Abstract
MicroRNAs (miRNAs) play an important role in drug resistance, and it is reported that miR-27a-3p regulated the sensitivity of cisplatin in breast cancer, lung cancer and ovarian cancer. However, the relationship between miR-27a-3p and chemosensitivity of cisplatin in hepatocellular carcinoma (HCC) was unclear, especially the underlying mechanism was unknown. In the present study, we analyzed miR-27a-3p expression levels in 372 tumor tissues and 49 adjacent tissues in HCC samples from TCGA database, and found that the miR-27a-3p was down-regulated in HCC tissues. The level of miR-27a-3p was associated with metastasis, Child–Pugh grade and race. MiR-27a-3p was regarded as a favorable prognosis indicator for HCC patients. Then, miR-27a-3p was overexpressed in HepG2 cell, and was knocked down in PLC cell. Next, we conducted a series of in vitro assays, including MTT, apoptosis and cell cycle assays to observe the biological changes. Further, inhibitor rate and apoptosis rate were detected with pre- and post-cisplatin treatment in HCC. The results showed that overexpression of miR-27a-3p repressed the cell viability, promoted apoptosis and increased the percentage of cells in G0/G1 phase. Importantly, overexpression of miR-27a-3p significantly increased the inhibitor rate and apoptosis rate with cisplatin intervention. Besides, we found that miR-27a-3p added cisplatin sensitivity potentially through regulating PI3K/Akt signaling pathway. Taken together, miR-27a-3p acted as a tumor suppressor gene in HCC cells, and it could be useful for modulating cisplatin sensitivity in chemotherapy.
Keywords: Cisplatin, Hepatocellular cancer, miR-27a-3p, PI3K/Akt pathway, Sensitivity
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and highly lethal malignant tumors of digestive system worldwide [1]. The 5-year overall survival rate is merely 12% for most of the patients who are already at the advanced stage of HCC at the time of diagnosis [2]. Therefore, systematic treatments are recommended by experts in the HCC guidelines. These options including targeted drugs of Sorafenib and Lenvima, as well as systematic chemotherapy. However, the treatment effect of the chemotherapy was generally unsatisfactory [3,4]. Several cytotoxic agents, including cisplatin, doxorubicin and 5-florouracil (5-FU) have shown multiple drug resistance which limit their therapeutic efficacy [5]. Therefore, it is urgent to explore the molecular targets to improve the sensitivity of these cytotoxic drugs in HCC.
MicroRNAs (miRNAs) refer to a group of small and non-coding RNAs, which are 22 nucleotides in length. Its main function is to regulate gene expression at the translation level. Recently, it is reported that aberrant expression of miRNAs can modulate cell growth, apoptosis as well as tumorigenesis [6]. Besides, miRNAs can also make contributions to the chemosensitivity in HCC [7,8]. However, the underlying molecular mechanisms of chemosensitivity have not been clarified.
MiR-27a-3p is located on chromosome 19 (19p13.1), which is expressed in multiple malignant tumors, such as renal carcinoma, oral squamous cell carcinoma and pancreatic cancer and so on [9–12]. In addition, it is reported that miR-27a-3p plays a vital role in invasion, metastasis and epithelial–mesenchymal transition in HCC [13]. Furthermore, miR-27a-3p also regulates the sensitivity of cisplatin in breast cancer, lung cancer and ovarian cancer [14–16]. Nevertheless, the relationship between miR-27a-3p and chemosensitivity of cisplatin in HCC is unknown, and its underlying mechanism needs to be explored.
Thus, in the present study, we intend to assess the effect of miR-27a-3p in cisplatin treatment of HCC, and try to identify its mechanism. We found that miR-27a-3p is an indicator of favorable prognosis in HCC patients. Beside in vitro assays, up-regulation of miR-27a-3p decreased the cell viability, promoted the apoptosis and blocked cells in G0/G1 phase. Importantly, overexpression of miR-27a-3p markedly increased the inhibitor rate and apoptosis rate when cisplatin was added in HCC cells.
In contrast, knockdown of miR-27a-3p significantly showed an opposite trend. In addition, Western blot revealed that miR-27a-3p plus cisplatin revealed weaker expressions of PI3K and p-Akt and stronger level of C-caspase-3. Thus, PI3K/Akt pathway probably mediated this process. Hence, miR-27a-3p added cisplatin sensitivity potentially through regulating PI3K/Akt signaling pathway.
Materials and methods
TCGA data analysis
The online accessible TCGA data portal (https://tcga-data.nci.nih.gov/tcga/) was used. We mainly focused on miR-27a-3p expression and clinical data of HCC patients, including age, sex, race, TNM stage, grade and Child–Pugh stage. All values were collected and analyzed from 372 HCC patients. MiR-27a-3p expression was quantified using RSEM based on the TCGA methods. The upper quartile data were normalized according to the TCGA normalization protocol.
Cell culture
Hepatoma cell lines (HepG2, Huh-7, PLC) and the human normal liver cell line LO2 were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). All cell lines were cultured in RPMI 1640 medium (Life Technologies, U.S.A.), supplemented with 10% fetal bovine serum (Life Technologies, U.S.A.) and cultured in a humidified atmosphere containing 5% CO2 at 37°C.
Reagents
The miExpress™ Precursor miRNA Expression (Lot No. 21895-1), inhibitor expression (Lot No. B302), clone of miR-27a-3p and control were purchased from GenePharma Company (Shanghai, China). The following antibodies were used in the study: anti-PI3K, anti-Akt, anti-p-Akt, anti-C-caspase were obtained from Cell Signaling Technology (Beverly, MA). The PI3K/p-Akt signaling inhibitor LY49002 was purchased from Apicent Biological Technology Company (Shanghai, China). β-actin was purchased from Bioworld Technology (CA, U.S.A.).
