Abstract
Objective:
To determine the dose-dependent effect of contemporary marijuana exposure on female menstrual cyclicity and reproductive endocrine physiology in a nonhuman primate model.
Design:
Research animal study.
Setting:
Research institute environment.
Animals:
Adult female rhesus macaques (6–12 years of age; n = 8).
Intervention(s):
Daily delta-9-tetrahydrocannabinol (THC) edible at medically and recreationally relevant contemporary doses.
Main Outcome Measure(s):
Menstrual cycle length (MCL), anti-Müllerian hormone, prolactin, basal follicle-stimulating hormone (FSH), estradiol (E2) and progesterone, luteinizing hormone (LH), and thyroid-stimulating hormone.
Result(s):
The average before THC weight was 6.9 kg (standard deviation, 0.8), and at the highest THC dosing, the average weight was 7.2 kg (standard deviation, 0.8). With increasing THC dosing, MCL and FSH concentrations increased, while basal E2 concentration was stable. The average MCL concentration increased 4.0 days for each mg/7 kg/day of THC (95% CI, 1.4–6.6 days). Follicle-stimulating hormone concentration increased significantly with increasing THC dose, 0.34 ng/mL for each mg/7 kg/day of THC (95% CI, 0.14–0.57 ng/mL). No significant trends were observed between THC dosing and average basal progesterone, anti-Müllerian hormone, prolactin, LH, or thyroid-stimulating hormone concentrations.
Conclusion(s):
In rhesus macaques, a dose response toward increased MCL and basal FSH concentrations but plateau of basal E2 and LH concentrations was observed with increasing THC dosing, suggesting ovulatory dysfunction. Further studies are needed to determine the effects of a longer duration of exposure and whether the significant increase in MCL and FSH concentrations results in reduced fecundity.
Keywords: Cannabis, female reproductive health, marijuana, menstrual cycle
Marijuana is the most commonly used psychotropic drug in the United States after alcohol (1) with growing popularity as both a recreational and medicinal drug. In 2019, 48.2 million people aged >12 years in the United States reported using marijuana in the past year (2). As the legal status of marijuana continues to evolve domestically and abroad, greater accessibility to the drug has led to an increase in the number of annual marijuana users with a concurrent decrease in the perceived risk of harm (2). Although marijuana use is on the rise across all age groups and especially in young adults, the 2017 National Survey on Drug Use and Health noted that the most significant increases were in reproductive-age women, ages 18–25 years old (3). Furthermore, recreational and medical marijuana has been used for the treatment of menstrual-related cramps, mood symptoms, and endometriosis-related pain, which may additionally increase with the decreased perceived risk of harm (4, 5). Despite this growing prevalence, several questions remain regarding the safety of frequent marijuana use. While it has been demonstrated that chronic exposure to marijuana or its principal psychoactive component, delta-9-tetrahydrocannabinol (THC), may impact reproductive and offspring health (6-9), the data available are limited and conflicted.
Previous literature suggests that exposure to THC interferes with reproductive function and decreased serum concentrations of pituitary gonadotropin. The endocannabinoid system is composed of cannabinoid receptors; the two most well-known cannabinoid receptors are CB1 and CB2, both G protein-coupled receptors. In prior rodent studies, THC exposure was found to activate hypothalamic endocannabinoid CB1 receptors and ultimately reduced gonadotropin concentrations via the suppression of pulsatile luteinizing hormone (LH) secretion and menstrual cyclicity in females (10-12). A high acute dose of THC administered intraperitoneal in rats significantly decreased serum LH and prolactin (PRL) concentrations (11) and suppressed ovulation (13). In regular cycling rhesus macaques, daily injected THC in the first 18 days of the menstrual cycle resulted in anovulation in 80% of subjects (14). However, most of the prior animal studies performed did not study the effects of THC through a delivery vehicle commonly used by humans, such as smoking, edibles, or vaping, nor at doses comparable to contemporary marijuana use.
Human studies on substance use are often limited by a retrospective or observational design, rely primarily on patient self-report, are confounded by polysubstance abuse, preclude the determination of a causal effect on female reproductive health from THC, and have not focused on edible routes of THC exposure (4, 15). In addition, prior studies revealed that women underreport their marijuana use (16), and there is no biologic validation for self-reported doses (8, 17). Even when use is reported accurately, it is difficult to quantify and compare reported usage. Unlike alcohol where a shot, a glass of wine, and a beer have roughly the same amount of alcohol, there is no such equivalency for marijuana because different strains and delivery mechanisms vary in potency and there is a lack of consistency in formulation and labeling (18). In addition, the variability in potency has resulted in limited dose-response data, which is relevant to counsel patients unable to abstain from use.
