Abstract
Background
Results of NRG Oncology RTOG 0825 reported adding bevacizumab to standard chemoradiation did not significantly improve survival endpoints and resulted in greater decline in neurocognitive function (NCF) and patient-reported outcomes (PRO) over time in bevacizumab-treated patients. The present report provides additional results of patient-centered outcomes over time and their prognostic association with survival endpoints.
Methods
NCF tests, MD Anderson Symptom Inventory - Brain Tumor Module (MDASI-BT), and European Organization for Research and Treatment of Cancer (EORTC) quality of life (QOL) questionnaire with brain cancer module (QLQ-C30/BN20) were completed in a subset of progression-free patients at baseline and longitudinally. The prognostic value of baseline and early changes in NCF and PROs and differences between treatments from baseline to follow-up assessments were evaluated.
Results
A total of 508 randomized patients participated. Baseline/early changes in NCF and PROs were prognostic for OS and PFS. No between-arm differences in time to deterioration were found. At week 6, patients treated with bevacizumab evidenced greater improvement on NCF tests of executive function and the MDASI-BT Cognitive Function scale, but simultaneously reported greater decline on the EORTC Cognitive Function Scale. At later time points (weeks 22, 34, and 46), patients treated with bevacizumab had greater worsening on NCF tests as well as PRO measures of cognitive, communication, social function, motor symptoms, general symptoms, and interference.
Conclusion
The collection of patient-centered clinical outcome assessments in this phase III trial revealed greater deterioration in NCF, symptoms, and QOL in patients treated with bevacizumab. Baseline and early change in NCF and PROs were prognostic for survival endpoints.
Keywords: glioma, neurocognitive function, patient-reported outcome
Key Points.
Baseline neurocognitive function and self-reported symptoms in glioblastoma (GBM) patients are prognostic.
Bevacizumab is associated with worse cognitive function and symptoms in newly diagnosed GBM patients.
Importance of the Study.
This report of the clinical outcomes assessment in RTOG 0825 is important in that it provides evidence that treatment with bevacizumab is associated with worse neurocognitive function and patient-reported symptoms during treatment as compared to standard of care. This report adds to the literature as it included both objective testing and self-report of symptoms in addition to assessment of quality of life, with all measures demonstrating lack of benefit from the addition of bevacizumab. In addition, the prognostic utility of baseline clinical outcomes assessment in this vulnerable patient population was reported. Finally, this report demonstrates that the collection of these measures in large trials is feasible and provides important data on the impact of treatment on how the person feels and functions.
Glioblastoma (GBM) is one of the most common malignant primary brain tumors. At diagnosis, standard treatment includes the use of concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide.1 Despite the improvement in overall survival (OS) with this approach, median survival remains poor with only 10%-15% of patients living 5 years or longer. Patients often experience neurocognitive deficits and neurologic symptoms from the time of diagnosis, and most patients experience progressive decline throughout the illness trajectory. As a consequence, evaluating the impact of new therapeutic approaches should include evaluation of the clinical benefit of the treatment; specifically addressing whether the treatment results in improvement of disease-related neurocognitive dysfunction and symptoms and/or if the treatment has unfavorable systemic or neurologic toxicity.
Studies evaluating the efficacy of bevacizumab in patients with recurrent GBM demonstrated an improved progression-free survival (PFS), a reduction in the use of corticosteroids, and stable or improved neurocognitive function (NCF) in the majority of patients prior to disease progression.2–4 These results led to the accelerated Food and Drug Administration approval of bevacizumab for patients with recurrent GBM. NRG Oncology RTOG 0825 was undertaken to evaluate whether adding bevacizumab to standard treatment at diagnosis, resulted in either improved OS or PFS in patients with GBM. NRG Oncology RTOG 0825 implemented a multi-method evaluation of patient-centered clinical outcome assessments to help evaluate the net clinical benefit of these treatment strategies. In NRG Oncology RTOG 0825, survival outcomes were not improved and patients randomized to the bevacizumab arm demonstrated greater decline in NCF, greater neurocognitive complaints, increased symptom burden and interference, and reduced quality of life (QOL) over time despite being judged to be radiographically and clinically free of tumor progression.5 The present report provides additional results of analyses evaluating changes in NCF, symptoms, and health-related quality of life (HRQOL).
Patients and Methods
Patients
Eligibility criteria included age ≥18 years of age, newly diagnosed GBM confirmed on central review, Karnofsky performance status ≥70, proficient in English (to participate in the NCF, symptoms, and HRQOL component of the trial), and provide written informed consent to participate in this optional portion of the trial. The study was approved by the institutional review board or the equivalent panel at each study center before patient enrollment. Participating centers consisted of both community and academic centers within the National Cancer Institute’s National Clinical Trials network in the United States, Canada, Europe, and Israel.
Study Design
NRG Oncology RTOG 0825 (NCT00884741; protocol available on CTSU.org) was a randomized, double-blind, placebo-controlled, multicenter phase III trial that was sponsored by the National Cancer Institute and supported by an unrestricted educational grant provided by Genentech. Details of the treatment schema were previously reported.5 Briefly, all patients were registered to the trial prior to fractionated, conformal, or intensity-modulated radiotherapy and chemotherapy with temozolomide. The NRG Oncology Statistics and Data Management Center randomized patients using permuted block randomization in a parallel fashion 1:1 to receive either intravenously administered bevacizumab or placebo every 2 weeks starting at week 4 of radiotherapy until disease progression, severe treatment-related toxicity, or completion of adjuvant therapy. Up to 12 cycles of temozolomide were permitted in the adjuvant maintenance phase. Patients were stratified by O6-methylguanine-DNA methyltransferase (MGMT) and a tumor-based molecular profile based on the expression of nine genes. Patients were unblinded after progression to receive bevacizumab as salvage treatment.
Patient Evaluation and Follow-Up
Patients who were clinically and radiographically stable without evidence of disease progression were scheduled to complete neurocognitive testing and patient-reported outcomes (PROs) at baseline, end of radiation (corresponding to week 6 from the start of treatment) at the time of imaging studies during treatment (weeks 10, 22, 34, 46, and 62 from the start of treatment), and after treatment completion (every 3 months for 1 year, then every 4 months for 1 year, then every 6 months). The current manuscript reports the results from the first 46 weeks of patient follow-up in order to have sufficient patient numbers to analyze. The NCF test battery consisted of the following measures assessing learning and memory, processing speed, and executive function: Hopkins Verbal Learning Test-Revised (HVLT-R),6 Trail Making Test (TMT),7 and Controlled Oral Word Association (COWA).8 NCF testing was conducted by a healthcare professional that completed structured training and was credentialed by the study neuropsychologist (J.S.W.). At the same visit, patients additionally completed two PRO measures: the MD Anderson Symptom Inventory - Brain Tumor Module (MDASI-BT) and the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire with a brain cancer module (EORTC QLQ-C30/BN20). The NCF and PRO instruments and their scoring have been previously described.2,9
Endpoints
The aims of this study were to (1) determine the differential acute effects associated with the addition of bevacizumab to temozolomide and radiation, as compared to the conventional arm, on measures of NCF, symptoms, and HRQOL during radiation and across the longitudinal progression-free interval and (2) determine the relationship of NCF, symptoms, and HRQOL with PFS and OS.
