 | 0.93 (0.78-1.12) | 0.44 |
| Pulmonary circulation disorders | 0.75 (0.53-1.05) | 0.10 | 0.68 (0.43-1.08) | 0.11 |
| Valvular disease | 0.75 (0.53-1.07) | 0.12 | 1.01 (0.64-1.60) | 0.96 |
| Weight loss | 0.91 (0.78-1.07) | 0.27 | 1.05 (0.97-1.13) | 0.21 |
| Medical procedure | ||||
| Renal replacement therapy | 0.98 (0.85-1.14) | 0.81 | 0.92 (0.68-1.25) | 0.59 |
| Liver transplantation | 0.33 (0.23-0.46) | < 0.001 | 0.15 (0.11-0.21) | < 0.001 |
| TIPS | 0.40 (0.18-0.90) | 0.03 | 0.23 (0.12-0.43) | < 0.001 |
| Paracentesis | 0.46 (0.41-0.53) | < 0.001 | 0.48 (0.43-0.53) | < 0.001 |
| Mechanical ventilation | 6.97 (5.66-8.59) | < 0.001 | 9.24 (7.90-10.81) | < 0.001 |
HIV/AIDS: Human immunodeficiency virus/acquired immunodeficiency syndrome; TIPS: Transjugular intrahepatic portosystemic shunt; OR: Odds ratio; 95%CI: 95% confidence interval.
Need for mechanical ventilation (OR: 9.24; P < 0.001) was associated with higher mortality, whereas liver transplantation (OR: 0.15; P < 0.001) and TIPS (OR: 0.23; P < 0.001), and abdominal paracentesis (OR: 0.48; P < 0.001) were associated with lower hospital mortality. Renal replacement therapy was not significantly associated with mortality risk.
DISCUSSION
In this study based on a large United States database of hospitalizations, the mortality rate for hospitalized patients with HRS decreased by approximately 50% during the 10-year study period. During the same period, there was a 2-fold increase in the incidence of HRS patients receiving a liver transplant and the incidence of in-hospital renal replacement therapy increased by 60%. Notably, there were also increase in length of hospital stay and a 2-fold increase in the estimated hospital cost, which is likely related to higher utilization of healthcare resources. This highlights the high economic burden of chronic liver disease in the United States[32,33].
The marked improvement in the in-hospital mortality rate for HRS is likely reflective of changes in both medical and surgical management during the study period. Our study shows that there was an apparent increase in the number of liver transplants and renal replacement therapy around 2007 to 2008. This trend coincided with overall changes in clinical practice over the preceding years[34]. Although the unique pathophysiology of HRS has long been recognized as a functional renal failure occurring as a result of advanced liver disease[35], its treatment, including the initiation of in-hospital dialysis, and the role for liver transplantation have significantly evolved[36]. Historically, the initiation of renal replacement therapy in patients with HRS was felt to be controversial and futile. Increasing experience with liver transplantation in the setting of HRS as well as improved access to continuous renal replacement have resulted in a change in practice and a decrease in mortality[37].
In 2007-2008, multiple randomized control trials on terlipressin were published and have influenced the medical management HRS as well as patient outcomes[9-11,14,15]. Studies have shown potential beneficial effects of terlipressin, a potent selective splanchnic and extrarenal vasoconstrictor, on kidney function among patients with HRS[10,38,39]. Additionally, non-response to vasoconstrictors can also predict HRS mortality[40,41]. Unfortunately, as of 2020, the FDA has not yet approved the use of terlipressin for HRS in the United States. Results from the phase 3 trial terlipressin did not show any significant survival benefit and its use was associated with adverse events, such as respiratory failure[42,43]. Although terlipressin is currently not yet available in the United States[1,26], the observed findings of decreasing mortality trends for HRS in the Unites States are likely due to improvements in healthcare, increased access and acceptance of chronic intermittent hemodialysis for patients with liver disease as well as increased acceptance of liver transplantation for patients with acute decompensation[44].