To address the key gaps in marijuana and reproductive health research, an animal model allows for standardization of subject variability, experimental manipulation, and precise marijuana exposure to elucidate direct biologic consequences of chronic marijuana use while methodologically controlling for potential confounders. Our study aimed to determine the effect of chronic THC use on menstrual cyclicity and female reproductive health in rhesus macaques. Rhesus macaques are an ideal surrogate for understanding THC effects on reproductive processes in women because of their highly similar physiological, endocrine, genetic, and anatomical properties (19).
MATERIALS AND METHODS
Experimental Design
A cohort of sexually mature, adult female rhesus macaques (Macaca mulatta) (n = 8) weighing 6–8 kg with regular menstrual cycles were used in this study. Animals were pair-housed indoors under controlled conditions. Animal ages ranged from 6.7 to 12.7 years, with a mean age of approximately 10 years old. All animals were in the Oregon National Primate Research Center’s (ONPRC) time-mated breeding program and considered fertile. Animals were considered infertile if they were no longer naturally cycling, if a pelvic ultrasound revealed very few ovarian follicles, or if anti-Müllerian hormone (AMH) concentrations were very low. All animal procedures were approved by the ONPRC Institutional Animal Care and Use Committee and conformed to all applicable regulations (IP0001389).
Animals were maintained on a standard chow diet (Test-Diet, St. Louis, Missouri) with a daily cookie containing THC (THC edible) made using research-grade THC obtained directly from the National Institute on Drug Abuse Drug Supply Program. Chow and tap water were available ad libitum. All cookies were administered before the animal’s daily chow to ensure they were consumed on an empty stomach and confirm complete ingestion. Animals were slowly titrated up to 2.5 mg/7 kg/day of THC over approximately a 3–4-month time period to model medical marijuana acclimation recommendations from Colorado (20). Specifically, animals were initially maintained on a dose of 0.5 mg/7 kg/day of THC for weeks 1–3, 1 mg/7 kg/day (moderate THC dose) for weeks 4–6, 2 mg/7 kg/day for weeks 7–9, and 2.5 mg/7 kg/day (heavy THC dose) for weeks 10–12. This THC dosage was calculated from the recommended THC starting dose of 5 mg for a 68-kg adult (the average rhesus macaque weighs approximately 6.5–7.5 kg), followed by titration to 10 mg for moderate users (standardized serving size for edible retail marijuana products in Colorado) and 20–30 mg for heavy users (20-22). Blood was sampled (2 mL) at each dose adjustment time point during THC induction, 3 hours (20) after edible consumption, to determine THC concentrations with each increase in THC dosage.
Data Collection
Animal weight was recorded before THC induction and at the end of each dosing time point before adjustment until animals reached the maximum THC dose of 2.5 mg/7 kg/day. Vaginal swabs were performed to detect the onset and duration of menses before and during THC induction. Menstrual cycle length (MCL) was determined by counting the number of days between the onset of menstruation in one month and the onset of menstruation in the next. Day 1 of each menstrual cycle was assigned to the first day of a positive vaginal swab for blood. On cycle day 3, peripheral blood samples (2 mL collected before THC and at each THC dose) were obtained for follicular phase estradiol (E2) and progesterone concentrations as measured by radioimmunoassay (RIA) on a Roche Cobas e411 system (Roche Diagnostics, Indianapolis, IN). Additionally, concentrations of AMH, PRL, follicle-stimulating hormone (FSH), LH, and thyroid-stimulating hormone (TSH) were quantified with assays detailed in the following that were performed by the Endocrine Technologies Core (ETC) at the ONPRC and Oregon Clinical and Translational Research Institute Laboratories.
Anti-Müllerian hormone.
Serum AMH concentrations were measured by enzyme-linked immunosorbent assay (Ansh Labs, Webster, TX). The assay range was 0.07–16.9 ng/mL. Intra-assay coefficient of variation (CV) is 0.5%–1.6% using internal controls that are provided by the manufacturer. In addition, the ETC includes an in-house nonhuman primate (NHP) serum quality control (QC) pool with each assay. Intra-assay CV for this pool in the AMH ELISA was 1.2%. Because all AMH concentrations were determined in a single assay, no inter-assay CV was determined.
Follicle-stimulating hormone and luteinizing hormone.
Follicle-stimulating hormone and LH concentrations were performed by the ETC using a double-antibody RIA procedure similar to that described by Niswender and Spies (23). The LH and FSH RIA kits were purchased from Dr. Albert Parlow (NHPP, Harbor-UCLA Medical Center, Los Angeles). These are homologous cynomolgus macaque assays with recombinant cynomolgus LH (AFP-6936A) or FSH (AFP-6940A) used for both iodination and standards. Rabbit anti-cynomolgus LH (AFP-342994) or FSH (AFP-782594) were used at final dilutions of 1:750,000 and 1:1,038,462 for LH and FSH, respectively. The standard curves ranged between 0.005 and 10 ng/tube for both assays. The detection limit of each assay was 0.005–0.02 ng/tube. Intra-assay variations were 2.6% and 5.4% for LH and FSH, respectively. Overall inter-assay variation for monkey gonadotropin RIAs in the ETC was less than 15%.