Cell transfection
Before transfection, a total of 1.5 × 105 HCC cells were seeded into six-well plates for 24 h. Lipofectamine 3000 (Invitrogen, U.S.A.) was used for the transient transfection according to the manufacturer’s instructions. HepG2 cells were chosen for miR-27a-3p overexpression by plasmid transfection and PLC cells for miR-27a-3p knockdown using siRNA transient transfection. Cells were divided into four groups, miR-27a-3p overexpression and control were referred to as miR-27a and miR-Con, respectively; miR-27a-3p inhibitor and inhibitor control were named as miR-inhibitor-27a and miR-inhibitor-Con, respectively. The expression levels of miRNAs were confirmed by qRT-PCR assay.
Cell viability and proliferation assay
Twenty-four hours after cell transfection, cell viability was identified by 3-(4,5-Dimethylthiazol-2-yl)-2-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay (MTT, Promega, U.S.A.). HepG2 and PLC cells were triplicate plated in a 96-well plate at the density of 5 × 103 cells/well and incubated overnight. Then, the cells were treated with different concentrations of cisplatin: 0, 3, 6, 9, 12 µg/ml for 48 h, respectively. Subsequently, the MTT reagent (20 µl) was added to each well, followed by incubation at 37°C in 5% CO2 atmosphere for 4 h. Lastly, the absorbance was read by using a Synergy 2 (BioTek, U.S.A.) plate reader.
Cell apoptosis and cell cycle analysis by FACS
Annexin V/propidium iodide (Av/PI) staining (Beyotime Biotechnology, China) was analyzed by flow cytometry. Cells were collected and washed twice with phosphate-buffered saline (PBS), followed by resuspension in 250 μl binding buffer. Five microliters of FITC–Annexin V and 10 μl PI (20 µg/ml) were added to each 100-μl cell suspension, and then the cells were incubated at room temperature for 15 min. Subsequently, 400 μl PBS was added to the cell suspensions, and the samples were detected by flow cytometry (Becton-Dickinson, U.S.A.). The percentage of the cells in different phases was counted and compared.
For cell cycle analysis, cells were cultured in serum-free medium for 24 h to induce cell cycle synchronization. Cells were harvested at different time points. For DNA content analysis, cells were fixed in 70% ethanol, rehydrated in PBS, treated with RNase A (10 mg/ml) for 30 min, then stained with PI (10 µg/ml) for 5 min. The percentage of cells in the S, G0/G1 and G2/M phases was counted and compared.
Western blot
Cells were harvested and washed twice with PBS (HyClone, Logan, UT). Total protein was extracted using RIPA cell lysis buffer (Beyotime Biotechnology, China). Protein concentration was determined by the bicinchoninic acid protein assay (Pierce, U.S.A.), and proteins were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and blotted on to polyvinylidene difluoride (PVDF) membranes (Millipore, MA). The membranes were incubated with primary antibodies in blocking buffer overnight at 4°C. The membrane was washed three times for 5 min each time with washing buffer and incubated with secondary antibodies (Invitrogen, U.S.A.) for 1.5 h at room temperature. The proteins were visualized with the Western Breeze Kit (WB7105, Invitrogen, U.S.A.) and analyzed with Quantity One software (Bio-Rad Laboratories, U.S.A.).
Statistical analysis
All statistical analysis was performed using SPSS software, version 17.0 (SPSS, Chicago, U.S.A.). The results are expressed as the mean ± standard deviation (SD). The data were compared among groups by one-way analysis of variance followed by Bonferroni’s correction. Each experiment was done independently at least three times. P-value <0.05 was considered as statistically significant difference.
Results
MiR-27a-3p was down-regulated in tumor tissue in HCC patients
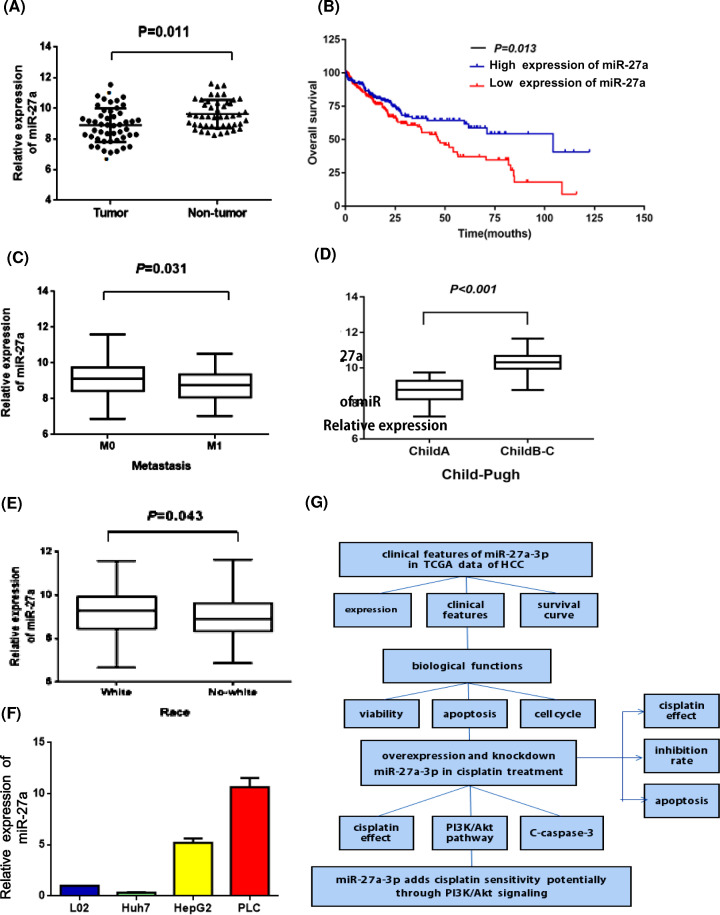
To explore the clinical significance of miR-27a-3p in HCC patients, we downloaded the clinical data and miR-27a-3p expression in TCGA dataset. A total of 372 tumor tissues and 49 adjacent normal tissues were involved. Results showed that miR-27a-3p was significantly low-expressed in tumor tissue (Figure 1A). And Kaplan–Meier survival curve revealed that high level of miR-27a-3p significantly correlated with better overall survival in HCC patients (Figure 1B). Moreover, miR-27a-3p expression was associated with metastasis, Child–Pugh grade and race (Figure 1C–E), but not with the T stage, N stage and differentiation grade. The correlation between miR-27a-3p level and clinicopathological features in HCC patients is shown in Tables 1 and 2. Taken together, these results reflected that miR-27a-3p level was an indicator for favorable prognosis of HCC patients.