In addition to liver transplantation, our study interestingly showed that TIPS and abdominal paracentesis were associated with lower hospital mortality among patients with HRS. Possible mechanisms underlying reduced mortality among patients who received paracentesis were that those who had abdominal paracentesis received more aggressive treatments such as albumin and vasopressors, TIPS, and liver transplantation than those who received palliative care. Furthermore, abdominal paracentesis may have led to the diagnosis and treatment for spontaneous bacterial peritonitis[45]. The use of TIPS in patients with HRS remains controversial, although there is increasing data suggesting there may be benefit[24,29]. According to current best practice recommendations, the presence of HRS is not an absolute contraindication for TIPS and the presence of other indications, such as ascites, should guide decision making[29]. Specific to this topic, there is a clear need for additional randomized controlled trials, however, in the interim, there are an increasing number of small studies demonstrating positive outcomes in select HRS patients receiving TIPS[24,46,47]. Since mortality in patients with HRS undergoing TIPS is driven mainly by poor liver function it may be possible that there was a population selection bias and these patients had initially better liver function resulting in better survival.
Our study also showed several risk factors associated with in-patient mortality for HRS. These factors include advanced age, history of alcohol use, coagulopathy and presence of a neurological disorder. It is well known that older age, coagulopathy, and neurological disorder are associated with poor outcomes in patients with HRS[11,18-21]. Hepatic encephalopathy is known to be associated with mortality[48], and thus this could be the underlying reason for association between neurological disorder and increased in-patient mortality for HRS. Although specific knowledge regarding the duration and timing of alcohol use prior to hospitalization is a limitation of this dataset, active alcohol use is a known decompensating event that can result in AKI and HRS. It is also possible that recent alcohol use prevented certain patients from being suitable for liver transplantation. In this foreseeable scenario, initiation of renal replacement therapy has increasingly been used as a bridge to liver transplant eligibility and liver compensation.
There are several limitations in our study. The NIS is a hospitalized database. Thus, we did not evaluate the long-term outcomes of HRS following hospitalization. Although our study showed a decreasing trend of in-hospital mortality rates, it should not be generalized to the overall survival of patients with HRS. Estimates of in-hospital mortality do not include deaths that occur after discharge. The database did not contain MELD score, which predicted mortality in HRS patients[48]. In addition, treatment of HRS was not assessed in this study[40,41]. Data on medications including midodrine, octreotide, vasopressor, albumin infusion were not available in the database. Thus, we could not assess the effects of these agents and the response to treatments on the outcomes of HRS. Lastly, HRS was identified by ICD-9 diagnosis code. Given definition of the HRS has changed over the years, these changes in definition may have affected the incidence of HRS in our study overtime.
CONCLUSION
In summary, our study showed a decreasing trend of in-hospital mortality rates in patients with HRS. These trends were likely related to advances in medicine, increased access and acceptance of renal replacement therapy, and increased utilization of liver transplantation which is the definitive treatment for HRS. Future studies are needed to understand if these trends are impacted by other factors such as facility performance, patient care teams, health insurance reimbursement policies, or other factors.
ARTICLE HIGHLIGHTS
Research background
Hepatorenal syndrome (HRS) is a serious complication of cirrhosis, associated with high morbidity, mortality, and resource utilizations. In recent decades, there have been significant advances in knowledge, treatment and optimal management of patients with HRS.
Research motivation
There has been improvement in overall care for patients with HRS. Data on trends of hospital mortality and resource utilization in hospital admissions for HRS were limited.
Research objectives
We aimed to evaluate patient characteristics, in-hospital treatments, mortality, resource use among hospital admissions for HRS s in the United States. We also assessed the temporal trend in mortality and identified the predictors for mortality.
Research methods
We used the national inpatient sample database to identify unweighted sample of 4938 hospital admissions primarily for HRS from 2005 to 2014 (weighted sample of 23973 admissions). The primary outcome was the temporal trend in and predictors for hospital mortality. We estimated odds ratio from multi-level mixed effect logistic regression to identify patient characteristics and treatments associated with hospital mortality.