Statistical Considerations
Sample size calculations for this trial were previously reported.5 All analyses were conducted as intent-to-treat by randomization arm. Results of the longitudinal repeated measures analysis using general linear models with fixed effects for study group and time and inclusion of MGMT status and RPA (recursive partitioning analysis) class to adjust for prognostic status were previously reported.5 The current manuscript reports further protocol-specified results of mean changes from baseline to each follow-up time point and time to deterioration (TTD) in patients that were clinically and radiographically determined to have no evidence of progressive disease. Changes from baseline to each subsequent time point were calculated by subtracting baseline from follow-up scores (ie, standardized NCF test scores, transformed EORTC scores, and raw MDASI-BT scores). Differences between arms were tested with a Wilcoxon-Mann-Whitney test due to departures from normality and effect size was calculated as the z score divided by the square root of the sample size.10Deterioration in NCF from baseline was determined based on the reliable change index (RCI)2,9,11; the minimally important difference (MID) for each PRO measure was used to determine deterioration from baseline in HRQOL ratings (10-point change)12 and MDASI-BT ratings (1-point change).9,13 TTD was defined for the NCF tests, HRQOL scales, and MDASI-BT factors individually. TTD in NCF was defined as the time required for a patient to evidence the first deterioration on any of the NCF tests. TTD in symptoms was defined as the time required for a patient to evidence the first deterioration on any of the MDASI-BT factors (ie, affective, cognitive, neurologic, treatment, generalized/disease, gastrointestinal [GI] factor). TTD in HRQOL was defined as the time required for a patient to evidence the first deterioration on any of the following EORTC QLQ-C30/BN20 scales (ie, global QOL, physical functioning, cognitive functioning, social functioning, motor dysfunction, and communication deficit). A cumulative incidence approach was used, treating death and progression as a competing risk, with testing between treatments arms done using Gray’s test.14 Cause-specific Cox proportional hazards models were used to determine hazard ratios and 95% confidence intervals (CI).15 A P value of .0167, calculated as 0.05/3 for each component of this study (NCF, QOL, and symptoms), was used to determine statistical significance for all analyses given that these substudy endpoints were considered tertiary. A sensitivity analysis was conducted in which TTD was evaluated in the same manner as above except death was counted as an event rather than as a competing risk.
Cox proportional hazards models were used to determine if baseline or early change (from baseline to week 6) scores on the NCF tests, the MDASI-BT, or the EORTC QLQ-C30/BN20 were prognostic for OS and PFS in univariable and multivariable analyses after accounting for RPA class (III vs IV vs V) and MGMT status (unmethylated vs methylated). Separate multivariable models were built for baseline and early changes with collinearity of covariates assessed using Spearman correlation coefficients. A significance level of 0.10 was used for univariable analyses due to the exploratory nature of model building using stepwise selection to reduce covariates in the models.
Results
Patient Characteristics
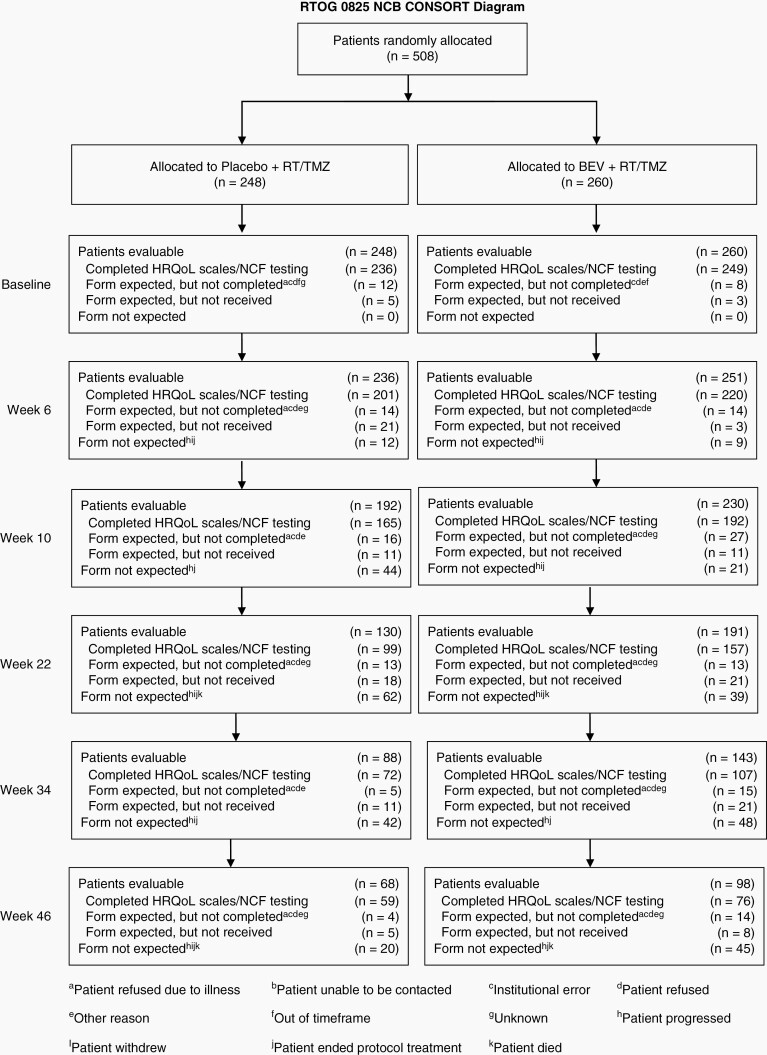
Detailed reports of participation rates in the NCF and PRO clinical outcome assessments and subject characteristics have been previously published.5 Between April 2009 and May 2011, when the target accrual was met, 978 patients were enrolled, and 637 patients were randomized on NRG Oncology RTOG 0825. Of the enrolled patients, 525 patients consented to participate in longitudinal evaluation of NCF, symptoms, and HRQOL as described in our previous publication.5 There was no difference between the 525 (81%) patients that consented to participate in the substudy and the 125 (19%) patients that did not on any sociodemographic or clinical characteristics.5 The substudy sample included 260 patients that were randomized to bevacizumab and 248 patients that were randomized to placebo; 17 patients were not randomized (Figure 1). There were no between-arm differences in sociodemographic or clinical characteristics for patients participating in the substudy5 (Table 1).
Fig. 1.
NRG Oncology RTOG 0825 substudy CONSORT diagram and completion rates.
Table 1.
