Dear Editor,
A 68‐year‐old, otherwise healthy, man presented to our dermatology department in late April 2021 with a history of a blistering eruption which commenced 3 days after his first dose of the Pfizer BioNTech COVID‐19 vaccine (2 March 2021) and worsened after the second dose given three weeks later. The blisters first appeared over the sternal area and were accompanied by intense, generalized pruritus which started a day before the blisters appeared. His family doctor prescribed acyclovir for a presumed diagnosis of herpes zoster with no improvement and later desloratadine and a 5‐day course of 20‐mg oral prednisolone. However, the blisters continued to increase in number and erupted over the right side of the chest and upper back particularly after the patient received the second dose of the Pfizer vaccine.
At presentation to dermatology, the patient had several healing crusted areas scattered over the right chest and back but no intact blisters and was prescribed clobetasol propionate ointment and an emollient cream. Routine blood tests, epidermal basal membrane antibodies and prickle cell desmosomes antibodies were normal and negative respectively.
At follow‐up, 2 weeks later, there was a recently ruptured blister arising in an area of urticated erythema over his back (Fig 1a,b) and an ulcer situated on the left buccal mucosa (Fig 1c). A biopsy taken from one of the truncal lesions revealed subepidermal blistering associated with a superficial dermal inflammatory infiltrate composed of eosinophils and hemosiderophages. The roof of the bulla consisted of thinned, viable epidermis whilst within the bulla, erythrocytes and scattered inflammatory cells including eosinophils were seen (Fig 1d). Direct immunofluorescence showed linear basal deposition of IgG and C3 (Fig 1e,f). All these findings were in keeping with a diagnosis of bullous pemphigoid (BP). He was advised to continue the previously prescribed topical treatment, and at follow‐up 3 months after the first dose of COVID‐19 vaccine, he was found to be completely asymptomatic with no new blisters present and only residual post‐inflammatory hyperpigmentation present at previously affected sites.
Figure 1.
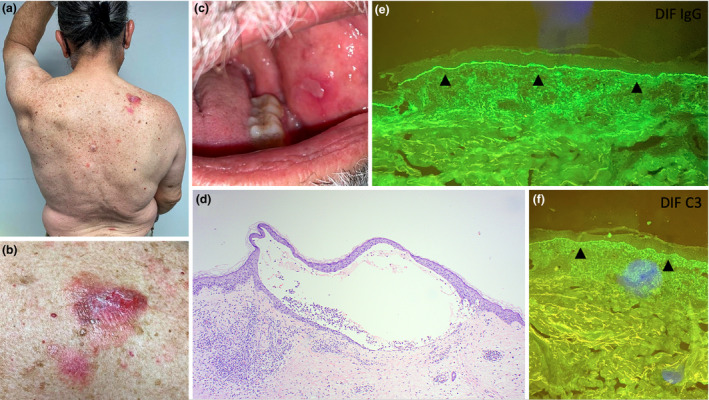
(a, b) Photos taken thirteen weeks after the first dose of the Pfizer COVID‐19 vaccine displaying four lesions over the patient’s back with a close‐up image of one such lesion located over the right upper back. (c) Left buccal ulcer seen at follow‐up 2 weeks later. (d) Photomicrograph showing a subepidermal blister roofed by normal epidermis. The blister contains scattered inflammatory cells, and a patchy inflammatory infiltrate is noted in the dermis adjacent to the blister. (H&E stain. Original magnification x 40) (e, f) Linear basal deposition of IgG and C3 noted on direct immunofluorescence (DIF) as indicated by the arrowheads.
This case is intriguing since to our knowledge it is the first reported case of BP related to the SARS‐CoV‐2 mRNA vaccine. We acknowledge that a possible differential diagnosis for this case would be epidermolysis bullosa acquisita (EBA). However, the histological features, particularly the eosinophilic‐predominant infiltrate, are not typical of EBA and are more in keeping with BP. A large number of cases of BP have been reported following other vaccines including the pneumococcal vaccine 1 and influenza vaccine. 2 However, the pathogenesis is not clear. It has been hypothesized that vaccine‐induced inflammation could lead to disruption of the basement membrane architecture with subsequent generation of anti‐basement membrane‐specific antibodies, and such vaccines may also increase the antigenicity of BP antigens. However, there are no known similarities between the basement membrane protein and the implicated vaccines; therefore, it is unlikely that the vaccine coupled with its respective antibody response is the sole cause of this phenomenon. In fact, it has also been postulated that these vaccines may precipitate a heightened immune response in individuals having either subclinical BP or immunological deposition. 2 Interestingly, vaccine‐induced‐adult BP hasbeen seen mainly in the elderly. This in part may be explained by the phenomenon of immunosenescence which occurs as a result of age‐induced thymic atrophy and can lead to autoimmune disease via breakdown of immune tolerance. 1
On further review of the literature, several articles document other cutaneous manifestations associated with the COVID‐19 vaccines (Table 1). 3 , 4 , 5 , 6 , 7 , 8 , 9 In a large‐scale study by McMahon et al., out of the 414 subjects, no cases of vaccine‐induced BP were reported. In fact, the commonest cutaneous manifestations excluding local site reactions were urticaria, morbilliform drug eruptions and erythromelalgia. 10
Table 1.