Research results
The overall hospital mortality was 32%. Hospital mortality decreased from 44% in 2005 to 24% in 2014 (P < 0.001), while there was an increase in the rate of liver transplantation (P = 0.02), renal replacement therapy (P < 0.001), length of hospital stay (P < 0.001), and hospitalization cost (P < 0.001). Multivariable analysis older age, alcohol abuse, coagulopathy, neurological disorder, and need for mechanical ventilation predicted higher hospital mortality, whereas liver transplantation, TIPs, and abdominal paracentesis were associated with lower hospital mortality.
Research conclusions
Although there was an increase in resource utilizations, hospital mortality among hospital admissions for HRS significantly improved.
Research perspectives
These trends were likely related to increased utilization of liver transplantation which is the definitive treatment for HRS. Future studies are needed to understand if these trends are impacted by other factors such as facility performance, patient care teams, health insurance reimbursement policies, or other factors.
Footnotes
Institutional review board statement: The Mayo Clinic Institutional Review Board approved this study (IRB number 21-007353 and date of approval; July 27, 2021).
Informed consent statement: The Mayo Clinic institutional review board approved this study and exempted the need for informed consent because the data in NIS database was publicly available and de-identified.
Conflict-of-interest statement: The authors deny any conflict of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 4, 2021
First decision: October 16, 2021
Article in press: November 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kowalewski G, Kulkarni AV, Singh SA, Zaghloul MS S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Wisit Kaewput, Department of Military and Community Medicine, Phramongkutklao College of Medicine, Bangkok 10400, Thailand.
Charat Thongprayoon, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
Carissa Y Dumancas, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
Swetha R Kanduri, Division of Nephrology, Department of Medicine, Ochsner Clinic Foundation, New Orleans, LA 70121, United States.
Karthik Kovvuru, Division of Nephrology, Department of Medicine, Ochsner Clinic Foundation, New Orleans, LA 70121, United States.
Chalermrat Kaewput, Division of Nuclear Medicine, Department of Radiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10400, Thailand.
Pattharawin Pattharanitima, Department of Internal Medicine, Faculty of Medicine, Thammasat University, Pathum Thani 12121, Thailand.
Tananchai Petnak, Division of Pulmonary and Pulmonary Critical Care Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Ploypin Lertjitbanjong, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Tennessee Health Science Center, Memphis, TN 13326, United States.
Boonphiphop Boonpheng, Division of Nephrology, University of Washington, Seattle, WA 98195, United States.
Karn Wijarnpreecha, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI 48109, United States.
Jose L Zabala Genovez, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
Saraschandra Vallabhajosyula, Section of Cardiovascular Medicine, Department of Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101, United States.
Caroline C Jadlowiec, Division of Transplant Surgery, Mayo Clinic, Phoenix, AZ 85054, United States.
Fawad Qureshi, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN 55905, United States.
Wisit Cheungpasitporn, Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic, Rochester, MN 55905, United States. wcheungpasitporn@gmail.com.
Data sharing statement
We conducted a retrospective cohort study of hospital admissions for HRS from 2005 to 2014 in the National Inpatient Sample (NIS) database (publicly available and de-identified).