Baseline Characteristics of Randomized Patients that Consented to the Substudy
| Placebo (n = 248) | Bevacizumab (n = 260) | Chi-Square Test P Value | |
|---|---|---|---|
| Age (y) | |||
| Median (min-max) | 57 (19-82) | 59 (21-82) | |
| >=50 | 194 (78.2%) | 213 (81.9%) | .30 |
| Gender | .17 | ||
| Male | 156 (62.9%) | 148 (56.9%) | |
| Race (n, %) | .59 | ||
| White | 235 (94.8%) | 251 (96.5%) | |
| Black or African American | 5 (2.0%) | 4 (1.5%) | |
| Other | 8 (3.2%) | 5 (2.0%) | |
| Education | .10 | ||
| Less than high school | 17 (6.9%) | 9 (3.5%) | |
| High school graduate/GED | 58 (23.4%) | 73 (28.1%) | |
| Some college/ vocational school/ associate degree | 74 (29.8%) | 58 (22.3%) | |
| Bachelor’s degree or higher | 75 (30.2%) | 92 (35.4%) | |
| Other | 24 (9.7%) | 28 (10.8%) | |
| KPS | .82 | ||
| 90-100 | 156 (62.9%) | 161 (61.9%) | |
| 70-80 | 92 (37.1%) | 99 (38.1%) | |
| Surgery | .40 | ||
| Gross total | 146 (58.9%) | 166 (63.8%) | |
| Subtotal | 94 (37.9%) | 89 (34.2%) | |
| Other | 8 (3.2%) | 5 (1.9%) | |
| Neurologic function | .75 | ||
| No symptoms | 87 (35.1%) | 89 (34.2%) | |
| Minor symptoms | 108 (43.5%) | 121 (46.5%) | |
| Moderate symptoms | 53 (21.4%) | 50 (19.2%) | |
| Laterality | Right vs Left | .60 | |
| Right | 135 (54.4%) | 147 (56.5%) | |
| Left | 108 (43.5%) | 107 (41.2%) | |
| Bilateral | 4 (1.6%) | 5 (1.9%) | |
| Unknown | 1 (0.4%) | 1 (0.4%) | |
| MGMT status | Methylated vs Unmethylated | .64 | |
| Methylated | 68 (27.4%) | 76 (29.2%) | |
| Unmethylated | 171 (69.0%) | 178 (68.5%) | |
| Invalid | 9 (3.6%) | 6 (2.3%) | |
| Molecular profile | Favorable vs Unfavorable | .87 | |
| Favorable | 66 (26.6%) | 62 (23.8%) | |
| Unfavorable | 160 (64.5%) | 175 (67.3%) | |
| Indeterminate | 20 (8.1%) | 20 (7.7%) | |
| Failed | 2 (0.8%) | 3 (1.2%) | |
| RPA class | .18 | ||
| III | 40 (16.1%) | 30 (11.5%) | |
| IV | 156 (62.9%) | 183 (70.4%) | |
| V | 45 (18.1%) | 44 (16.9%) | |
| Unknown | 7 (2.8%) | 3 (1.2%) | |
| Tumor location | |||
| Frontal | 97 (39.1%) | 100 (38.5%) | |
| Temporal | 104 (41.9%) | 111 (42.7%) | |
| Parietal | 92 (37.1%) | 95 (36.5%) | |
| Occipital | 26 (10.5%) | 45 (17.3%) | |
| Other | 24 (9.7%) | 21 (8.1%) |
Abbreviations: MGMT, O6-methylguanine-DNA methyltransferase; GED, General Education Development; KPS, Karnofsky Performance Status; RPA, recursive partitioning analysis.
At baseline, analyzable (defined as measures completed without error) NCF test results were available for ≥94% of patients; ≥94% of patients had symptom measures, and ≥95% had HRQOL measures that were analyzable.5
Baseline NCF, Symptoms, and HRQOL
As can be seen in Table 2, patients presented with a wide range of performances on the NCF tests with the majority of patients performing below the healthy population, consistent with mild or greater impairment at baseline. Likewise, patients presented with symptom severity reported across the range of severity with the majority providing ratings <2 (in the minimal range) (Table 3). Most patients reported their HRQOL to be high and similar to the general population.16 The greatest exception to this was numerically lower ratings on the Cognitive Functioning and Social Functioning scales. There were no between-arm differences on any NCF test or the MDASI-BT at baseline. Patients randomized to the bevacizumab arm reported less Communication Deficit on the EORTC QLQ-BN20 (14.3 vs 17.9, P = .03) at baseline.
Table 2.
Baseline Neurocognitive Test Scores
| Placebo | Bevacizumab | Mann-Whitney Test P Value (Effect Sizec) | |
|---|---|---|---|
| Neurocognitive testa | |||
| HVLT-R Total Recall | (n = 235) | (n = 250) | |
| Mean (SD) | −1.4 (1.6) | −1.4 (1.5) | |
| Median (min, max) | −1.3 (−6.3 to 1.8) | −1.3 (−5.7 to 1.7) | .89 (0.006) |
| HVLT-R Delayed Recall | (n = 237) | (n = 249) | |
| Mean (SD) | −1.5 (1.8) | −1.4 (1.7) | |
| Median (min, max) | −1.3 (−6.1 to 1.2) | −1.3 (−5.5 to 1.2) | .95 (0.003) |
| HVLT-R Delayed Recognition | (n = 236) | (n = 249) | |
| Mean (SD) | −1.0 (2.3) | −0.8 (1.8) | |
| Median (min, max) | −0.2 (−14.9 to 0.9) | −0.4 (−10.2 to 0.9) | .64 (0.021) |
| TMT Part A | (n = 237) | (n = 244) | |
| Mean (SD) | −3.4 (13.1) | −3.0 (7.7) | |
| Median (min, max) | −0.9 (−160.2 to 2.2) | −1.2 (−96.2 to 2.1) | .18 (0.062) |
| TMT Part B | (n = 236) | (n = 238) | |
| Mean (SD) | −4.1 (9.3) | −4.8 (14.4) | |
| Median (min, max) | −1.3 (−97.4 to 4.0) | −1.4 (−194.2 to 2.3) | .56 (0.027) |
| COWA | (n = 239) | (n = 247) | |
| Mean (SD) | −0.9 (1.4) | −1.0 (1.2) | |
| Median (min, max) | −0.9 (−4.1 to 5.0) | −1.0 (−3.9 to 2.6) | .29 (0.048) |
| CTB Compositeb | (n = 236) | (n = 244) | |
| Mean (SD) | −2.0 (3.5) | −2.0 (3.2) | |
| Median (min, max) | −1.2 (−33.4 to 2.1) | −1.3 (−35.2 to 1.0) | .37 (0.041) |
Abbreviations: COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery; HVLT-R, Hopkins Verbal Learning Test-Revised; SD, standard deviation; TMT, Trail Making Test.
aNeurocognitive test scores are presented as standardized z scores (mean = 0, SD = 1) where negative scores represent worse neurocognitive function. bCTB Composite score is the mean of standardized scores (HVLT-R Total Recall, HVLT-R Delayed Recall, HVLT-R Delayed Recognition, TMT Part A and B, COWA). At least 5 scores had to be available to calculate this variable. cEffect size calculated as Wilcoxon z score divided by the square root of the sample size.
