Abstract
Background
Few studies have evaluated the prevalence of post‐extubation dysphagia and associated factors in patients with coronavirus disease 2019 (COVID‐19) . Our study assessed the prevalence of post‐extubation dysphagia and body composition in patients with COVID‐19 discharged from an intensive care unit (ICU).
Methods
A prospective cohort study was performed in post‐ICU extubated patients with acute respiratory distress syndrome related to COVID‐19 in two referral hospitals. A total of 112 patients were evaluated and included; swallowing assessment and bioelectrical impedance analysis (BIA) were performed after extubation and discharge from the ICU. To identify associations between dysphagia, lower phase angle (PhA) (<4.8°) and hydration (extracellular water/total body water < 0.390) logistic and linear regression analyses were conducted.
Results
The incidence of post‐extubation dysphagia was 41% (n = 46). From these, 65% (n = 30) had severe swallowing impairment. Overhydration and PhA were significantly different in patients with dysphagia, and segmental hydration in the trunk and legs was higher than in arms. PhA <4.8° (odds ratio [OR], 12.2; 95% CI, 4.3–34.1; P < .05) and overhydration measured by BIA (OR, 9.1; 95% CI, 3.4–24.5; P < .05) were associated with post‐extubation dysphagia in multivariate analysis. PhA (<4.8°) was associated with a lower rate of swallowing recovery at hospital discharge (log‐rank test = 0.007).
Conclusions
A high incidence of post‐extubation dysphagia was found in patients with COVID‐19. Low PhA and overhydration were associated with the presence of dysphagia. Lower PhA was an independent factor for swallowing recovery at discharge.
Keywords: bioelectrical impedance, COVID‐19, dysphagia, intensive care unit, mechanical ventilation, overhydration, phase angle
INTRODUCTION
One of the main concerns of coronavirus disease 2019 (COVID‐19) is the progression to respiratory failure. 1 It has been documented that severe patients develop acute respiratory distress syndrome (ARDS) and may need an intensive care unit (ICU). 2 Additionally, it has been reported that the prevalence of patients requiring mechanical ventilation (MV) could be around 43%–69%. 3 Unfortunately, intubation and MV are associated with the risk of swallowing impairment, 4 and post‐extubation dysphagia is observed in 30%–62% of the general population at the ICU, which may require modified‐texture diets or enteral tube feeding to make food intake safer when severe swallowing impairment is present. 5 , 6
Dysphagia is associated with compromised patient outcomes, including malnutrition, sarcopenia, aspiration pneumonia, prolonged length of stay (LOS), and increased mortality risk. 7 The intubation process, post‐extubation, critical illness, use of tracheostomies, and delirium appear to be common variables in these patients; however, the causes, prevalence, and management of dysphagia in patients with COVID‐19 are yet to be understood. In post‐extubation patients, a systematic swallowing assessment is necessary to identify those dysphagia cases and prevent worsening outcomes correctly. Nonetheless, this assessment in patients with COVID‐19 represents a significant challenge because of the high‐risk situation for healthcare professionals, as it is considered an aerosol‐generating procedure. 8
Any healthcare provider who cares for patients with COVID‐19 has to perform their interventions to patients in a way that minimizes the risk of contagion and spreading the disease to other patients, coworkers, and people near them. Therefore, the use of technologies such as bioelectrical impedance analysis (BIA) offers an advantage in the nutrition assessment of patients, given the minimum time of contact required to perform it. BIA is a noninvasive technique used to estimate body composition through resistance and reactance mechanisms. It has been recognized as an excellent alternative to dual‐energy x‐ray absorptiometry. 9 , 10 One of the useful parameters that BIA gives is phase angle (PhA), an important prognosis indicator and a marker of cell membrane integrity. 11 A lower PhA is consistent with muscle mass loss, cell breakdown, and the cell aging process, whereas higher PhA is associated with large quantities of healthy cell membranes and body muscle mass. 11 , 12
To our knowledge, few studies have evaluated the prevalence of post‐extubating dysphagia and associated factors in patients with COVID‐19. Therefore, our study aimed to assess the prevalence of post‐extubation dysphagia and body composition in patients with COVID‐19 discharged from an ICU.
MATERIALS AND METHODS
A prospective cohort study was performed in post‐ICU and extubated patients with ARDS related to COVID‐19 in two referral centers. The study was conducted from September to December 2020. Swallowing assessment and BIA analysis were performed on the same day once patients were extubated and discharged from the ICU. Demographic and clinical parameters were obtained from the electronic file. Patients were informed about the study and the procedure of the utilized techniques, and those who agreed to participate in the study were screened and evaluated. This work has been carried out in accordance with the Code of Ethics of the World Medical Association and the Clinical Research and Bioethics Committee. The study was in adherence to the Declaration of Helsinki.