Literature review of the case reports documenting adverse cutaneous reactions as a result of a COVID‐19 vaccine 3 , 4 , 5 , 6 , 7 , 8 , 9
| Age | Gender | Comorbidities | Vaccine | Presentation | Onset | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 55 | Male | Nil of note | Pfizer (1st dose) |
Maculopapular rash Face, Trunk, Upper extremities and Thighs |
3 h | Topical steroids |
Resolution within days Did not take 2nd dose |
3 |
| 20s | Female | Alopecia | Pfizer (1st dose) |
Pityriasis rosea‐like eruptions Trunk and Proximal extremities |
2 days | Topical steroids | Resolution after 2 weeks | 4 |
| 40s | Male | Nil of note | Pfizer (2nd dose) |
Pityriasis rosea‐like eruptions Trunk and Proximal extremities |
2 days | Doxycycline and Bilastine | Resolution after 3 weeks | 4 |
| 60 | Male | DM, HTN on Tenegliptin, Metformin, Amlodipine | Unknown (1st dose) |
Steven Johnson Syndrome Extensive involvement with oral lesions |
3 days | Ciclosporin |
Resolution after 7 days Did not take 2nd dose |
5 |
| 30 | Male | Nil of note | Pfizer (both doses) | Pruritic erythematous morbilliform eruption | 2 days† | Nil | Resolution after 24 h | 6 |
| 83 | Female |
HTN Hypothyroidism Breast cancer on Palbociclib, Letrozole, Vitamin D |
Johnson & Johnson (single dose) |
Pruritic annular patches with central clearing Trunk and Axilla |
2 days | Antihistamines and Topical steroids | Resolution after 2 weeks | 7 |
| 56 | Female | Lichen planus | Pfizer (2nd dose) |
Lichen planus Extensive involvement |
2 days | Topical steroids | N/A | 8 |
| 26 | Female | Nil of note | Pfizer (both doses) |
Fixed drug eruption Near injection site |
14 days‡ | N/A | N/A | 9 |
Data documented in the study by McMahon et al. are excluded in this table.
DM, diabetes mellitus; HTN, hypertension; N/A, not available.
Cutaneous reaction occurred 2 days after the first dose and recurred 2 days after the second dose.
Fixed drug eruption occurred 14 days after the first dose and reappeared 14 days after the second dose.
Although it is possible that the appearance of BP after COVID vaccination in our patient was coincidental, the onset of symptoms so soon after the first dose, worsening after the second dose, complete resolution within a few weeks with only topical treatment, and no subsequent recurrence suggest a true association in this case.
Conflict of interest
None.
Funding source
None.
Acknowledgement
The patient gave written informed consent to the publication of his case details.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Maki N, Hashimoto T, Yamada T, Ishii N, Tsuruta D, Demitsu T. Case of pemphigoid with immunoglobulin G antibodies to BP180 C‐terminal domain and laminin‐γ1 (p200) developed after pneumococcal vaccination. J Dermatol 2020; 48: 101–105. [DOI] [PubMed] [Google Scholar]
- 2. Downs AM, Lear JT, Bower CP, Kennedy CT. Does influenza vaccination induce bullous pemphigoid? a report of four cases. Br J Dermatol 1998; 138: 363–373. [DOI] [PubMed] [Google Scholar]
- 3. Ackerman M, Henry D, Finon A, Binois R, Esteve E. Persistent maculopapular rash after the first dose of Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e423–e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cyrenne B, Al‐Mohammedi F, DeKoven J, Alhusayen R. Pityriasis rosea‐like eruptions following vaccination with BNT162b2 mRNA COVID‐19 Vaccine. J Eur Acad Dermatol Venereol 2021; 35: e546–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dash S, Sirka CS, Mishra S, Viswan P. Covid‐19 vaccine induced Steven‐Johnson syndrome. Clin Exp Dermatol 2021. 10.1111/ced.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jedlowski PM, Jedlowski MF. Morbilliform rash after administration of Pfizer‐BioNTech COVID‐19 mRNA vaccine. Dermatol Online J 2021; 27: 13030/qt4xs486zg 20. https://pubmed.ncbi.nlm.nih.gov/33560802/ [PubMed] [Google Scholar]
- 7. Song EJ, Wong AJS. Widespread annular eruption after Ad26.COV2.S COVID‐19 vaccine. JAAD Case Rep 2021; 13: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hiltun I, Sarriugarte J, Martínez‐de‐Espronceda I et al. Lichen planus arising after COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e414–e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mintoff D, Pisani D, Betts A, Scerri L. SARS‐CoV‐2 mRNA vaccine‐associated fixed drug eruption. J Eur Acad Dermatol Venereol 2021; 35: e560–e563. [DOI] [PubMed] [Google Scholar]
- 10. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8024548/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