Table 3.
Baseline HRQOL and Symptom Scores
| Placebo | Bevacizumab | Mann-Whitney Test P Value (Effect Sizef) | |
|---|---|---|---|
| EORTC QLQ-C30/BN20a | |||
| Global QOLb | (n = 236) | (n = 248) | |
| Mean (SD) | 68.6 (24.4) | 70.4 (21.3) | |
| Median (min, max) | 75.0 (0.0-100.0) | 75.0 (0.0-100.0) | .70 (0.018) |
| Physical functioningb | (n = 235) | (n = 249) | |
| Mean (SD) | 85.6 (17.5) | 85.7 (16.6) | |
| Median (min, max) | 93.3 (13.3-100.0) | 93.3 (13.3-100.0) | .85 (0.008) |
| Cognitive functioningb | (n = 236) | (n = 249) | |
| Mean (SD) | 72.7 (24.1) | 76.0 (22.9) | |
| Median (min, max) | 83.3 (0.0-100.0) | 83.3 (0.0-100.0) | .12 (0.071) |
| Social functioningb | (n = 236) | (n = 249) | |
| Mean (SD) | 69.6 (24.1) | 70.1 (22.9) | |
| Median (min, max) | 66.7 (0.0-100.0) | 66.7 (0.0-100.0) | .79 (0.012) |
| Motor dysfunctionc | (n = 235) | (n = 248) | |
| Mean (SD) | 16.3 (19.9) | 13.4 (18.4) | |
| Median (min, max) | 11.1 (0.0-100.0) | 11.1 (0.0-100.0) | .09 (0.078) |
| Communication deficitc | (n = 235) | (n = 249) | |
| Mean (SD) | 17.9 (21.5) | 14.3 (20.6) | |
| Median (min, max) | 11.1 (0.0-100.0) | 0.0 (0.0-100.0) | .03 (0.101) |
| MDASI-BTd | |||
| Symptom Burdene | (n = 234) | (n = 244) | |
| Mean (SD) | 1.4 (1.4) | 1.2 (1.1) | |
| Median (min, max) | 1.0 (0.0-9.0) | 0.9 (0.0-5.7) | .28 (0.050) |
| Symptom Interference - Globale | (n = 234) | (n = 243) | |
| Mean (SD) | 2.0 (2.3) | 1.9 (2.1) | |
| Median (min, max) | 1.2 (0.0-9.2) | 1.2 (0.0-9.0) | .79 (0.012) |
| Symptom Interference - Activitiese | (n = 234) | (n = 243) | |
| Mean (SD) | 2.2 (2.5) | 2.2 (2.4) | |
| Median (min, max) | 1.0 (0.0-10.0) | 1.3 (0.0-10.0) | .49 (0.031) |
| Symptom Interference - Moode | (n = 234) | (n = 242) | |
| Mean (SD) | 1.8 (2.3) | 1.6 (2.0) | |
| Median (min, max) | 1.0 (0.0-9.0) | 1.0 (0.0-9.0) | .75 (0.015) |
| Affective Factore | (n = 235) | (n = 244) | |
| Mean (SD) | 2.3 (2.2) | 2.1 (1.9) | |
| Median (min, max) | 1.6 (0.0-10.0) | 1.4 (0.0-8.4) | .48 (0.032) |
| Cognitive Factore | (n = 234) | (n = 244) | |
| Mean (SD) | 1.5 (2.0) | 1.4 (1.8) | |
| Median (min, max) | 0.8 (0.0-9.3) | 0.8 (0.0-8.3) | .52 (0.029) |
| Neurologic Factore | (n = 235) | (n = 243) | |
| Mean (SD) | 1.2 (1.7) | 0.9 (1.2) | |
| Median (min, max) | 0.7 (0.0-9.3) | 0.3 (0.0-6.7) | .09 (0.077) |
| Treatment Factore | (n = 234) | (n = 244) | |
| Mean (SD) | 1.4 (1.6) | 1.4 (1.6) | |
| Median (min, max) | 1.0 (0.0-9.3) | 0.7 (0.0-8.0) | .88 (0.007) |
| Generalized/Disease Factore | (n = 232) | (n = 243) | |
| Mean (SD) | 1.1 (1.5) | 0.9 (1.1) | |
| Median (min, max) | 0.5 (0.0-9.5) | 0.5 (0.0-7.0) | .09 (0.078) |
| GI Factore | (n = 231) | (n = 240) | |
| Mean (SD) | 0.3 (1.2) | 0.2 (0.6) | |
| Median (min, max) | 0.0 (0.0-9.5) | 0.0 (0.0-4.5) | .90 (0.006) |
Abbreviations: GI, gastrointestinal; HRQOL, health-related quality of life; MDASI-BT, MD Anderson Symptom Inventory - Brain Tumor Module; SD, standard deviation.
Activities: work, general activity, walking; Mood: relationships with other people, enjoyment of life, mood.
aEORTC QLQ-C30/BN20 Scale scores are presented as transformed scores; bHigher scores represent better HRQOL; cHigher scores represent worse HRQOL/symptoms; dMDASI-BT Factor scores are presented as raw scores; eHigher scores represent worse symptoms; fEffect size calculated as Wilcoxon z score divided by the square root of the sample size.
Deterioration in NCF, Symptoms, and HRQOL Relative to Baseline
Across the first 46 weeks of follow-up, the frequency of deterioration at each time point relative to baseline for each measure based on RCI/MID criterion is reported in Table 4 and Supplementary Table 1. At week 6, patients treated with bevacizumab evidenced less frequent deterioration on TMTB (Trail Making Test Part B), COWA, and MDASI-BT (Cognitive Factor), and more frequent deterioration on the EORTC (Cognitive Function Scale) and MDASI-BT (Affective Factor). At week 10, patients treated with placebo evidenced greater worsening on the MDASI-BT (GI Factor). At week 22, patients treated with bevacizumab evidenced greater worsening on the MDASI-BT (Symptom Interference - Mood). At week 34, patients treated with bevacizumab evidenced greater worsening on TMTA (Trail Making Test Part A) and TMTB, MDASI-BT (Affective Factor, Treatment Factor, Generalized/Disease Factor), and EORTC (Motor Dysfunction Scale). At week 46, patients treated with bevacizumab evidenced greater worsening on TMTA, COWA, MDASI-BT (Symptom Interference - Activities), and EORTC (Cognitive Function Scale, Communication Deficit Scale, and Social Functioning Scale). Changes scores and corresponding effect sizes are located in Supplementary Tables 2–4.
Table 4.