Swallowing function
At extubation time, the Yale Swallow Protocol was performed. 13 The criteria for success were uninterrupted drinking (assisted or independent) of 89 ml of water from a cup without coughing. Criteria for failure were inability to drink the entire amount, interrupted drinking, or coughing during or immediately after drinking. A trained researcher performed a volume‐viscosity swallow test (V‐VST) 14 in patients who failed the test.
The V‐VST assesses the ability to drink safely and effectively with different types of viscosity and volume. The following clinical signs of ineffective swallowing were observed during the test: impaired labial seal, oral residue, and multiple swallows per bolus. According to V‐VST, the following clinical signs of unsafe swallowing are voice‐quality changes, cough, or decrease in oxygen saturation ≥3% to detect silent aspiration if a patient presented a decline of ≥5% in oxygen saturation. If any of these were observed, the test was interrupted because of the high risk of aspiration.
Body composition analysis
Patients were first weighed with a chair scale (SECA 954; SECA Co, Hamburg, Germany); afterward, the body composition analysis was performed using multifrequency BIA equipment (InBody S10; InBody Co, Ltd, Seoul, Korea) with the standard technique. All participants were supine with limbs slightly spread apart from the body. The area where the electrodes were to be placed was cleaned with alcohol, and then the electrodes were placed on both hands and feet according to the manufacturer's instructions (InBody Co). A standardized healthcare professional performed the study using the same device to avoid interobserver and interdevice variability. The InBody S10 uses segmental impedance and reactance at multiple frequencies to determine total body water (TBW), (segmental) extracellular water (ECW), and the individual ECW/TBW ratio. High‐frequency currents pass predominantly through the TBW, whereas low‐frequency currents cannot penetrate cell membranes and flow predominantly through the ECW. A standardized ECW/TBW ratio of 0.380 is the reference value for normal hydration. An ECW/TBW ratio >0.380 is considered an overhydration status. 15 In addition, a 50‐kHz PhA was assessed. A PhA <4.8° is considered an independent predictor of mortality in the ICU. 16
Statistical analysis
Data are presented as mean ± standard deviation or median with interquartile range (25th–75th percentile)], depending on its distribution. The Shapiro‐Wilk or Kolmogorov‐Smirnov test for the proper parametric or nonparametric test was performed. A Student t‐test or Mann‐Whitney U test was used for continuous variables to compare groups with dysphagia and those without dysphagia. Categoric variables are presented as percentages and proportions between groups and were compared using the chi‐squared test. We hypothesized that there would be an association between age, MV, PhA, and dysphagia. A logistic and linear regression analysis was conducted to identify potential associations between dysphagia with lower PhA (<4.8°) and hydration. A P‐value <.05 was considered statistically significant. Additional analysis was performed to separate survival analysis regarding the time to swallowing recovery associated with PhA using log‐rank test; Kaplan‐Meier curves are displayed for most significant results.
The data were analyzed using SPSS, version 25.
RESULTS
A total of 112 patients were evaluated and included in this study, the mean age of the patients was 54 ± 12 years, and 18% (20 of 112) were female. Table 1 shows the demographics and clinical characteristics of our study population. The incidence of post‐extubation dysphagia at the evaluation was 41% (46 of 112). A total of 65% (30 of 46) of the patients had severe swallowing impairment, and a feeding tube was placed to feed and hydrate the patient. The mean duration of MV of the study population was 20 ± 9 days. The most common comorbidities among patients with COVID‐19 were diabetes mellitus, hypertension, and obesity.
TABLE 1.