References
- 1.Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. doi: 10.1136/bmj.m2687. [DOI] [PubMed] [Google Scholar]
- 2.Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, Graupera I, Ariza X, Pereira G, Alfaro I, Cárdenas A, Fernández J, Poch E, Ginès P. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, Lin YF, Lu KC. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006;65:28–33. doi: 10.5414/cnp65028. [DOI] [PubMed] [Google Scholar]
- 4.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, Angeli P. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Sujan R, Cruz-Lemini M, Altamirano J, Simonetto DA, Maiwall R, Axley P, Richardson T, Desai V, Cabezas J, Vargas V, Kamath PS, Shah VH, Sarin SK, Bataller R, Singal AK. A Validated Score Predicts Acute Kidney Injury and Survival in Patients With Alcoholic Hepatitis. Liver Transpl. 2018;24:1655–1664. doi: 10.1002/lt.25328. [DOI] [PubMed] [Google Scholar]
- 6.Makar M, Reja D, Chouthai A, Kabaria S, Patel AV. The impact of acute kidney injury on mortality and clinical outcomes in patients with alcoholic cirrhosis in the USA. Eur J Gastroenterol Hepatol. 2021;33:905–910. doi: 10.1097/MEG.0000000000001947. [DOI] [PubMed] [Google Scholar]
- 7.Pant C, Jani BS, Desai M, Deshpande A, Pandya P, Taylor R, Gilroy R, Olyaee M. Hepatorenal syndrome in hospitalized patients with chronic liver disease: results from the Nationwide Inpatient Sample 2002-2012. J Investig Med. 2016;64:33–38. doi: 10.1136/jim-d-15-00181. [DOI] [PubMed] [Google Scholar]
- 8.Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–156. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 9.Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, Siringo S, Castellino P. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830–835. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V, Rodés J, Ginès P TAHRS Investigators. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 12.He X, Su F, Taccone FS, Laporte R, Kjølbye AL, Zhang J, Xie K, Moussa MD, Reinheimer TM, Vincent JL. A Selective V(1A) Receptor Agonist, Selepressin, Is Superior to Arginine Vasopressin and to Norepinephrine in Ovine Septic Shock. Crit Care Med. 2016;44:23–31. doi: 10.1097/CCM.0000000000001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579–1589.e2. doi: 10.1053/j.gastro.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Khemichian S, Francoz C, Nadim MK. Advances in management of hepatorenal syndrome. Curr Opin Nephrol Hypertens. 2021;30:501–506. doi: 10.1097/MNH.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline vs terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 16.Mauro E, Garcia-Olveira L, Gadano A. End-stage liver disease: Management of hepatorenal syndrome. Liver Int. 2021;41 Suppl 1:119–127. doi: 10.1111/liv.14866. [DOI] [PubMed] [Google Scholar]
- 17.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 18.Wong F, Pappas SC, Boyer TD, Sanyal AJ, Bajaj JS, Escalante S, Jamil K REVERSE Investigators. Terlipressin Improves Renal Function and Reverses Hepatorenal Syndrome in Patients With Systemic Inflammatory Response Syndrome. Clin Gastroenterol Hepatol. 2017;15:266–272.e1. doi: 10.1016/j.cgh.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Wong F, Boyer TD, Sanyal AJ, Pappas SC, Escalante S, Jamil K. Reduction in acute kidney injury stage predicts survival in patients with type-1 hepatorenal syndrome. Nephrol Dial Transplant. 2020;35:1554–1561. doi: 10.1093/ndt/gfz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Hüsing-Kabar A, Solà E, Gerbes A, Bernardi M, Alessandria C, Scheiner B, Tonon M, Maschmeier M, Solè C, Trebicka J, Gustot T, Nevens F, Arroyo V, Gines P, Angeli P EASL CLIF Consortium, European Foundation for the Study of Chronic Liver Failure (EF Clif) Association Between Grade of Acute on Chronic Liver Failure and Response to Terlipressin and Albumin in Patients With Hepatorenal Syndrome. Clin Gastroenterol Hepatol. 2018;16:1792–1800.e3. doi: 10.1016/j.cgh.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019;39:1246–1255. doi: 10.1111/liv.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis vs hepatorenal syndrome. Liver Transpl. 2012;18:539–548. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- 24.Charilaou P, Devani K, Petrosyan R, Reddy C, Pyrsopoulos N. Inpatient Mortality Benefit with Transjugular Intrahepatic Portosystemic Shunt for Hospitalized Hepatorenal Syndrome Patients. Dig Dis Sci. 2020;65:3378–3388. doi: 10.1007/s10620-020-06136-2. [DOI] [PubMed] [Google Scholar]