Deterioration Frequencies
| Placebo | Bevacizumab | Chi-Square Test P Value | |
|---|---|---|---|
| Neurocognitive testa | |||
| HVLT-R Total Recall | |||
| Week 6 | (n = 185) | (n = 207) | .24 |
| Deteriorated | 18.4% | 23.2% | |
| Week 10 | (n = 154) | (n = 178) | .10 |
| Deteriorated | 27.9% | 20.2% | |
| HVLT-R Delayed Recall | |||
| Week 6 | (n = 186) | (n = 207) | .78 |
| Deteriorated | 20.4% | 19.3% | |
| Week 10 | (n = 155) | (n = 178) | .75 |
| Deteriorated | 27.1% | 28.7% | |
| HVLT-R Delayed Recognition | |||
| Week 6 | (n = 185) | (n = 207) | .82 |
| Deteriorated | 28.1% | 27.1% | |
| Week 10 | (n = 155) | (n = 176) | .16 |
| Deteriorated | 20.0% | 14.2% | |
| TMTA | |||
| Week 6 | (n = 183) | (n = 197) | .10 |
| Deteriorated | 25.1% | 18.3% | |
| Week 10 | (n = 154) | (n = 169) | .24 |
| Deteriorated | 10.4% | 14.8% | |
| TMTB | |||
| Week 6 | (n = 179) | (n = 193) | .04 |
| Deteriorated | 26.3% | 17.6% | |
| Week 10 | (n = 151) | (n = 162) | .12 |
| Deteriorated | 25.2% | 17.9% | |
| COWA | |||
| Week 6 | (n = 188) | (n = 205) | .002 |
| Deteriorated | 11.7% | 3.4% | |
| Week 10 | (n = 156) | (n = 175) | .78 |
| Deteriorated | 4.5% | 5.1% | |
| CTB Composite* | |||
| Week 6 | (n = 183) | (n = 201) | .16 |
| Deteriorated | 36.6% | 29.9% | |
| Week 10 | (n = 154) | (n = 170) | .19 |
| Deteriorated | 35.1% | 28.2% | |
| MDASI-BT | |||
| Symptom Burden | |||
| Week 6 | (n = 186) | (n = 206) | .45 |
| Deteriorated | 28.5% | 32.0% | |
| Week 10 | (n = 158) | (n = 180) | .65 |
| Deteriorated | 20.9% | 18.9% | |
| Symptom Interference - Global | |||
| Week 6 | (n = 187) | (n = 206) | .42 |
| Deteriorated | 35.8% | 39.8% | |
| Week 10 | (n = 158) | (n = 180) | .74 |
| Deteriorated | 31.6% | 33.3% | |
| Symptom Interference - Activities | |||
| Week 6 | (n = 187) | (n = 206) | .22 |
| Deteriorated | 35.3% | 41.3% | |
| Week 10 | (n = 158) | (n = 180) | .68 |
| Deteriorated | 35.4% | 33.3% | |
| Symptom Interference - Mood | |||
| Week 6 | (n = 187) | (n = 205) | .13 |
| Deteriorated | 32.1% | 39.5% | |
| Week 10 | (n = 158) | (n = 179) | .62 |
| Deteriorated | 31.0% | 33.5% | |
| Cognitive Factor | |||
| Week 6 | (n = 186) | (n = 206) | .05 |
| Deteriorated | 33.5% | 24.3% | |
| Week 10 | (n = 158) | (n = 180) | .51 |
| Deteriorated | 24.7% | 21.7% | |
| Neurologic Factor | |||
| Week 6 | (n = 187) | (n = 205) | .83 |
| Deteriorated | 18.2% | 19.0% | |
| Week 10 | (n = 159) | (n = 180) | .21 |
| Deteriorated | 18.9% | 13.9% | |
| Treatment Factor | |||
| Week 6 | (n = 186) | (n = 206) | .22 |
| Deteriorated | 46.8% | 52.9% | |
| Week 10 | (n = 158) | (n = 180) | .74 |
| Deteriorated | 41.8% | 40.0% | |
| Generalized Disease Factor | |||
| Week 6 | (n = 185) | (n = 206) | .58 |
| Deteriorated | 27.6% | 30.1% | |
| Week 10 | (n = 156) | (n = 180) | .64 |
| Deteriorated | 24.4% | 22.2% | |
| Affective Factor | |||
| Week 6 | (n = 187) | (n = 206) | .04 |
| Deteriorated | 27.8% | 37.4% | |
| Week 10 | (n = 159) | (n = 180) | .36 |
| Deteriorated | 28.3% | 23.9% | |
| GI Factor | |||
| Week 6 | (n = 181) | (n = 201) | .59 |
| Deteriorated | 29.3% | 31.8% | |
| Week 10 | (n = 156) | (n = 176) | .04 |
| Deteriorated | 28.8% | 19.3% | |
| EORTC QLQ-C30/BN20 | |||
| Global HRQOL Scale | |||
| Week 6 | (n = 191) | (n = 212) | .19 |
| Deteriorated | 32.5% | 38.7% | |
| Week 10 | (n = 159) | (n = 184) | .89 |
| Deteriorated | 34.6% | 35.3% | |
| Cognitive Functioning Scale | |||
| Week 6 | (n = 192) | (n = 213) | .01 |
| Deteriorated | 29.2% | 40.8% | |
| Week 10 | (n = 159) | (n = 184) | .56 |
| Deteriorated | 27.0% | 29.9% | |
| Physical Functioning Scale | |||
| Week 6 | (n = 191) | (n = 211) | .64 |
| Deteriorated | 36.1% | 38.4% | |
| Week 10 | (n = 157) | (n = 184) | .46 |
| Deteriorated | 32.5% | 28.8% | |
| Social Functioning Scale | |||
| Week 6 | (n = 191) | (n = 213) | .23 |
| Deteriorated | 35.1% | 40.8% | |
| Week 10 | (n = 158) | (n = 184) | .43 |
| Deteriorated | 36.7% | 32.6% | |
| Motor Dysfunction | |||
| Week 6 | (n = 190) | (n = 211) | .57 |
| Deteriorated | 30.5% | 28.0% | |
| Week 10 | (n = 156) | (n = 184) | .31 |
| Deteriorated | 27.6% | 32.6% | |
| Communication Deficit | |||
| Week 6 | (n = 190) | (n = 212) | .34 |
| Deteriorated | 25.3% | 21.2% | |
| Week 10 | (n = 156) | (n = 184) | .67 |
| Deteriorated | 27.6% | 25.5% |
Abbreviations: COWA, Controlled Oral Word Association; CTB, Clinical Trial Battery; HRQOL, health-related quality of life; HVLT-R, Hopkins Verbal Learning Test-Revised; MDASI-BT, MD Anderson Symptom Inventory - Brain Tumor Module; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B.
Deterioration is calculated using the change score.
aChange score is calculated using standardized z score.