Prolactin.
Serum PRL concentrations were determined by automatic immunoassay on a Roche Cobas e411 system (Roche Diagnostics, Indianapolis, IN). Intra-assay CV using an in-house NHP serum QC pool was 2.9%. Because all PRL concentrations were determined in a single assay, no inter-assay CV was determined.
Thyroid-stimulating hormone.
Thyroid-stimulating hormone concentrations were measured using an IMMULITE 1000 (Siemens Medical Diagnostics) solid-phase two-site chemiluminescent immunometric assay (LKTS1).
THC Testing
Chemicals and reagents.
The Strata Impact protein precipitation plate and 2-mL collection plates were from Phenomenex (Torrance, CA). Oasis Prime elution plates and 1-mL round collection plates were from Waters (Milford, MA). Tetrahydrocannabinol and metabolites as well as their deuterated internal standards were purchased from Cerilliant (Round Rock, TX). Acetonitrile, methanol, and water were purchased from Honeywell (Mexico City, Mexico), and formic acid along with sample vials and other high performance liquid chromatography supplies were purchased from Fisher Scientific (Rockwood, TN). Human ethylenediaminetetraacetic acid plasma for standards was purchased from Innovative research (Novi, Michigan) with a voluntary drug-free affidavit, although multiple samples were tested before a drug-free matrix was obtained. Control rhesus macaque plasma was used for initial testing and QCs.
Preparation of plasma and calibrators.
For calibration, a commercial standard at 100 ng/μL was diluted into use stocks at 2,000, 1,000, 200,100, 20, 10, and 2 ng/mL. A volume of 50 μL of each stock was added to 950 μL of plasma, except for the highest standard where 100 μL of use stock was added to 900 μL of plasma for standard curves in the range of 0.1–200 ng/mL. Quality controls were prepared from separate dilutions at 25 and 2.5 ng/mL using 500 and 50 ng/mL of stocks. Internal standard mix was prepared using deuterated versions of all compounds at a final concentration of 1 ng/sample. Samples were thawed, and 50 μL of standards, QCs, or samples was pipetted into an Impact Protein Precipitation plate, followed by the addition of 5 μL of internal standard mix (1 ng) and 150 μL of 0.1% formic acid in acetonitrile. Each sample was pipetted up and down two to three times to mix. The cleaned extract was then eluted into a 2-mL collection plate on a Bio-tage 96-well positive pressure device. The capture plate was removed from the device, and 325 μL of water was added to each well and mixed and then transferred to an Oasis HLB Prime 2-mg u-elution plate and pushed through the plate. The plate was then washed with 2 × 250 μL of 25:75 ratio of methanol to water. The plate was then placed onto a mass spec-compatible 1-mL collection plate, and the compounds were eluted using 2 × 25 μL of 90:10 ratio of acetonitrile to methanol. Moreover, 50 μL per well of water was added, and the plate was analyzed.
Liquid chromatography with tandem mass spectrometry analysis of cannabinoid metabolites.
Tetrahydrocannabinol and metabolites were analyzed using a 5500 QTRAP hybrid/triple quadrupole linear ion trap mass spectrometer (SCIEX, Framingham, MA) with electrospray ionization in positive mode. The mass spectrometer was interfaced to a Shimadzu (Columbia, MD) SIL-20AC XR autosampler, followed by two liquid chromatography (LC)-20AD XR LC pumps. The instrument was operated with the following settings: source voltage, 5,000 kV; GS1, 40; GS2, 50; CUR, 15; TEM, 700; and CAD gas, HIGH. Compounds were infused individually, and instrument parameters were optimized for each multiple reaction monitoring transition. The gradient mobile phase consisted of two solvents: A, 0.1% formic acid in water, and B, 0.1% formic acid in acetonitrile. The column used was a Phenomenex Kinetex 2.6 μ XB-C18 100 × 3 mm, with a flow rate of 0.35 mL/min. The gradient consisted of an initial concentration of organic of 55% B. Start conditions were held for 1 min, followed by an increase to 95% B over 4 min, held at 95% B for 3 min, and decreased to start condition of 55% B over 0.1 min and re-equilibration for 1.9 min. The standard curves ranged from 0.1 to 200 ng/mL; the lower limit of detection was 0.1 ng/mL, and the lower limit of quantification was 0.5 ng/mL for all compounds.
Statistical Analysis
We compared the mean animal weights at baseline and at the highest THC dose (2.5 mg/7 kg/day) using a paired t test. We assessed the average association between THC dose and plasma THC concentrations, MCL, and hormone concentrations using linear mixed-effects modeling with random intercepts by animal. All statistical tests were two-sided and used an alpha of 0.05. All analyses were performed using Stata version 15.1 (StataCorp, College Station, TX).
