Dear Editor,
Immune checkpoint inhibitors (ICIs) have dramatically changed the prognosis of advanced melanoma patients, but also exposed them to immune‐related adverse events (irAEs). 1 Furthermore, with the combination ipilimumab (IPI) plus nivolumab (NIVO), some rare irAEs, such as myositis and neuromuscular diseases, are increasingly emerging. 2 Similar manifestations have been reported during coronavirus disease 2019 (COVID‐19) 3 and more rarely with the vaccination. 4 Because cancer patients exhibit severe and fatal forms of the COVID‐19 disease, vaccination is recommended.
Herein, we report a case of severe necrotizing myopathy after COVID‐19 vaccination, in a man with advanced melanoma treated with IPI plus NIVO.
A 41‐year‐old man, without medical history, was diagnosed in November 2015 with stage IIIB melanoma (defined by the American Joint Committee on Cancer) on the left shoulder. He was subsequently enrolled in January 2016 in the EORTC MK‐3475 trial. 5 He received pembrolizumab/placebo for 1 year, without any irAEs. In January 2021, computed tomography (CT) revealed unresectable, latero‐aortic and common iliac lymph node masses; biopsy confirmed melanoma metastasis, and molecular testing revealed a BRAFV600E mutation. Despite unblinding revealed that he was in the pembrolizumab arm, we decided to initiate NIVO (1 mg/kg) plus IPI (3 mg/kg) every 3 weeks. He received his first and second injections on 25 February and 19 March 2021 respectively. On March 12, the first dose of COVID‐19 vaccine (BTN162b2, Pfizer‐BioNTech) was performed into his deltoid. Three days after the second infusion of ICIs, he was hospitalized for severe muscle pain, weakness of the limbs, bulbar symptoms and dyspnoea. Physical examination revealed asymmetric and severe weakness of the quadriceps, psoas, deltoids, biceps and neck flexor muscles. Blood tests revealed increased creatine phosphokinase (CPK) levels (12647 IU/L, normal, < 190 IU/L), without myocarditis. Autoantibodies associated with myasthenia gravis and paraneoplastic myositis were negative. Magnetic resonance imaging (MRI) was consistent with a myositis (Fig. 1a–d). Electromyography (EMG) showed severe myogenic syndrome in the four limbs (Fig. 1e), and muscle biopsy revealed a necrotizing myopathy (Fig. 2a–f).
Figure 1.
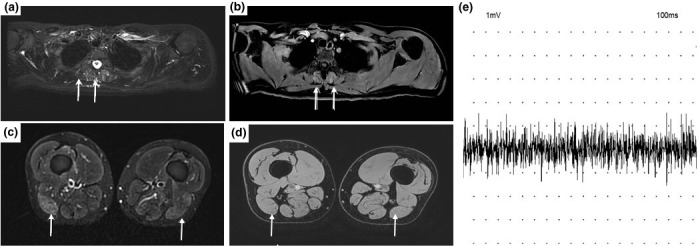
COVID‐19 vaccine and necrotizing myopathy in a 41‐year‐old man receiving immune checkpoint inhibitors: Magnetic resonance imaging (MRI) in a 41‐year‐old man with COVID‐19 vaccine‐related showed: (a–d) increasing signal intensity in STIR sequences of paraspinal muscle (a, arrows), of biceps femoris (c, arrows). A postgadolinium sequence (b–d) shows enhancement of paraspinal muscle (b, arrows), and biceps femoris muscle does not show significant enhancement (d, arrow). (e) Electroneuromyography (EMG) of supraspinatus muscle showed a rapidly interferential pattern for modest muscular effort, with reduced amplitude of motor unit potentials (MUPs).
Figure 2.