Figure 1. MiR-27a-3p was down-regulated in tumor tissue in HCC patients.
(A) The scatter diagram represented the expression of miR-27a-3p in 49 paired HCC tissues and adjacent non-tumor tissues, which showed that miR-27a-3p was significantly low-expressed in tumor tissue. (B) The Kaplan–Meier curve revealed that high level of miR-27a-3p significantly correlated with better overall survival in HCC patients. (C–E) The correlation analysis reflected that miR-27a-3p expressions were associated with metastasis, Child–Pugh grade and race in HCC patients. (F) The miR-27a-3p expressions in different cell lines were measured by RT-qPCR. (G)The research flow chart of the article.
Table 1. The correlation between clinicopathological features and miR-27a-3p expression in HCC patients.
| Clinical characteristics | Expression | χ2 | P-value | |||
|---|---|---|---|---|---|---|
| Low | High | |||||
| 186 | 50.00% | 186 | 50.00% | |||
| Age (years) | ||||||
| ≤60 | 86 | 23.10% | 91 | 24.50% | 0.269 | 0.604 |
| >60 | 100 | 26.90% | 95 | 25.50% | ||
| Gender | ||||||
| Female | 56 | 15.10% | 63 | 16.90% | 0.605 | 0.437 |
| Male | 130 | 34.90% | 123 | 33.10% | ||
| Race | ||||||
| White | 79 | 21.20% | 103 | 27.70% | 6.196 | 0.013* |
| No-white | 107 | 28.80% | 83 | 22.30% | ||
| T stage | ||||||
| T1–2 | 147 | 39.50% | 132 | 35.50% | 3.226 | 0.072 |
| T3–4 | 39 | 10.50% | 54 | 14.50% | ||
| N stage | ||||||
| N0 | 161 | 43.30% | 170 | 45.70% | 2.220 | 0.136 |
| N1 | 25 | 6.70% | 16 | 4.30% | ||
| M stage | ||||||
| M0 | 159 | 42.70% | 172 | 46.20% | 4.633 | 0.031* |
| M1 | 27 | 7.30% | 14 | 3.80% | ||
| Differentiation grade | ||||||
| G1–2 | 117 | 31.50% | 118 | 31.70% | 0.012 | 0.914 |
| G3–4 | 69 | 18.50% | 68 | 18.30% | ||
| Child–Pugh | ||||||
| Grade A | 134 | 36.00% | 160 | 43.00% | 10.966 | 0.001* |
| Grade B–C | 52 | 14.00% | 26 | 7.00% | ||
| Tumor stage | ||||||
| I–II | 113 | 30.40% | 112 | 30.10% | 0.011 | 0.916 |
| III–IV | 73 | 19.60% | 74 | 19.90% | ||
P<0.05.
Table 2. Univariate and multivariate analyses of overall survival in patients with HCC.
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| χ2 | P-value | HR | (95% CI) | P-value | ||
| Age | 1.600 | 0.206 | 1.321 | 0.384 | 3.989 | 0.721 |
| Gender | 1.165 | 0.280 | 1.645 | 0.381 | 5.542 | 0.285 |
| Race | 0.990 | 0.320 | 1.725 | 0.426 | 5.892 | 0.361 |
| AFP | 0.093 | 0.761 | 0.895 | 0.325 | 2.462 | 0.829 |
| Cirrhosis | 1.080 | 0.299 | 1.895 | 0.578 | 6.200 | 0. 291 |
| Tumor size | 28.902 | <0.001* | 0.135 | 0.042 | 0.052 | <0.001* |
| Lymph node metastasis | 0.163 | 0.686 | 0.285 | 0.426 | 1.892 | 0.648 |
| Distant metastasis | 4.031 | 0.045* | 0.047 | 0.232 | 0.067 | 0.011* |
| Differentiation grade | 0.192 | 0.661 | 0.296 | 0.431 | 1.910 | 0.653 |
| Child–Pugh Grade | 8.018 | 0.005* | 1.103 | 0.061 | 0.461 | 0.015* |
| Tumor stage | 24.084 | <0.001* | 0.723 | 0.057 | 1.201 | 0.061 |
| miR-27a-3p | 6.088 | 0.014* | 1.117 | 0.013 | 0.291 | 0.010* |
P<0.05.
MiR-27a-3p acted as a tumor suppressor gene in HCC
As miR-27a-3p was low-expressed in HCC tumor tissue, we speculated that miR-27a-3p may function as a tumor suppressor gene. Thus, we conducted a series of in vitro assays to explore its biological function. First, we detected the expression of miR-27a-3p in several hepatoma cell lines (HepG2, Huh-7 and PLC) and one normal liver cell line (L02). RT-qPCR results showed that miR-27a-3p expressions differed in these cells. HepG2 cells had a relatively low level whereas PLC cells had a relatively high expression (Figure 1F). Therefore, HepG2 cells were chosen for miR-27a-3p overexpression and PLC cells for miR-27a-3p knockdown.