RESULTS
Of the eight female rhesus macaques in this study, all were of reproductive age (mean, 10 years; standard deviation [SD], 2.2), all but one had proven fecundity, and none were previously exposed to THC or any other significant, known environmental exposures (Table 1). All animals consumed the daily THC edible before their regular chow. The average baseline weight of all animals was 6.9 kg (SD, 0.8), and all but one animal gained weight after starting the THC treatment (Table 1). At the highest THC dosing, there was an increase in average weight of all animals by 0.3 kg (SD, 0.4; P = .083) that was not significant. The animals’ behavior after THC treatment was not formally assessed but was not noted to be grossly different per veterinary and animal support staff.
TABLE 1.
Characteristics of the female rhesus macaques used in this study (n = 8).
| Characteristic | Mean ± standard deviation |
|---|---|
| Age (years) | 10.0 ± 2.2 |
| Gravidity | 2.6 ± 2.1 |
| Parity | 2.6 ± 2.1 |
| Before THC weight (kg) | 6.9 ± 0.8 |
| After THC weight (kg) | 7.2 ± 0.8 |
| Before THC menstrual cycle length (days) | 28.3 ± 2.8 |
| After THC menstrual cycle length (days) | 37.8 ± 15.8a |
Note: Average increase of 4.0 days per mg/7 kg/day of THC dose increase
P = .002. THC = tetrahydrocannabinol.
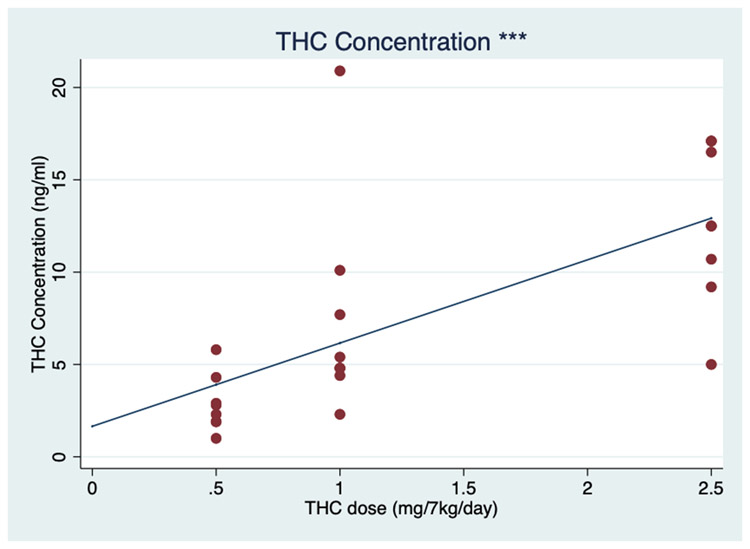
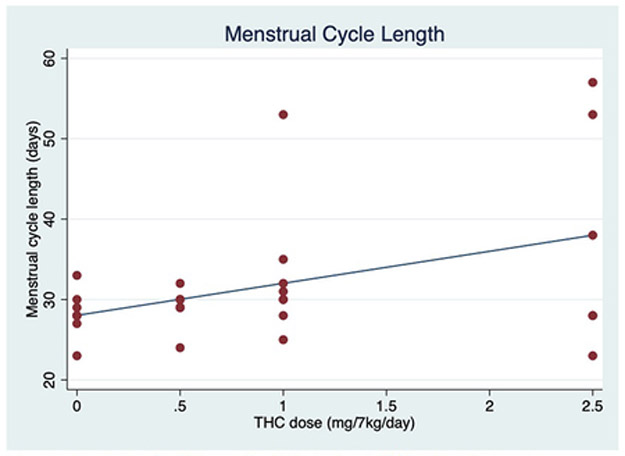
During the THC induction, the average plasma THC concentrations increased by 4.5 ng/mol for each mg/7 kg/day increase in THC (95% CI, 2.6–6.5 ng/mol; P<.001) (Fig. 1). With increasing THC edible dosing, a positive dose-dependent effect on the average MCL was observed. The average cycle length increased 4.0 days for each mg/7 kg/day of THC (95% CI, 1.4–6.6 days; P = .002) (Fig. 2).
FIGURE 1.
Plasma tetrahydrocannabinol (THC) concentrations with increasing THC dosing. Individual (red dots) and average fixed-effect (blue line) plasma THC concentration (ng/mol) in response to increasing oral THC dosage (0–2.5 mg/7 kg/day) among eight female rhesus macaques. ***P≤.001.