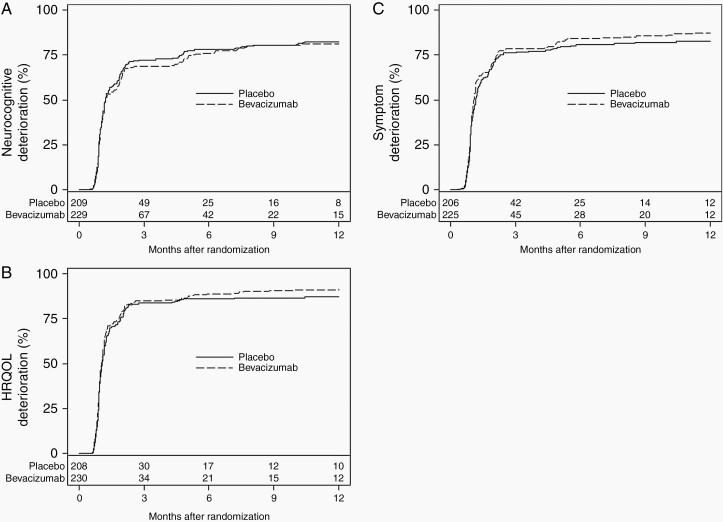
TTD in NCF, Symptoms, and HRQOL
There was no statistically significant difference between arms in the time to neurocognitive, symptom, or HRQOL deterioration. As can be seen in Figure 2A and C, patients in both arms deteriorated very early. Median time to neurocognitive deterioration for patients treated with placebo was 1.22 months (95% CI: 1.15-1.41) vs 1.24 months (95% CI: 1.12-1.81) for patients treated with bevacizumab. Median time to symptom deterioration (MDASI-BT) was 1.15 (95% CI: 1.08-1.25) and 1.12 (95% CI: 1.02-1.15) for patients treated with placebo vs bevacizumab, respectively. Median TTD in HRQOL (EORTC) was 1.08 months (95% CI: 1.02-1.15) and 1.05 months (95% CI: 0.99-1.12) for patients treated with placebo vs bevacizumab, respectively. In the sensitivity analyses where death was treated as a deterioration event, there was a trend for patients treated with bevacizumab to have more rapid deterioration in Global Health Status and Social Functioning on the EORTC QLQ-C30 (Supplementary Figures 1 and 2). There was no statistically significant difference in the TTD between arms on any other HRQOL subscale (results not shown) or overall NCF, symptom, or HRQOL (Supplementary Figures 3–6).
Fig. 2.
A, Time to neurocognitive deterioration. B, Time to symptom worsening. C, Time to HRQOL deterioration. Abbreviation: HRQOL, health-related quality of life.
Baseline and Early Worsening Prediction of PFS and OS
In univariable analyses, baseline and early changes in numerous NCF tests, symptom factors, and HRQOL scales were associated with OS (Supplementary Table 5). In terms of PFS, numerous baseline NCF tests, symptom factors, and HRQOL scales were associated with PFS but only early changes in NCF tests and symptom factors were associated with PFS (Supplementary Table 6). Multivariable analyses demonstrated baseline and early changes in the TMT Part B score from the NCF tests (baseline: HR = 1.30, 95% CI: 1.03-1.65, P = .029; early changes: HR = 1.48, 95% CI: 1.05-2.10, P = .026); baseline Neurologic factor (HR = 1.12, 95% CI: 1.03-1.21, P = .005) and early changes in the Cognitive factor score (HR = 1.61, 95% CI: 1.18-2.22, P = .003) from the MDASI-BT; and, baseline Physical Function and Motor Dysfunction scale (HR = 0.99, 95% CI: 0.98-0.99, P < .001) and early changes in Communication Deficit (HR = 1.51, 95% CI: 1.12-2.04, P = .007) from the EORTC QLQ-C30/BN20 were associated with OS (Supplementary Table 7). Multivariable analyses only demonstrated baseline MDASI-BT Neurologic factor score (HR = 1.10, 95% CI: 1.02-1.18, P = .015) and baseline EORTC Physical Function scale (HR = 0.99, 95% CI: 0.98-0.99, P < .001) were associated with PFS (Supplementary Table 8).
Discussion
As has been reported previously, treatment with bevacizumab did not increase OS time in patients with GBM that participated in NRG Oncology RTOG 0825; PFS time was longer but did not exceed the pre-specified criterion. In addition, longitudinal repeated measures modeling in patients that were clinically and radiographically progression-free demonstrated greater worsening in aspects of NCF, symptoms, and HRQOL for patients in the bevacizumab arm compared to the patients in the placebo arm.5
The current report extends the work examining differences in NCF, symptoms, and HRQOL found in NRG Oncology RTOG 0825. Completion rates of NCF testing and PROs were excellent at baseline (≥94%). At baseline, most patients present with neurocognitive dysfunction while HRQOL on average is similar to the healthy population. The majority of patients endorsed minimal symptoms at baseline through a wide range of symptom burden can be appreciated. Both baseline and early worsening in select measures of NCF, symptoms, and HRQOL were predictive of both PFS and OS, highlighting the importance of these measures to survival outcomes and the potential utility as either stratification factors or as study entry criteria in future clinical trials.
Through 46 weeks, completion rates of NCF testing and PROs were>75%, which is quite favorable relative to previous clinical trials. It is hypothesized that this improved participation rate and reduction in attrition and missing data are due to substantial efforts from the study team to inform sites of the importance of and to increase familiarity with this component of the trial. The successful integration and participation in this aspect of the trial builds on previous work utilizing the same measures,9 and represents a critical milestone in neuro-oncology that allows investigators to design studies with fewer concerns that these important endpoints will be inevaluable due to excessive, unplanned missing data. Clearly, there remains room to improve (particularly in terms of obtaining these data over time as patients begin to develop increased brain damage), which is needed both to address the pressing scientific and clinical questions posed in these trials and because regulatory authorities have recognized these outcomes as being critical in the appraisal of treatments for patients with brain tumors.17
In patients that were clinically and radiographically free from progressive disease, the median time to decline in all Net Clinical Benefit (NCB) assessments was <2 months in both treatment arms with no appreciable differences between the arms. This demonstrates the early and relentless neurodegenerative course experienced by patients with GBM, which is seen both on tests of NCF and patient self-reports of symptoms and HRQOL. Unfortunately, patients treated with bevacizumab did not experience a delay in time to decline in NCF or worsening in symptom burden or HRQOL. Importantly, worsening of multiple measures of NCF, symptoms, and HRQOL occurred at multiple later time points compared to baseline. This highlights that, on average, the adverse effects of the additional therapy may be additive to that of the disease and the importance of evaluating not only the disease-related effects but also those of the therapy to better understand the clinical impact of the treatment on the individual.