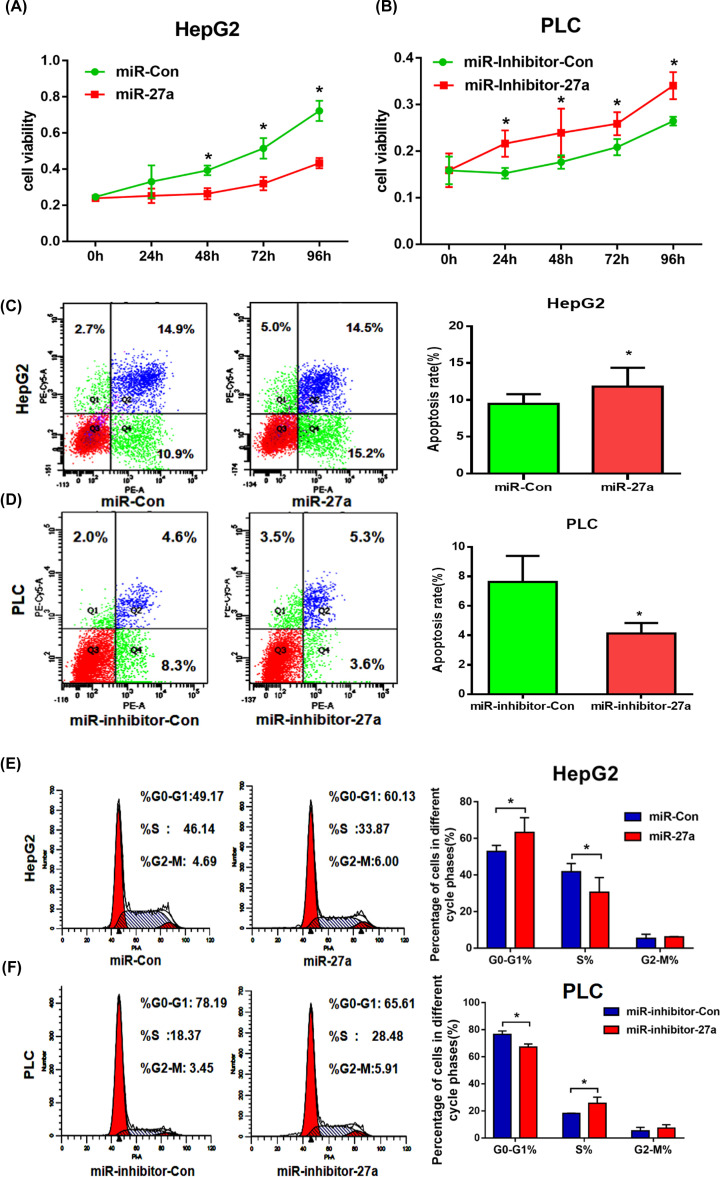
Second, MTT assay showed that high expression of miR-27a-3p impaired the cell viability in HepG2, whereas low level of miR-27a-3p added viability in PLC (Figure 2A,B). Besides, flow cytometry showed that overexpression of miR-27a-3p increased the apoptosis rate compared with miR-Con group in HepG2. In contrast, knockdown of miR-27a-3p significantly reduced the apoptosis rate in PLC (Figure 2C,D). In addition, cell cycle analysis indicated that the overexpression of miR-27a-3p led to an increase in G0/G1 phase and a decrease in S-phase in HepG2, while knockdown of miR-27a-3p resulted in a reverse trend in PLC (Figure 2E,F).
Figure 2. MiR-27a-3p acted as a tumor suppressor gene in HCC.
HepG2 cells were chosen for miR-27a-3p overexpression and PLC cells were selected for miR-27a-3p knockdown. (A,B) MTT assays showed that high level of miR-27a-3p impaired the cell viability in HepG2, whereas its low level added viability in PLC. (C,D) Up-regulation of miR-27a-3p increased the apoptosis rate in HepG2. In contrast, knockdown of miR-27a-3p significantly reduced the apoptosis rate in PLC. (E,F) The cell cycle assays revealed that overexpression of miR-27a-3p led to an increase in G0/G1 phase and a decrease in S-phase in HepG2, while its knockdown resulted in a reverse trend in PLC. All experiments were performed in triplicate. *P<0.05.
For miR-27a-3p has the function of inhibiting cell viability, inducing cell apoptosis, as well as affecting the cell cycle progression, it suggests that miR-27a-3p plays a key role in tumor suppression in the development of HCC.