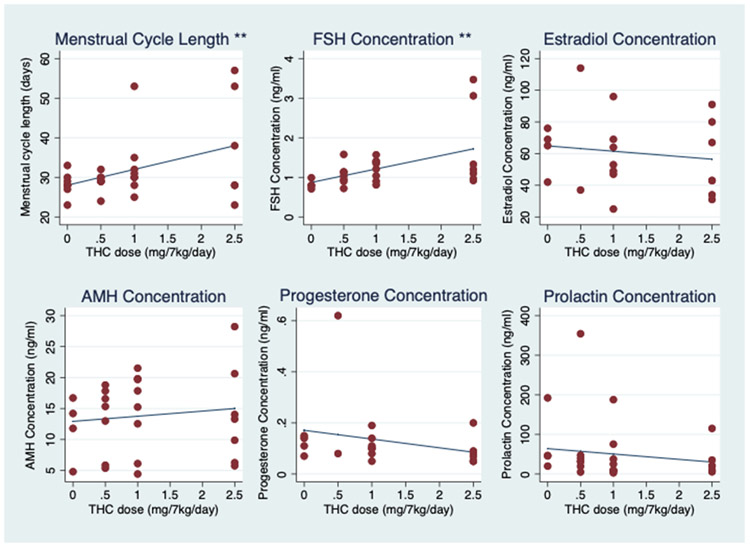
FIGURE 2.
Menstrual cycle length, basal follicle-stimulating hormone (FSH), estradiol, anti-Müllerian hormone (AMH), progesterone, and prolactin concentrations with increasing tetrahydrocannabinol (THC) dosing. Individual (red dots) and average fixed-effect (blue lines) menstrual cycle length (days) and hormone concentrations (ng/mL) in response to increasing oral THC dosage (0–2.5 mg/7 kg/day) among eight female rhesus macaques. **P ≤.01. All other hormone associations are not significant at the 0.05 level.
In terms of the reproductive endocrine axis, serum FSH concentration increased significantly with increasing THC dose (0.34 ng/mL for each mg/7 kg/day of THC) (95% CI, 0.14–0.57 ng/mL; P = .004), while serum E2 concentration remained stable, with an average change of −3.4 pg/mL (95% CI, −11.4–4.7 pg/mL; P = .416) and within expected values for day 3 of the macaque cycle with increasing THC dosing (Fig. 2). In addition, luteinizing hormone concentrations remained flat, with an average change of 0.0 ng/mL with increasing THC dose (95% CI, −0.1–0.1 ng/mL; P = .819). Hence, there was an increase in FSH concentration without a corresponding change in basal E2 concentration as well as stable LH concentrations across increasing THC doses (Figs. 2 and 3). With increasing THC dosing, AMH concentrations trended upward, although the average change was not significant (0.8 ng/mL; 95% CI, −0.7–2.3 ng/mL; P = .278). As anticipated, there was no change in progesterone concentrations because blood samples were collected in the follicular phase of the menstrual cycle (Fig. 2).
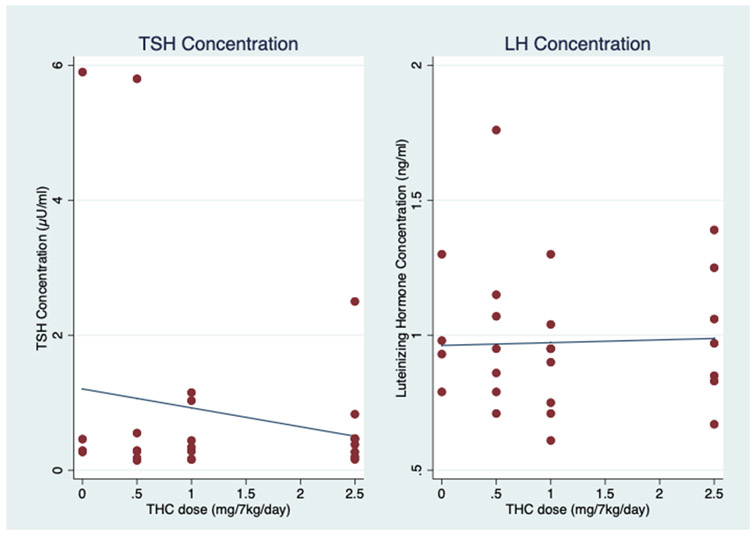
FIGURE 3.
Thyroid-stimulating hormone (TSH) and luteinizing hormone (LH) concentrations with increasing tetrahydrocannabinol (THC) dosing. Individual (red dots) and average fixed-effect (blue lines) TSH concentration (μU/mL) and LH concentration (ng/mL) in response to increasing oral THC dosage (0–2.5 mg/7 kg/day) among eight female rhesus macaques. Neither hormone association is significant at the 0.05 level.