When each measure was examined at individual time points, patients treated with bevacizumab demonstrated greater improvements in executive function on objective testing at week 6 as well as improved self-reported cognitive function on the MDASI-BT, although they also self-reported greater neurocognitive complaints on the HRQOL measure. At week 10, patients that received placebo reported greater worsening on the GI Factor of the MDASI-BT. Patients treated with bevacizumab did not demonstrate or report improved NCF, symptoms, or HRQOL at any other time point compared to patients that received placebo. By week 34, patients treated with bevacizumab demonstrated worse NCF test results and/or greater neurocognitive complaints on at least one of the clinical outcome assessment measures. Additionally, patients treated with bevacizumab reported more worsening on multiple components of the symptom measure, reflective of both disease- and treatment-associated symptoms, and primarily cognitive and social role functioning changes on the HRQOL measure. While it is not possible to isolate the reason for the early and time-limited benefit in tested executive function and self-reported cognitive function on the MDASI-BT at week 6 in patients that received a single dose of bevacizumab, it may be analogous to some beneficial effects of steroids on symptoms of edema and is consistent with anecdotal experience of an immediate benefit on mental status for a subgroup of patients. However, patients’ subjective global appraisal of their NCF on the HRQOL measure was opposite this result. One possible explanation for this difference is the specific measures of executive function that worsened and consideration of the individual items in each PRO tool. Importantly, it is the COWA and TMTB objective tests at week 6 that show less deterioration. The MDASI-BT cognitive factor includes the patients’ perceived severity of the following symptoms: difficulty understanding, remembering, speaking, and concentrating. The EORTC Cognitive scale includes only difficulty in concentrating, reading a newspaper or watching television, and difficulty remembering things, with trouble finding the right words to express yourself, difficulty speaking and communicating your thoughts included in a separate item grouping referred to as the “communication scale.” This summary score did align with the findings from week 6 on other tests and measures and may indicate how these items are represented in the scales leads to this difference. For clinicians who employ these tools to monitor patients, it is important to understand the individual symptoms that are included in summary scores as well as the domains evaluated for any test that is employed to ensure understanding of these results.
Efforts to develop predictive models to identify patients that may experience at least early benefit from bevacizumab may be of value. However, caution must be applied to the interpretation of these results as corrections for multiple comparisons were not applied given the exploratory nature of these analyses.
Despite the similarities between NRG Oncology RTOG 0825 and the AvaGlio trial,18 there are numerous differences in study design, outcome measures, and statistical analytical approaches employed that complicate a simple “head-to-head” comparison. The most immediate and obvious are differences in the patient-centered clinical outcome assessments: NRG Oncology RTOG 0825 used 3 tests of NCF that measure episodic learning and memory, processing speed, and executive function, while AvaGlio used a dementia screening instrument (Mini Mental State Examination); RTOG used a multi-symptom inventory (MDASI-BT), AvaGlio had no such measure; and both RTOG and AvaGlio used the EORTC QLQ-C30/BN20 HRQOL measures. While it is tempting to compare the results from the HRQOL measure across studies, differences in the statistical analysis and study design hamper the validity of such cross-trial comparisons of the published results. A particularly compelling finding within NRG Oncology RTOG 0825 was the consistency of findings across both objective tests of NCF and subjective ratings of cognitive function in both the longitudinal repeated measures analysis and at the later time points in the change from baseline analyses.
As the analyses of the patient-centered outcomes were dependent on the clinical and radiographic status of the patient, examination of these outcomes again after central review of the imaging studies of the brain are of high interest. Such analyses may help clarify the hypothesis that the greater declines in NCF and symptom report over time in the bevacizumab patients was due to the imaging criteria in NRG Oncology RTOG 0825 failing to detect tumor progression as early in the bevacizumab group as it does in the placebo group leading to more patients in the bevacizumab group at each time point with progressing brain tumor/brain damage and thus measurably greater neurocognitive dysfunction.
The current study has several limitations including imperfect compliance with NCF test and PRO completion as described earlier, the absence of NCF test and PRO data after the patient experienced progressive disease, application of imaging criterion that may not have been able to detect tumor progression equally well in both arms, and limitations in generalizing the results observed in the clinical trial population to all patients with GBM. As described previously, we have made tremendous strides in improving compliance to these endpoints and are confident that the neuro-oncology clinical and research community will continue to improve in this area. One of the most common reasons for noncompliance with NCF testing and PRO completion is disease progression; however, collecting this critical data at the time of and/or after progression would allow examination of the extent of contribution of tumor progression to changes in NCF and PROs. As the scans for patients on trial were collected and banked there will be opportunity for reanalysis of the imaging with newer criterion that may further enhance our understanding of the impact of disease and treatment on patients’ NCF, symptoms, and HRQOL. As is true with most phase III clinical trials in newly diagnosed patients with GBM, the eligibility criteria generally limit access to the subpopulation of patients in better health, which will limit the generalizability of the findings to the broader and often less well population of patients with this disease.
NRG Oncology RTOG 0825 has firmly established that it is feasible and highly relevant to the interpretation of therapeutic efficacy and safety in brain tumor trials to measure critical aspects of NCF, symptoms, and HRQOL. We are encouraged by the widespread acceptance and implementation of these clinical outcome assessments at the sites within the NRG Oncology RTOG network and are certain that these outcomes will offer deeper insights into the optimal therapies for patients that maximize both life span and health span.