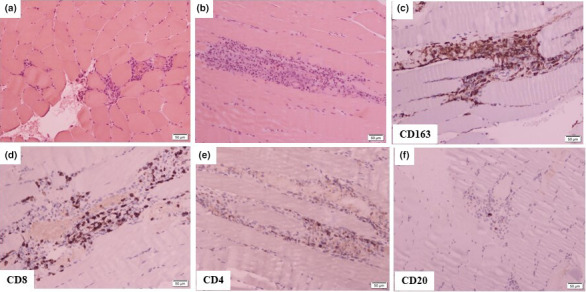
COVID‐19 vaccine and necrotizing myopathy in a 41‐year‐old man receiving immune checkpoint inhibitors: Histopathological analysis of skeletal muscle from the left deltoid of patient as stained by haematoxylin and eosin (a): frozen section, (b): paraffin section shows necrotic fibres with macrophagic infiltrate and lymphocytes. Immunohistochemical staining reveals; (c) numerous macrophagic cells, (d–f) lymphocytes are mainly T and CD8+ with few CD4+ and CD20+ cells.
Considering all these data, a diagnosis of necrotizing myopathy grade 4 according to the Common Terminology Criteria for Adverse Events ((version 4) was made. Immunotherapy was discontinued, and high dose of glucocorticoids (1 g/day intravenously) was administered for 3 days, followed by oral prednisone, progressively reduced. Daily physical examination revealed progressive improvement. The CPK levels, MRI and EMG were normalized within 2 months. In this context and as IgG against SARS‐CoV‐2 was highly positive (807 IU/L, normal, < 190 IU/L), the patient did not receive the second injection of the vaccine. Six months after, he had a near‐complete clinical recovery and a partial oncological response.
Clinical trials with the BNT162b2 mRNA COVID‐19 vaccine reported a good safety profile. 6 Nevertheless, several cases of myositis following COVID‐19 vaccination with RNA vaccines (Pfizer‐BioNTech or Moderna) and adenovirus vaccine (Oxford‐AstraZeneca) have also been registered in the international pharmacovigilance database. To date, the relationship between COVID‐19 vaccination and an increase of irAEs during ICI therapy has not been investigated. Data exist with the inactivated influenza vaccine (IV), but caution is warranted because they are contrasted. 7 , 8
Myositis is a rare but already reported adverse event of IPI+NIVO treatment. 1 We cannot exclude that it is also the case in our patient. However, we hypothesize that vaccination by an immune‐boosting effect acts as the trigger of necrotizing myopathy in our patient, with ICIs as the potential contributors. This scenario was remarkably exposed by Au et al., who reported a cytokine release syndrome in a patient with colorectal cancer treated with anti‐PD‐1 after vaccination with BNT162b2. 9
As an increasing number of patients treated with ICIs will be vaccinated in the coming months, clinicians should be aware of this potential association between COVID‐19 vaccines and the exacerbation of irAEs. Further prospective pharmacovigilance data are needed in this population.
Conflicts of interest
Manon Blaise, Fanny Rocher, Hélène Spittler, Adrien Sanchez, Elisabeth Lanteri, Lucia Coco, Angela Puma, Arnaud Martel, Geraldine Gonfrier, Thierry Passeron and Henri Montaudié declare to have no conflict of interest.
Funding sources
None.
Ethics statement
Informed consent statement: The patient provided informed written consent prior to the study.
Acknowledgements
The authors thank the patient for consenting to the publication of this work. We thank ‘editage’ for the English editing.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Five‐year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 2. Moreira A, Loquai C, Pföhler C et al. Myositis and neuromuscular side‐effects induced by immune checkpoint inhibitors. Eur J Cancer 2019; 106: 12–23. [DOI] [PubMed] [Google Scholar]
- 3. Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID‐19. Neurol Sci 2020; 41: 3039–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Theodorou DJ, Theodorou SJ, Axiotis A, Gianniki M, Tsifetaki N. COVID‐19 vaccine‐related myositis. QJM 2021; 114: 424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eggermont AMM, Blank CU, Mandala M et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018; 378: 1789–1801. [DOI] [PubMed] [Google Scholar]
- 6. Oliver SE, Gargano JW, Marin M et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer‐BioNTech COVID‐19 vaccine ‐ United States, December 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis 2020; 70: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Läubli H, Balmelli C, Kaufmann L et al. Influenza vaccination of cancer patients during PD‐1 blockade induces serological protection but may raise the risk for immune‐related adverse events. J Immunother Cancer 2018; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Au L, Fendler A, Shepherd STC et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 2021; 27: 1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