Additionally, with increasing THC dosing, there were no significant average changes in PRL (−13.6 ng/mL; 95% CI, −31.9–4.7 ng/mL; P = .146) (Fig. 2) and TSH (−0.3 μU/mL; 95% CI, −0.8–0.2 μU/mL; P = .284) concentrations (Fig. 3).
DISCUSSION
Our study design utilized a THC induction schedule that modeled published medical marijuana acclimation recommendations for humans (20). Using this model, we found a positive correlation between oral THC dosage and plasma THC concentration confirming that the dosing regimen was successful in the NHP. Additionally, the average plasma THC concentrations at the highest oral THC edible dose were within the expected contemporary dosing range reported in humans 3 hours after consuming a similar oral THC dose (22, 24). There was no significant THC effect on weight gain observed, which is consistent with the existing literature that demonstrates an appetite stimulating effect but not always clinically meaningful weight gain (25, 26).
A notable impact of chronic THC consumption on MCL was observed in a relatively short period of exposure time. Females were exposed for a total of 3 months, with only exposure in the last month to an equivalent, heavy medical marijuana dose. This resulted in a dose response of longer MCL as well as greater variation in interanimal MCL with increased THC edible dosing. There have only been few studies examining the effects of marijuana on MCL, but the limited data suggests an association between marijuana use and disrupted menstrual cycles, including a slightly higher rate of anovulatory cycles (4, 15). A prior rhesus macaque study utilizing daily intramuscular injections of THC of 2.5 mg/kg induced longer anovulatory cycles (14), but this dose or mode of THC delivery is not reflective of contemporary human marijuana use.
With increasing THC dose, we noted a dose response of increased FSH concentrations. In our study, FSH concentrations in the follicular phase of the menstrual cycle (i.e., 3 days after the appearance of menses) increased, whereas E2 concentrations were unchanged. This suggests an effect on hypothalamic neurons (e.g., kisspeptin) or hypothalamic endocannabinoid CB1 receptors, which would be an area for further study. Although follicular development can occur with persistent estrogen production, follicular development can be arrested before full maturation (27). This can lead to or represent an underlying disruption in ovulation and alterations in MCL and ultimately impact fecundability. Similarly, prior studies of intravenously administered acute doses of THC in rhesus macaques have additionally described significant disruption in cycle length and increasing FSH resulting in anovulation (14, 28).
Our results suggest that at increasing doses of THC, there was no significant change in AMH concentrations. Because of the animal cohort size of this pilot study and interanimal variability, there was not enough power to demonstrate a statistically significant difference. Anti-Müllerian hormone is an established predictor of ovarian reserve, with nomograms previously established for the rhesus macaques (29). It has a significant role in folliculo-genesis, with overexpression of AMH within human populations such as those with polycystic ovarian syndrome in which women are oligo-ovulatory or anovulatory (30). Although in our study AMH values remained in the previously established nomograms, the nonsignificant rise in AMH concentrations we observed could indicate follicular arrest with increasing THC doses leading to an increased number of follicles in the preantral or antral stage. Existing literature in humans suggests that there are adverse effects of chronic marijuana use implicating a shortened luteal phase (15) and reduced fertility (4, 5, 15) because of menstrual cycle disturbances.
Serum PRL concentrations, conversely, did not significantly change with increasing THC doses. Although the existing literature on the neuroendocrine effects of chronic marijuana use is limited, this result is consistent with published findings in prior humans and rodent studies (11, 31-33). Ranganathan et al. (31) reported that frequent chronic marijuana users have a lower plasma PRL concentration relative to healthy controls. Interestingly, in a small cohort of human females studying the acute effects of marijuana smoking on PRL concentrations, there were no significant changes in plasma PRL during the follicular phase of the menstrual cycle observed, but PRL concentrations were significantly lower at 60–120 and 150–180 min after marijuana smoking during the luteal phase of the menstrual cycle (34). However, these findings of acute marijuana-induced suppression of plasma PRL concentrations in females have not been observed in human males when the administration of marijuana compounds was either orally or through smoking (34).
There was a slight decrease in TSH concentrations with increasing THC dosing, but this was not significant. This result is similar to reported findings in human and rat studies after recent or acute THC exposure. A prior cross-sectional study from the National Health and Nutrition Examination Survey noted that recent marijuana use was not associated with thyroid dysfunction but was significantly associated with lower TSH concentrations (35). In a rat model, reduced TSH secretion was observed after systemic injection of THC (36).
