Dear Editor,
In March 2020, the Word Health Organization announced the COVID‐19 outbreak as a pandemic. A vaccine was the main solution to stop the infection from spreading. In December 2020, the Food and Drug Administration authorized the first, the Pfizer–BioNTech (BNT162b2). It consists of a messenger RNA (mRNA) which is administered intramuscularly, as a double dose vaccine.
It has been shown to be safe and effective by conferring 95% protection against COVID‐19. 1 However, there are few adverse events; the most described are pain at the injection site, fatigue, and headache. 2 Herein, we report two cases of herpes zoster ophthalmicus (HZO) following COVID‐19 vaccination.
1. CASE 1
An 80‐year‐old woman with a history of breast cancer diagnosed in 2018 was referred to us for erythematous, pruritic, and painful blistering rash localized to the right side of the forehead and scalp. She also presented eyelid edema of the right eye (Figure 1). As for her breast cancer, the patient had undergone a quadrantectomy and radiotherapy 3 years earlier and was currently on adjuvant therapy with Anastrozole. She reported that she had received the second dose of the BNT162b2 COVID‐19 vaccine 3 days before the onset of the skin manifestations, diagnosed as HZO. An urgent eye examination ruled out corneal involvement The patient was treated with acyclovir therapy and eye drops for 10 days. After 1 week, the skin lesions resolved with the presence of serous crusts. The patient reported improvement in itching and pain.
Figure 1.
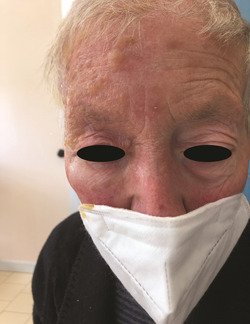
Vescicular rash that involves the right ophthalmic division of the fifth cranial nerve and edema of the right eyelid
2. CASE 2
A 69‐year‐old woman who had received the second dose of the BNT162b2 COVID‐19 vaccine 7 days before, presented to us for ocular pain and small papules and blisters on his left forehead. She had no significant past medical history. On physical examination, the tip of the nose was involved (Hutchinson's sign) (Figure 2). We diagnosed HZO without ocular complications and started treatment with acyclovir. After 1 week, ophthalmalgia and the skin eruption improved significantly.
Figure 2.
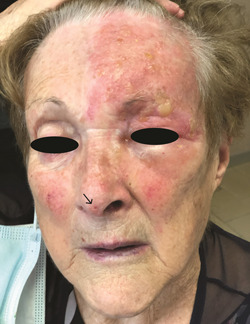
Erythema and blisters on the left forehead, edema of the left eye, and Hutchinson's sign (involvement of the tip of the nose) (black arrow)
Varicella‐zoster virus (VZV) is responsible for a primary infection (i.e., chickenpox); subsequently, the virus remains dormant at the level of the spinal dorsal root and cranial ganglia. In conditions of stress or immunosuppression, it can reactivate and cause secondary herpes zoster (HZ) infection. HZO accounts for 10%–20% of HZ cases and is characterized by involvement of the ophthalmic branch of the fifth cranial nerve. It is considered a dangerous condition that could lead to severe consequences such as blindness in 20%–70% of the cases. 3 The main risk factors for the reactivation of VZV are a compromise of the cell‐mediated immunity (CMI) that presents itself in old age, in some chronic diseases such as diabetes, autoimmune disease, HIV, and during immunosuppressive therapies. 4
Several cases of HZ have been described in Pfizer vaccine recipients, however, only one of them with ophthalmic localization, in a 56‐year‐old woman with rheumatoid arthritis. 5
We report two cases of postvaccine HZO although a rarely reported adverse event with potentially serious consequences.
HZO was also diagnosed in four patients suffering from a moderate form of COVID‐19 infection 6 that were effectively treated with antivirals without any visual sequelae. In these cases, the triggering factor for viral reactivation is probably due to lymphopenia secondary to SARS‐CoV‐2.
The close temporal proximity between the administration of the vaccine and the onset of symptoms has assumed a causal relationship between the two events. The underlying mechanism could be identified in vaccine‐induced immunomodulation. In fact, the mRNA vaccine determines an abundant production of I INF which can lead to a dysfunctional TCD8+ response and TCD8+ exhaustation. 7
Further epidemiological studies are needed to confirm the link between mRNA vaccination and HZ reactivation.
It can be concluded that the vaccination is safe since in neither case, the disease did not show complications. However, it is necessary to take into account this possible side effect to start immediately the correct therapy.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Nicoletta Bernardini (conceptualization), Nevena Skroza (writing the original review), Alessandra Mambrin (writing the original review), Ilaria Proietti (writing editing), Anna Marchesiello (writing editing), Federica Marraffa (conceptualization), Giovanni Rossi (writing original draft), Salvatore Volpe (writing original draft), Ersilia Tolino (writing original draft), Concetta Potenza (supervision). All individuals listed as authors have contributed substantially to the design, performance, analysis, or reporting of the work.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Clinical trial group safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diotallevi F, Campanati A, Radi G, et al. Vaccination against SARS‐CoV‐2 and psoriasis: the three things every dermatologist should know. J Eur Acad Dermatol Venereol. 2021;35(7):e428‐e430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis AR, Sheppard J. Herpes zoster ophthalmicus review and prevention. Eye Contact Lens. 2019;45(5):286‐291. [DOI] [PubMed] [Google Scholar]
- 4. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 suppl):S3‐S12. [DOI] [PubMed] [Google Scholar]
- 5. Nofal A, Fawzy MM, Sharaf El Deen SM, El‐Hawary EE. Herpes zoster ophthalmicus in COVID‐19 patients. Int J Dermatol. 2020;59:1545‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID‐19 vaccine. J Med Virol. 2021;93(9):5231‐5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Beuckelaer A, Grooten J, De Koker S. Type I interferons modulate CD8+ T cell immunity to mRNA vaccines. Trends Mol Med. 2017;23(3):216‐226. [DOI] [PubMed] [Google Scholar]