MiR-27a-3p enhanced the cisplatin sensitivity of HCC cells
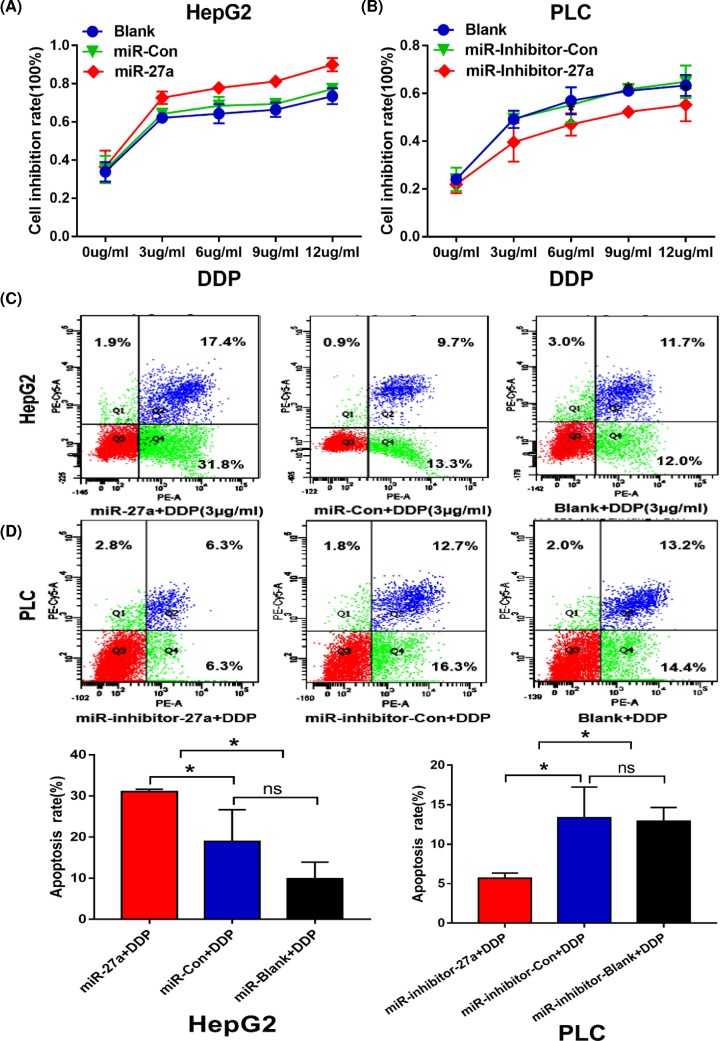
To explore whether miR-27a-3p could affect the chemosensitivity of cisplatin in HCC, we treated HepG2 and PLC cells with different concentrations of cisplatin (0, 3, 6, 9 and 12 µg/ml) and examined the effects of miR-27a-3p on cisplatin treatment. Firstly, we observed that the cell inhibition rates were gradually increased with the elevated concentration of cisplatin in blank group in both HepG2 and PLC. It indicated that the killing effect of cisplatin (Figure 3A,B). Interestingly, with cisplatin stimulation, MTT assay showed that overexpression of miR-27a-3p had the higher inhibition rate than that in miR-Con group in HepG2. On the contrary, knockdown of miR-27a-3p significantly decreased the inhibition rate compared with miR-inhibitor-Con group in PLC (Figure 3A,B).
Figure 3. MiR-27a-3p enhanced the cisplatin sensitivity of HCC cells.
(A,B) MTT assays showed that overexpression of miR-27a-3p increased the inhibition rate in HepG2. On the contrary, knockdown of miR-27a-3p significantly decreased the inhibition rate in PLC. (C,D) MiR-27a+DDP group had higher apoptosis rate than that in miR-Con+DDP group in HepG2 (*P<0.05). MiR-inhibitor-27a+DDP group had lower apoptosis rate than that in miR-inhibitor-Con+DDP group in PLC (*P<0.05).
Subsequently, we selected a moderate concentration of cisplatin (3 µg/ml DDP) for further intervention. As revealed in Figure 3C,D, cisplatin had the effects of inducing apoptosis in blank+DDP group in both HepG2 and PLC. When miR-27a-3p was overexpressed, the apoptosis rate in miR-27a+DDP group (31.8%) was higher than miR-Con+DDP group (13.3%, P<0.05). When miR-27a-3p was knocked down, the apoptosis rate in miR-inhibitor-27a+DDP group (6.3%) was lower than miR-inhibitor-Con+DDP group (16.3%, P<0.05). Collectively, we found that more inhibition rate and more apoptosis rate exhibited when miR-27a-3p was overexpressed in treatment of cisplatin in HCC cells. Thus, it implies that high level of miR-27a-3p obviously enhances cisplatin sensitivity in HCC cells.
MiR-27a-3p added the cisplatin sensitivity potentially by inhibiting PI3K/Akt pathway
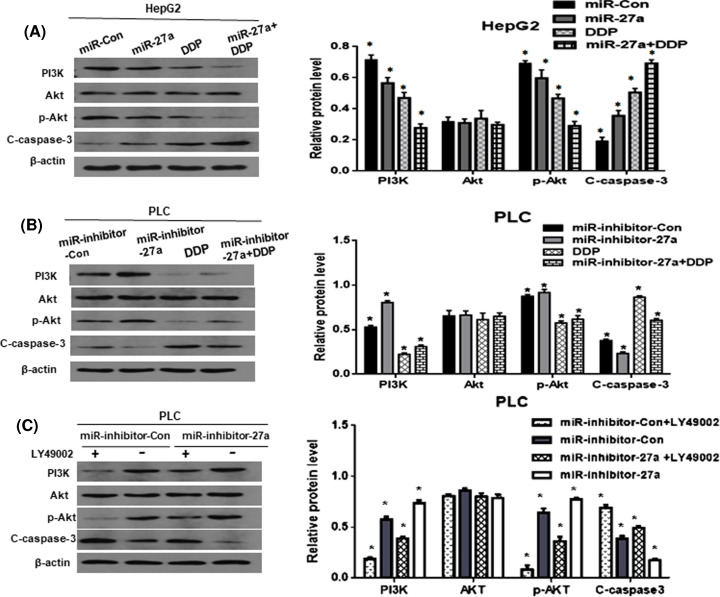
In order to show the potential mechanism by which miR-27a-3p exerted anti-cancer effects in HCC cells, the signaling pathway proteins were assessed. Firstly, as revealed in Figure 4A, compared with miR-Con group, overexpression of miR-27a-3p inhibited PI3K/p-Akt signaling and elevated C-caspase-3 in HepG2. Nevertheless, PLC cells exhibited an opposite trend, compared with miR-inhibitor-Con group, silencing of miR-27a-3p activated the PI3K/p-Akt signaling and resulted in reduced C-caspase-3 level (Figure 4B). In addition, it is known that cisplatin has anti-cancer effect. And DDP group exhibited the suppression of PI3K/p-Akt pathway and up-regulation of C-caspase-3. Interestingly, miR-27a+DDP group showed the identical effect, and which was more obvious than that in DDP group in HepG2. It indicated that overexpression of miR-27a-3p facilitated the anti-cancer effect of cisplatin. However, the miR-inhibitor-27a+DDP group revealed stronger expressions of PI3K and p-Akt protein and weaker level of C-caspase-3, compared with DDP group in PLC. Silencing of miR-27a-3p attenuated the cisplatin effect.