To our knowledge, this is the first study to use a relevant, translational rhesus macaque model to study a dose-response effect from THC on female reproductive health. Tetrahydrocannabinol edibles were used to simulate marijuana consumption and corresponding blood concentrations in humans. Compared with other animal models, the NHP offers several advantages including a similar plasma disposition of THC (37), and rhesus macaques have a similar 28-day menstrual cycle that is regulated by mechanisms akin to women (38-40). In addition, our rhesus model allows precise marijuana exposure and experimental manipulation, not feasible or ethical in humans, to elucidate direct, dose-dependent biologic consequences from chronic marijuana use while methodologically controlling for potential confounders. Hence, this NHP pilot study provides otherwise unattainable insights into the relationship between increasing THC dose on MCL and reproductive endocrine function that is translatable and applicable to humans. In addition, it provides additional informative data concerning female endocrine hormone concentrations after chronic marijuana use, which has been an underinvestigated area of research. Limited existing studies have largely focused on the effects of acute marijuana exposure (5, 14, 26, 34, 41). It is possible that because some reproductive hormones are not secreted continuously and have episodic secretion patterns, some effects of chronic marijuana use were not detected because only one blood sample was obtained at each THC dose increase time point. In addition, seasonal changes in MCL have been reported in the NHP, but this has largely been observed in animals that are outdoor housed. All of the animals in this study were indoor housed and had regularly tracked cycles before undergoing THC induction.
In summary, these pilot data strongly suggest a dose-response impact on female reproductive health from chronic contemporary THC use. Further studies are needed to determine whether the increases in MCL and FSH concentrations are indicative of other reproductive abnormalities such as reduced fecundity as well as the effect of a longer duration of exposure. Given the dearth of existing literature, the larger impact of chronic THC on ovarian function and fertility is significant. Our study findings may be helpful to healthcare providers when counseling patients regarding the health consequences of chronic marijuana use.
In addition, these data indicate that increasing doses of chronic THC consumption results in a dose-response relationship for increased MCL and serum FSH concentrations, suggestive of ovulation dysfunction.
Menstrual cycle length with increasing THC dosing. Individual (red dots) and average (blue lines) menstrual cycle length (days) in response to increasing oral THC dosage (0 to 2.5 mg/7kg/day) among 8 female rhesus macaques.
Acknowledgments:
The investigators thank the veterinary and husbandry staff that provided excellent care for the animals used in this study, in particular Dr. Lauren Drew Martin and Travis Hodge. Additionally, the investigators thank the Endocrine Technologies Core and Assisted Reproductive Core at the ONPRC as well as the Bioanalytical Shared Resource/Pharmacokinetics Core and Oregon Clinical and Translational Research Institute Laboratories at Oregon Health & Science University.
For the financial support, all Oregon National Primate Research Center (ONPRC) cores and units were supported by the National Institutes of Health (NIH) Grant P51 OD011092. Research reported in this publication was supported by the Reproductive Scientist Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute on Drug Abuse (NIDA), March of Dimes Foundation, and Silver Family Innovation Award under Award Numbers K12 HD000849 (to J.L.) and R03 HD097116 (to J.L.). The contents of this study are solely the responsibility of the investigators and do not necessarily represent the official view of NIH NICHD and NIDA.
REFERENCES
- 1.National Institute on Drug Abuse. What is the scope of marijuana use in the United States? Available at: https://www.drugabuse.gov/publications/research-reports/marijuana/what-scope-marijuana-use-in-united-states. Accessed May 14, 2021. [Google Scholar]
- 2.Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. Available at: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTMI72019NSDUHFFR090120.htm. Accessed May 14, 2021. [Google Scholar]
- 3.Results from the 2017 National Survey on Drug Use and Health: Detailed Tables, SAMHSA, CBHSQ. Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.pdf. Accessed May 14, 2021. [Google Scholar]
- 4.Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. J Womens Health (Larchmt) 2007;16:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wise LA, Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Sorensen HT, et al. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health 2018;72:208–15. [DOI] [PubMed] [Google Scholar]
- 6.Brents LK. Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med 2016;89:175–91. [PMC free article] [PubMed] [Google Scholar]
- 7.Kasman AM, Thoma ME, McLain AC, Eisenberg ML. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertil Steril 2018;109:866–71. [DOI] [PubMed] [Google Scholar]