Supplementary Material
Acknowledgment
Sincere thanks to the participating centers USON-Texas Oncology-Sugar Land, Arizona Oncology Services Foundation, Southeast Cancer Control Consortium, Inc., CCOP, The University of Texas MD Anderson Cancer Center, Emory University, University of Wisconsin Hospital, Intermountain Medical Center, Cleveland Clinic Foundation, Thomas Jefferson University Hospital, Michigan Cancer Research Consortium CCOP, Kaiser Permanente Santa Clara Medical Center, University of Utah Health Science Center, Ohio State University Medical Center, Virginia Mason CCOP, Washington University, Florida Hospital, UPMC-Shadyside Hospital, Indiana University Health Methodist Hospital, Erlanger Health System, Florida Radiation Oncology Group - Baptist Regional, Penrose Cancer Center, Penrose-St. Francis Health Services, Robert Wood Johnson University Hospital, St. Joseph Hospital, University Hospitals of Cleveland, Allan Blair Cancer Centre, Allegheny-Singer Research Institute, St. Vincent Hospital and Health Care Centers, Inc., University of Texas Southwestern Medical School, Columbia River CCOP, Kansas City CCOP, Maine Medical Center, Methodist Cancer Center, UCSD - University of California, San Diego, University of Chicago, University of Cincinnati, Northern Indiana Cancer Research Consortium CCOP, Abington Memorial Hospital, Akron General Medical Center, Dartmouth Hitchcock Medical Center, Erlanger Health System, McGill University, Montana Cancer Consortium CCOP, Nevada Cancer Research Foundation CCOP, New Mexico Oncology Hematology Consultants, Northwestern Memorial Hospital, Poudre Valley Hospital Radiation Oncology, Radiation Oncology Center of Walnut Creek - John Muir Med Ct, Saint Barnabas Medical Center, Swedish Cancer Institute, Upstate Carolina CCOP - Gibbs Regional Cancer Center, University of Kansas Comprehensive Cancer Center, University of Maryland Medical Systems, University of Rochester, University of Vermont, Akron City Hospital, Anne Arundel Medical Center, Aultman Hospital, Baystate Health, Beaumont CCOP, Boulder Community Hospital, Cape Radiation Oncology, City of Hope Medical Center, Community Hospitals of Indiana Regional Cancer Care North, Good Samaritan Hospital & Medical Center, Lancaster General Hospital, Loyola University Medical Center, Main Line CCOP, Massachusetts General Hospital, Memorial Medical Center, Memorial Sloan-Kettering Cancer Center, Metro-MN CCOP, Parkview Cancer Center - Parkview Hospital, Penn State University and The Milton S. Hershey Medical Center, Piedmont Hospital, Rhode Island Hospital, Saint Alphonsus Regional Medical Center, Saint Elizabeth Regional Medical Center, St. Lukes Hospital, Toledo Community Hospital Oncology Program CCOP, Thompson Cancer Survival Center, University of California San Francisco, University of Nebraska Medical Center, University of Texas Medical Branch, Wesley Medical Center, Yale University, Alegent Health Immanuel Medical Center, Bay Area Tumor Institute CCOP, Cancer Care Associates of Fresno Medical Group, Centracare Health System/Coborn Cancer Center, Centre Hospitalier de l'Université de Montréal -Notre Dame, Columbus Community Clinical Oncology Program, Community Hospital, Diagnostic & Treatment Center, Good Samaritan Health Systems, Geisinger Medical Center, Grant/Riverside Methodist Hospital, Gulf Coast Mb-CCOP-Cancer Center at Providence Hospital, Harrison Memorial Hospital, Hartford Hospital, Hartford Hospital, Hudson Valley Oncology Associates, Ingalls Memorial Hospital, Inova Alexandria Hospital, James Graham Brown Cancer Center at University of Louisville, John Muir Medical Center - Concord Campus, Kalamazoo CCOP-West Michigan Cancer Center, Martin Memorial Medical Center, Memorial Regional Hospital, Mercy Hospital Medical Center, Meritcare Clinic Bemidji, Meritcare Hospital, MD Anderson Cancer Center - Orlando, Montefiore Medical Center, Naval Medical Center/Portsmouth, North Broward Medical Center - Cancer Center, Northeast Georgia Medical Center, Northeast Radiation Oncology Center, Northeastern Ontario Regional Cancer Centre, Northwest, Peacehealth St. Joseph Cancer Center, Pomona Valley Hospital Medical Center, Providence Cancer Therapy Center, Radiological Associates of Sacramento, Reading Hospital and Medical Center, Rocky Mountain Center - Thornton, Sacred Heart Hospital, Sentara Norfolk General Hospital, Samaritan North Health Care Center, Southern Regional Medical Center, St. Agnes Healthcare, St. Vincent’s Hospital, State University of NY At Stony Brook, The Hospital of Central Connecticut, The Schiffler Cancer Center, Trinitas Comprehensive Cancer Center, Trinity Medical Center, University of Oklahoma Health Sciences Center, Warren Cancer Research Foundation-Oklahoma CCOP, Waukesha Memorial Hospital, and Yakima Valley Memorial Hospital.
Funding
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, UG1CA189867, and R01NR014195 (to J.S.W.) from the National Cancer Institute (NCI) and Genentech.
Conflict of interest statement. T.S.A., M.R.G., G.H., H.I.R., K.S.R., M.M.W., and M.W. have no disclosures. Dr. D.G.B. reports employed as Chief Technology Officer and board member at GT Medical Technologies, a medical device manufacturer that has no direct or indirect relationship to this specific work but has an FDA-cleared device used for recurrent brain tumors including GBM. Dr. P.D.B. reports personal fees from UpToDate (contributor). Dr. I.R.C. reports personal fees and consulting; Speakers’ Bureau from Varian Medical Systems. Dr. M.P.M. reports Consultant for IBA, Varian, Celgene, Abbvie, AstraZeneca, Tocagen, Blue Earth Diagnostics and Board of Directors: Oncoceutics. Dr. S.L.P. reports funding paid to institution from Genetech-Roche, Pfizer-Astellas, and Millennium. Dr. J.S.W. reports personal fees from Roche, personal fees from Genentech, during the conduct of the study; personal fees from Angiochem, Bayer, Blueprint Medicines, Juno, Novocure, Vanquish Oncology, and INSYS Therapeutics.
Authorship statement. Conception and design: T.S.A., M.R.G., and J.S.W. Collection and assembly of data: D.G.B., I.R.C., M.R.G., H.I.R., K.S.R., J.S.W., M.M.W., and M.W. Data analysis and interpretation: T.S.A., D.G.B., P.D.B., M.R.G., G.H., M.P.M., S.L.P., J.S.W., and M.W. Manuscript writing: T.S.A., D.G.B., P.D.B., I.R.C., M.R.G., G.H., M.P.M., S.L.P., H.I.R., K.S.R., J.S.W., M.M.W., and M.W. Final approval of the manuscript: T.S.A., D.G.B., P.D.B., I.R.C., M.R.G., G.H., M.P.M., S.L.P., H.I.R., K.S.R., J.S.W., M.M.W., and M.W.
Previous presentation: Portions of this manuscript were presented at the 2013 American Society of Clinical Oncology Meeting and at the 2013 4th Quadrennial Meeting of the World Federation of Neuro-Oncology held in conjunction with the Scientific Meeting and Education Day of the Society for Neuro-Oncology.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Wefel JS, Cloughesy T, Zazzali JL, et al. . Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman HS, Prados MD, Wen PY, et al. . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 4. Kreisl TN, Kim L, Moore K, et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benedict RH, Schretten D, Groninger L, et al. . Hopkins Verbal Learning Test-Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–45. [Google Scholar]
- 7. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. [DOI] [PubMed] [Google Scholar]
- 8. Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 9. Armstrong TS, Wefel JS, Wang M, et al. . Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1): 2–18. [DOI] [PubMed] [Google Scholar]
- 11. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. [DOI] [PubMed] [Google Scholar]
- 12. Maringwa J, Quinten C, King M, et al. . Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol. 2011;22(9):2107–2112. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong TS, Mendoza T, Gning I, et al. . Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 14. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1998;16:1141–1154. [Google Scholar]
- 15. Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 16. Scott NW, Fayers PM, Aaronson NK, et al. . EORTC QLQ-C30 Reference Values. Brussels, Belgium: EORTC Quality of Life Group; 2008. [Google Scholar]
- 17. Sul J, Kluetz PG, Papadopoulos EJ, Keegan P. Clinical outcome assessments in neuro-oncology: a regulatory perspective. Neurooncol Pract. 2016;3(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.