Figure 4. MiR-27a-3p added the cisplatin sensitivity potentially by inhibiting PI3K/Akt pathway.
(A,B) MiR-27a group inhibited PI3K/p-Akt signaling and elevated C-caspase-3 in HepG2. MiR-inhibitor-27a group exhibited the opposite trend in PLC cells. And DDP group showed suppression of PI3K/p-Akt pathway and up-regulation of C-caspase-3. MiR-27a+DDP group showed more obvious trend than DDP group in HepG2. MiR-inhibitor-27a+DDP group revealed stronger expression in PI3K protein and weaker level of C-caspase-3, compared with DDP group in PLC. Silencing of miR-27a-3p attenuated the cisplatin effect. (C) The PI3K/Akt pathway inhibitors LY49002 was used, a significant suppression of PI3K/Akt signaling and an increased expression of C-caspase-3 was observed. When miR-27a-3p was knocked down, the above trend was attenuated in miR-inhibitor-27a group, compared with miR-inhibitor-Con group. β-actin was used as a loading control. All of the experiments were performed in triplicate. *P<0.05.
With LY49002 intervention, compared with miR-inhibitor-Con group,miR-inhibitor-27a group showed only a slightly weakened expression of C-caspase-3, the effect of knockdown miR-27a-3p was attenuated (Figure 4C). This suggests that PI3K/Akt signaling is the main pathway affecting cisplatin sensitivity. And miR-27a-3p adds its sensitivity potentially through the inhibition of PI3K/Akt signaling.
Discussion
In the present study, clinically, we mainly found that miR-27a-3p was down-regulated in HCC tissue and could be regarded as an indicator for favorable prognosis of HCC patients. And in in vitro assays, consistent with current studies, we noticed that miR-27a-3p acted as a tumor suppressor gene in HCC. For it had the functions of inhibiting cell viability, inducing the apoptosis and affecting the cell cycle. However, whether miR-27a-3p contributed to the sensitivity of cisplatin was unclear. Importantly, for the first time, we reported that overexpression of miR-27a-3p significantly increased the inhibition rate and apoptosis rate with cisplatin treatment in HCC cells. It suggests that miR-27a-3p play a vital role in regulating cisplatin sensitivity. Besides, we preliminarily found that miR-27a-3p added cisplatin sensitivity potentially through regulating PI3K/Akt signaling pathway. Our data highlight a novel molecular target for chemotherapy of HCC.
In clinical samples, the TCGA analysis showed that miR-27a-3p was down-regulated in HCC tissues, and was associated with metastasis, Child–Pugh Grade and race. HCC patients with a high level of miR-27a-3p had better prognosis than those with a low level. This was consistent with Zhao et al.’s findings [13]. He detected the level of miR-27a by qRT-PCR in 42 cases of HCC tissues and 35 cases of adjacent tissues. Identically, they also found that miR-27a was significantly lower in HCC tissues and it could be considered as an indicator for favorite prognosis in HCC patients.
In in vitro assays, we firstly verified its basic biological function in HCC cells. We demonstrated that overexpression of miR-27a-3p inhibited cell viability, promoted apoptosis and blocked the cell cycle in G0/G1 phase. It means that miR-27a-3p plays a key role in tumorigenesis of HCC. However, in other types of cancer, miR-27a-3p exerted different functions. In gastric cancer, Liu et al. reported that miR-27a worked as an oncogene of promoting cell growth [17]. And in nasopharyngeal carcinoma, Li et al. found that miR-27a-3p had the function of promoting cell proliferation, facilitating migration and invasion by targeting Mapk10 protein [18]. Besides, Mertens-Talcott et al. observed that the oncogenic role of miR-27a, which increased the percentage of breast cancer cells in G2-M phase by inducing target gene Myt-1 [19]. The above evidence reflects that miR-27a-3p plays different roles in different types of cancer.
Current researches have reported that some miRNAs could regulate the sensitivity of chemotherapy drug cisplatin in multiple types of cancer, including HCC. For example, Let-7 affected the sensitivity of cisplatin by IL-6/STAT3 pathway in esophageal cancer [20]. And miR-214 led to the cell survival and cisplatin resistance by targeting PTEN in ovarian cancer [21]. Furthermore, Li et al. observed that up-regulation of miR-27a contributed to the chemoresistance of cisplatin by suppressing RKIP expression in lung cancer cells [15]. And in HCC, some studies have confirmed that several specific miRNAs affect the chemosensitivity of cisplatin in HCC, which includes miR-33a, miR-34a-5p, miR-133a, miR-193b and so on [22–25]. However, the relationship between miR-27a-3p and cisplatin sensitivity in HCC was unknown.