- 25.Jamil K, Pappas SC, Devarakonda KR. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2. J Exp Pharmacol. 2018;10:1–7. doi: 10.2147/JEP.S146034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818–828. doi: 10.1056/NEJMoa2008290. [DOI] [PubMed] [Google Scholar]
- 27.Ponzo P, Campion D, Rizzo M, Roma M, Caviglia GP, Giovo I, Rizzi F, Bonetto S, Saracco GM, Alessandria C. Transjugular intrahepatic porto-systemic shunt in cirrhotic patients with hepatorenal syndrome - chronic kidney disease: Impact on renal function. Dig Liver Dis. 2021 doi: 10.1016/j.dld.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Song T, Rössle M, He F, Liu F, Guo X, Qi X. Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:323–330. doi: 10.1016/j.dld.2018.01.123. [DOI] [PubMed] [Google Scholar]
- 29.Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thongprayoon C, Kaewput W, Petnak T, O'Corragain OA, Boonpheng B, Bathini T, Vallabhajosyula S, Pattharanitima P, Lertjitbanjong P, Qureshi F, Cheungpasitporn W. Impact of Palliative Care Services on Treatment and Resource Utilization for Hepatorenal Syndrome in the United States. Medicines (Basel) 2021;8 doi: 10.3390/medicines8050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 32.Zou B, Yeo YH, Jeong D, Park H, Sheen E, Lee DH, Henry L, Garcia G, Ingelsson E, Cheung R, Nguyen MH. A Nationwide Study of Inpatient Admissions, Mortality, and Costs for Patients with Cirrhosis from 2005 to 2015 in the USA. Dig Dis Sci. 2020;65:1520–1528. doi: 10.1007/s10620-019-05869-z. [DOI] [PubMed] [Google Scholar]
- 33.Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, Chalasani N, Fallon MB, Calhoun EA. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019;10:e00062. doi: 10.14309/ctg.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suneja M, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. Population Based Trends in the Incidence of Hospital Admission for the Diagnosis of Hepatorenal Syndrome: 1998-2011. Int J Nephrol. 2016;2016:8419719. doi: 10.1155/2016/8419719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witzke O, Baumann M, Patschan D, Patschan S, Mitchell A, Treichel U, Gerken G, Philipp T, Kribben A. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369–1373. doi: 10.1111/j.1440-1746.2004.03471.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Gülberg V, Sigal S, Bexon AS, Teuber P Terlipressin Study Group. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17:1328–1332. doi: 10.1002/lt.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magan AA, Khalil AA, Ahmed MH. Terlipressin and hepatorenal syndrome: what is important for nephrologists and hepatologists. World J Gastroenterol. 2010;16:5139–5147. doi: 10.3748/wjg.v16.i41.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin AA, Alabsawy EI, Jalan R, Davenport A. Epidemiology, Pathophysiology, and Management of Hepatorenal Syndrome. Semin Nephrol. 2019;39:17–30. doi: 10.1016/j.semnephrol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Maiwall R, Sarin SK, Moreau R. Acute kidney injury in acute on chronic liver failure. Hepatol Int. 2016;10:245–257. doi: 10.1007/s12072-015-9652-y. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni A, Sowmya T, Sharma M, Kumar P, Vasireddy S, Tevethia H, Padaki NR, Nageshwar Reddy D. IDDF2020-ABS-0192 Terlipressin non-response predicts mortality in acute-on-chronic liver failure-a prospective cohort study. Gut. 2020:A87. [Google Scholar]
- 42.Food and Drug Administration. Cardiovascular and Renal Drugs Advisory Committee Meeting. [cited 20 July 2020]. Available from: https://www.fda.gov/
- 43.Kulkarni AV, Arab JP, Premkumar M, Benítez C, Tirumalige Ravikumar S, Kumar P, Sharma M, Reddy DN, Simonetto DA, Rao PN. Terlipressin has stood the test of time: Clinical overview in 2020 and future perspectives. Liver Int. 2020;40:2888–2905. doi: 10.1111/liv.14703. [DOI] [PubMed] [Google Scholar]
- 44.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallée JC. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 45.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 46.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55–64. doi: 10.1002/hep.20262. [DOI] [PubMed] [Google Scholar]
- 47.Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H, Sauerbruch T. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheng XY, Lin FY, Wu J, Cao HC. Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study. World J Gastroenterol. 2021;27:2615–2629. doi: 10.3748/wjg.v27.i20.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We conducted a retrospective cohort study of hospital admissions for HRS from 2005 to 2014 in the National Inpatient Sample (NIS) database (publicly available and de-identified).