- 8.Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol 2015;213:761–78. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez CE, Sheeder J, Allshouse AA, Scott S, Wymore E, Hopfer C, et al. Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. BJOG 2019;126:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besch NF, Smith CG, Besch PK, Kaufman RH. The effect of marihuana (delta-9-tetrahydrocannabinol) on the secretion of luteinizing hormone in the ovariectomized rhesus monkey. Am J Obstet Gynecol 1977;128:635–42. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarty I, Sheth AR, Ghosh JJ. Effect of acute delta9-tetrahydrocannabinol treatment on serum luteinizing hormone and prolactin levels in adult female rats. Fertil Steril 1975;26:947–8. [DOI] [PubMed] [Google Scholar]
- 12.Dalterio SL, Mayfield DL, Bartke A. Effects of delta 9-THC on plasma hormone levels in female mice. Subst Alcohol Actions Misuse 1983;4:339–45. [PubMed] [Google Scholar]
- 13.Nir I, Ayalon D, Tsafriri A, Cordova T, Lindner HR. Letter: Suppression of the cyclic surge of luteinizing hormone secretion and of ovulation in the rat by delta 1-tetrahydrocannabinol. Nature 1973;243:470–1. [DOI] [PubMed] [Google Scholar]
- 14.Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ. Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab 1981;52:50–5. [DOI] [PubMed] [Google Scholar]
- 15.Bauman J. Marihuana and the female reproductive system testimony before the subcommittee on criminal justice of the committee on the judiciary. Available at: http://babel.hathitrust.org/cgi/pt?id=pur1.32754078039595;view=1up;seq=1. Accessed May 14, 2021. [Google Scholar]
- 16.Fendrich M, Johnson TP, Wislar JS, Hubbell A, Spiehler V. The utility of drug testing in epidemiological research: results from a general population survey. Addiction 2004;99:197–208. [DOI] [PubMed] [Google Scholar]
- 17.National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017, PMCID: PMC Journal – In process. [PubMed] [Google Scholar]
- 18.Elsohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry 2016;79:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stouffer RL, Woodruff TK. Nonhuman primates: a vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR J 2017;58:281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannabis Clinicians Colorado. Medical marijuana new patient success guide. Available at: http://coscc.org/wp-content/uploads/2016/07/CCC-General-CannabisAsMedicine-2017DRAFT.pdf. Accessed May 14, 2021. [Google Scholar]
- 21.Colorado Department of Public Health and Environment. Retail Marijuana Public Health Advisory Committee’s monitoring health concerns related to marijuana in Colorado. Available at: https://www.colorado.gov/pacific/marijuana/safety-edibles. Accessed May 14, 2021. [Google Scholar]
- 22.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niswender GD, Spies HG. Serum levels of luteinizing hormone, follicle-stimulating hormone and progesterone throughout the menstrual cycle of rhesus monkeys. J Clin Endocrinol Metab 1973;37:326–8. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 1980;28:409–16. [DOI] [PubMed] [Google Scholar]
- 25.Sansone RA, Sansone LA. Marijuana and body weight. Innov Clin Neurosci 2014;11:50–4. [PMC free article] [PubMed] [Google Scholar]
- 26.Natale BV, Gustin KN, Lee K, Holloway AC, Laviolette SR, Natale DRC, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci Rep 2020;10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ESHRE Capri Workshop Group. Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update 2012;18:586–99. [DOI] [PubMed] [Google Scholar]
- 28.Asch RH, Smith CG. Effects of delta 9-THC, the principal psychoactive component of marijuana, during pregnancy in the rhesus monkey. J Reprod Med 1986;31:1071–81. [PubMed] [Google Scholar]
- 29.Long H, Wang Y, Wang L, Lu Y, Nie Y, Cai Y, et al. Age-related nomograms of serum anti-Mullerian hormone levels in female monkeys: comparison of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys. Gen Comp Endocrinol 2018;269:171–6. [DOI] [PubMed] [Google Scholar]
- 30.La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64:603–10. [DOI] [PubMed] [Google Scholar]
- 31.Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, et al. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolodny RC, Masters WH, Johnson VE. Textbook of sexual medicine. 1st ed. Boston: Little, Brown and Company; 1979. [Google Scholar]
- 33.Dornbush RL, Kolodony RC, Bauman JE, Webster SK. Human female chronic marijuana use and endocrine functioning. Soc Neurosci 1978;4:490. [Google Scholar]
- 34.Mendelson JH, Ellingboe J, Mello NK. Acute effects of natural and synthetic cannabis compounds on prolactin levels in human males. Pharmacol Biochem Behav 1984;20:103–6. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra S, Heptulla RA, Homel P, Motaghedi R. Effect of marijuana use on thyroid function and autoimmunity. Thyroid 2017;27:167–73. [DOI] [PubMed] [Google Scholar]
- 36.Lomax P The effect of marihuana on pituitary-thyroid activity in the rat. Agents Actions 1970;1:252–7. [DOI] [PubMed] [Google Scholar]
- 37.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther 2018;182:133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner RM, Slayden OD. Molecular and functional aspects of menstruation in the macaque. Rev Endocr Metab Disord 2012;13:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenner RM, West NB, McClellan MC. Estrogen and progestin receptors in the reproductive tract of male and female primates. Biol Reprod 1990;42:11–9. [DOI] [PubMed] [Google Scholar]
- 40.Smith CG, Asch RH. Acute, short-term, and chronic effects of marijuana on the female primate reproductive function. NIDA Res Monogr 1984;44:82–96. [PubMed] [Google Scholar]
- 41.Mendelson JH, Mello NK, Ellingboe J. Acute effects of marihuana smoking on prolactin levels in human females. J Pharmacol Exp Ther 1985;232:220–2. [PubMed] [Google Scholar]