Thus, we aimed to explore whether miR-27a-3p could affect the sensitivity of cisplatin. We treated HCC cells with different concentrations of cisplatin. An interesting phenomenon emerged, the cell viability was obviously inhibited and the apoptosis rate was significantly added when miR-27a-3p was overexpressed with cisplatin intervention in HepG2. Yet, the reverse trend was noticed in PLC cells when miR-27a-3p was knocked down. Collectively, it proved that high level of miR-27a-3p sensitized the effect of cisplatin by suppressing cell viability and inducing cell apoptosis. As we know that multidrug resistance (MDR) is the main problem that limits the therapeutic efficiency of chemotherapy drug. And P-gp protein and its encoded gene MDR1 have been verified to play a vital role in drug resistance. Li et al. found that miR-27a may be involved in drug resistance by regulating MDR1/P-gp protein expression in ovarian cancer cells [16].
It is reported that a variety of extracellular stimuli such as insulin and chemotherapy drugs can activate signal pathways, apart from the Smad-dependent signaling, Smad-independent signaling, including the PI3K/Akt, JAK/STAT3 and MAPK pathways [26]. These pathways exert their functions by signal cascade amplification [27]. And among them, PI3K/Akt signaling plays a critical role in affecting the sensitivity of chemotherapy drug [28]. The apoptosis rate detection is one of important methods to determine the drug sensitivity. The apoptosis pathway is mainly mediated by the caspase family protein, which determines whether the cell continues to survive or die through the internal or mitochondrial apoptosis pathway [29].
We all know that cisplatin has anti-cancer effect. In our study, DDP resulted in down-regulation of PI3K, p-Akt proteins and up-regulation of C-caspase-3 level. Notably, miR-27a+DDP showed more obvious trend than that in DDP alone, it implies that cisplatin inhibits the PI3K/Akt signaling and induces cell apoptosis, and miR-27a-3p increases the cytotoxicity of cisplatin. In contrast, when miR-27a-3p was knocked down, it showed the activation of PI3K/Akt signaling and suppression of C-caspase-3. It indicates that silencing of miR-27a-3p attenuates the cisplatin effect by affecting PI3K/Akt signaling.
To negatively validate whether miR-27a-3p affects cisplatin sensitivity by PI3K/Akt signaling, we used the pathway inhibitors LY49002. As shown in Figure 4C, when LY49002 was added, it caused a significant suppression of the PI3K/AKT signaling and an obvious increased expression of C-caspase-3. This suggests that PI3K/Akt signaling is the main pathway in increasing cisplatin sensitivity. Whereas knockdown of miR-27a-3p, the above trend was attenuated in miR-inhibitor-27a group, compared with miR-inhibitor-Con group. Thus, we infer that miR-27a-3p adds cisplatin sensitivity potentially through regulating PI3K/Akt signaling. This is supported by Zhu et al.’s results [30], he found that miR-27a-3p might be associated with resistance of breast cancer cells to adriamycin treatments, by targeting BTG2 and promoting the PI3K/Akt pathway in breast cancer cells. And the shortcoming of the present paper is that we have not determined the target gene of miR-27a-3p that involved in cisplatin intervention, and we will continue to explore in future study.
In conclusion, miR-27a-3p acts as a tumor suppressor gene in HCC. Importantly, overexpression of miR-27a-3p adds cisplatin sensitivity potentially through PI3K/Akt signaling in hepatoma cell lines. Our data highlight a molecular target in chemotherapy strategy of HCC.
Supplementary Material
Abbreviations
- HCC
hepatocellular carcinoma
- MDR
multidrug resistance
- miRNA
microRNA
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay
- PBS
phosphate-buffered saline
- PI
propidium iodide
- TCGA
the cancer genome atlas
- TNM
tumor node metastasis
Contributor Information
Hua Zhang, Email: 657015630@qq.com.
Yongxing Bao, Email: baoyx@vip.sina.com.
Data Availability
All data generated or analyzed during the present study are included in this published article and its Supplementary files.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University [grant number SKL-HIDCA-2020-41]; and The Supporting School Funding of Clinical Medicine Peak Discipline of Xinjiang Medical University [grant number 33-0104006020801].
CRediT Author Contribution
Ying Yang: Writing—original draft. Zhifang Yang: Investigation. Ruili Zhang: Investigation. Chunli Jia: Writing—review and editing. Rui Mao: Writing—review and editing. Sha Ya: Methodology. Yuefen Zhang: Investigation. Ge Wu: Methodology. Yan na Sun: Investigation. Xiao yan Jia: Investigation. Ainiwaer Aimudula: Formal analysis. Hua Zhang: Conceptualization. Yongxing Bao: Conceptualization.
References
- 1.Torre L.A., Bray F., Siegel R.L.et al. (2015) Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Nault J.C. (2017) The end of almost 10 years of negative RCTs in advanced hepatocellular carcinoma. Lancet 389, 4–6 10.1016/S0140-6736(16)32480-1 [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki M., Miyata H., Tanaka K.et al. (2011) Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology 80, 307–313 10.1159/000329806 [DOI] [PubMed] [Google Scholar]
- 4.Graf D., Vallböhmer D., Knoefel W.T.et al. (2014) Multimodal treatment of hepatocellular carcinoma. Eur. J. Intern. Med. 25, 430–437 10.1016/j.ejim.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H., Arimura Y., Yamashita K.et al. (2010) Phase I/II study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. Thorac. Oncol. 5, 122–128 10.1097/JTO.0b013e3181c1ffd5 [DOI] [PubMed] [Google Scholar]
- 6.Di Leva G., Garofalo M. and Croce C.M. (2014) MicroRNAs in cancer. Annu. Rev. Pathol. 9, 287–314 10.1146/annurev-pathol-012513-104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J., Luo M., Qian H.et al. (2014) Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene 538, 342–347 10.1016/j.gene.2013.12.043 [DOI] [PubMed] [Google Scholar]
- 8.Giovannetti E., Erozenci A., Smit J.et al. (2012) Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit. Rev. Oncol. Hematol. 81, 103–122 10.1016/j.critrevonc.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Wang W.S., Liu L.X., Li G.P.et al. (2013) Combined serum CA19-9 and miR-27a-3p in peripheral blood mononuclear cells to diagnose pancreatic cancer. Cancer Prev. Res. (Phila.) 6, 331–338 10.1158/1940-6207.CAPR-12-0307 [DOI] [PubMed] [Google Scholar]
- 10.Nakata W., Uemura M., Sato M.et al. (2015) Expression of miR-27a-3p is an independent predictive factor for recurrence in clear cell renal cell carcinoma. Oncotarget 6, 21645–21654 10.18632/oncotarget.4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W., Liu M., Peng X.et al. (2013) miR-24-3p and miR-27a-3p promote cell proliferation in glioma cells via cooperative regulation of MXI. Int. J. Oncol. 42, 757–766 10.3892/ijo.2012.1742 [DOI] [PubMed] [Google Scholar]
- 12.Zeng G., Xun W., Wei K.et al. (2016) MicroRNA-27a-3p regulates epithelial to mesenchymal transition via targeting YAP1 in oral squamous cell carcinoma cells. Oncol. Rep. 36, 1475–1482 10.3892/or.2016.4916 [DOI] [PubMed] [Google Scholar]
- 13.Zhao N., Sun H., Sun B.et al. (2016) miR-27a-3p suppresses tumor metastasis and VM by down-regulating VE-cadherin expression and inhibiting EMT: an essential role for Twist-1 in HCC. Sci. Rep. 6, 23091–23107 10.1038/srep23091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou S., Huang Q., Zheng S.et al. (2016) miR-27a regulates the sensitivity of breast cancer cells to cisplatin treatment via BAK- SMAC/DIABLO –XIAP axis. Tumour Biol. 37, 6837–6845 10.1007/s13277-015-4500-1 [DOI] [PubMed] [Google Scholar]
- 15.Li J., Wang Y., Song Y.et al. (2014) miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol. Cancer 13, 193–202 10.1186/1476-4598-13-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Hu S., Wang J.et al. (2010) MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol. Oncol. 119, 125–130 10.1016/j.ygyno.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 17.Liu T., Tang H., Lang Y.et al. (2009) MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 273, 233–242 10.1016/j.canlet.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 18.Li L. and Luo Z. (2017) Dysregulated miR-27a-3p promotes nasopharyngeal carcinoma cell proliferation and migration by targeting Mapk10. Oncol. Rep. 37, 2679–2687 10.3892/or.2017.5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertens-Talcott S.U., Chintharlapalli S., Li X.et al. (2007) The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 67, 11001–11011 10.1158/0008-5472.CAN-07-2416 [DOI] [PubMed] [Google Scholar]
- 20.Sugimura K., Miyata H., Tanaka K.et al. (2012) Let-7 expression is a significant determinant of response to chemotherapy through the regulation of IL-6/STAT3 pathway in esophageal squamous cell carcinoma. Clin. Cancer Res. 18, 5144–5153 10.1158/1078-0432.CCR-12-0701 [DOI] [PubMed] [Google Scholar]
- 21.Yang H., Kong W., He L.et al. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 68, 425–433 10.1158/0008-5472.CAN-07-2488 [DOI] [PubMed] [Google Scholar]
- 22.Meng W., Tai Y., Zhao H.et al. (2017) Downregulation of miR-33a-5p in hepatocellular carcinoma: a possible mechanism for chemotherapy resistance. Med. Sci. Monit. 23, 1295–1304 10.12659/MSM.902692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.Y., Wen J.Y., Jia C.C.et al. (2015) MicroRNA-34a-5p enhances sensitivity to chemotherapy by targeting AXL in hepatocellular carcinoma MHCC-97L cells. Oncol. Lett. 10, 2691–2698 10.3892/ol.2015.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J., Wang T., Guo R.et al. (2015) MicroRNA-133a and microRNA-326 co-contribute to hepatocellular carcinoma 5-fluorouracil and cisplatin sensitivity by directly targeting B-cell lymphoma-extra large. Mol. Med. Rep. 12, 6235–6240 10.3892/mmr.2015.4134 [DOI] [PubMed] [Google Scholar]
- 25.Yin W., Nie Y., Zhang Z.et al. (2015) miR-193b acts as a cisplatin sensitizer via the caspase-3-dependent pathway in HCC chemotherapy. Oncol. Rep. 34, 368–374 10.3892/or.2015.3996 [DOI] [PubMed] [Google Scholar]
- 26.Guo L., Wu H., Zhu J.et al. (2015) Genetic variations in the PI3K/AKT pathway predict platinum-based neoadjuvant chemotherapeutic sensitivity in squamous cervical cancer. Life Sci. 143, 217–224 10.1016/j.lfs.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 27.Dimitrova V. and Arcaro A. (2015) Targeting the PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr. Mol. Med. 15, 82–93 10.2174/1566524015666150114115427 [DOI] [PubMed] [Google Scholar]
- 28.Hafsi S., Pezzino F.M., Candido S.et al. (2012) Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of drug-resistance (review). Int. J. Oncol. 40, 639–644 [DOI] [PubMed] [Google Scholar]
- 29.Lee H.G., Lee J.M., Shin S.J.et al. (2014) Salinomycin inhibited cell proliferation and induced apoptosis in human uterine leiomyoma cells. Obstet. Gynecol. Sci. 57, 501–506 10.5468/ogs.2014.57.6.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B., Chen W., Fu Y.et al. (2020) MicroRNA-27a-3p reverses adriamycin resistance by targeting BTG2 and activating PI3K/Akt pathway in breast cancer cells. Onco Targets Ther. 13, 6873–6884 10.2147/OTT.S256153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study are included in this published article and its Supplementary files.