Demographics and clinical characteristics of study patients at extubation time
| Total, n = 112 | Dysphagia group, n = 46 | Nondysphagia group, n = 66 | P‐value | |
|---|---|---|---|---|
| Sex a | ||||
| Males, n (%) | 92 (82) | 36 (78) | 56 (85) | .37 |
| Age, years b | 54 ± 12 | 58 ± 11 | 50 ± 11 | <.001 |
| Comorbidities, n (%) a | ||||
| Diabetes mellitus | 51 (46) | 22 (48) | 29 (43) | .68 |
| Hypertension | 49 (44) | 19 (41) | 30 (46) | .66 |
| Obesity | 35 (30) | 14 (34) | 20 (33) | .89 |
| Chronic kidney disease | 3 (3) | 2 (3) | 3 (3) | .73 |
| Clinical variables | ||||
| MV, b days | 20 ± 9 | 28 ± 20 | 14 ± 8 | <.001 |
| Tracheal cannula placement, n (%) | 17 (15) | 16 (35) | 1 (2) | <.001 |
| Weight at admission, b kg | 86 ± 21 | 84 ± 24 | 88 ± 17 | .53 |
| Height, b cm | 164 ± 9 | 163 ± 10 | 166 ± 9 | .28 |
| BMI, b kg/m2 | 29 ± 5.9 | 30 ± 6.8 | 29 ± 6.2 | .66 |
| Weight at extubation, b kg | 79 ± 19 | 78 ± 21 | 80 ± 17 | .66 |
| Weight loss, b kg | 8 ± 5.9 | 7 ± 5.2 | 9 ± 6.5 | .91 |
| BIA measurements | ||||
| PhA, b ° | 4.8 ± 1.1 | 4.0 ± 0.96 | 5.2 ± 0.93 | <.001 |
| ECW/TBW b | 0.395 ± 0.138 | 0.402 ± 0.014 | 0.389 ± 0.010 | <.001 |
Abbreviations: BIA, bioelectrical impedance analysis; BMI, body mass index; ECW/TBW, extracellular water/total body water ratio; MV, mechanical ventilation; PhA, 50‐kHz phase angle.
Proportions were compared by using the chi‐squared test.
A t‐test was used for comparisons between groups.
A statistically significant difference was found in age, days of MV, and frequency of tracheal cannula placement between dysphagia patients and those with safe swallowing. In the BIA measurements, the PhA in the dysphagia patients group was lower than that in the nondysphagia group (4.0° ± 1.0° vs 5.2° ± 0.9°, P < .001). The frequency of overhydration measured by BIA analysis was higher in the dysphagia group. The anthropometric variables such as weight, body mass index, and weight loss were similar between study groups, and no statistically significant difference was found. The LOS of all patients was 28 ± 17 days; the dysphagia group had a higher LOS compared with the nondysphagia group (36 ± 20 vs 22 ± 11 days, respectively; P < .001).
Additionally, we evaluated PhA and overhydration in each segment of the body (arms, trunk, and legs) and assessed whether there was a difference between those with or without dysphagia (Figure 1).
FIGURE 1.
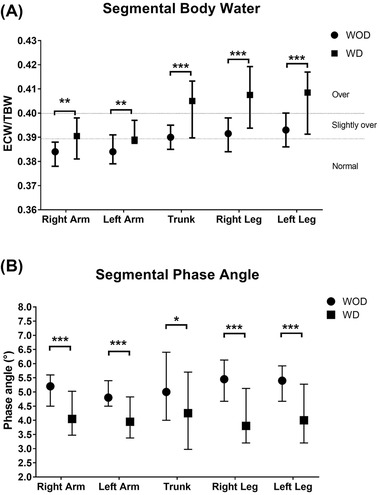
Segmental water ratios (A) and phase angle (PhA) (B) in patients with and without dysphagia. Patients without dysphagia (WOD) had better hydration and PhA in all the segments compared with patients with dysphagia (WD). Segmental body water and PhA differ between patients WD or WOD, and upper extremities had less overhydration than the trunk and lower extremities. *P < .05, **P < .01, and ***P < .001. ECW/TBW, extracellular water/total body water ratio
The factors associated with dysphagia are presented in Table 2. In the bivariate analysis, age >60 years, MV, LOS, PhA <4.8°, and overhydration measured by BIA analysis were associated with the presence of dysphagia; when results were adjusted by age and sex, only PhA <4.8° and overhydration measured by BIA analysis were associated with post‐extubation dysphagia in the multivariate analysis.
TABLE 2.
Factors associated with dysphagia
| Bivariate analysis | Multivariate analysis b | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age (>60 years) | 2.6 | 1.2–5.9 a | ||
| MV (days) | 12.4 | 3.4–45.9 a | ||
| LOS (days) | 5.7 | 1.2–27.8 a | ||
| PhA (<4.8°) | 15.2 | 5.6–41.4 a | 12.2 | 4.3–34.1 a |
| Overhydration | 18.0 | 5.4–60.8 a | 9.1 | 3.4–24.5 a |
Abbreviations: LOS, length of stay; MV, mechanical ventilation; PhA, phase angle.
Significant P‐value < .05.
Multivariate analysis adjusted by sex and age.
Finally, a Kaplan‐Meier analysis is presented in Figure 2 and shows that a lower PhA (< 4.8°) was associated with a lower rate of swallowing recovery at the time of hospital discharge compared with those patients with higher PhA (log‐rank test = 0.007). A total of 69% (n = 30) of the patients diagnosed with swallowing impairment at the time of extubation persisted with swallowing abnormalities at the time of hospital discharge.
FIGURE 2.
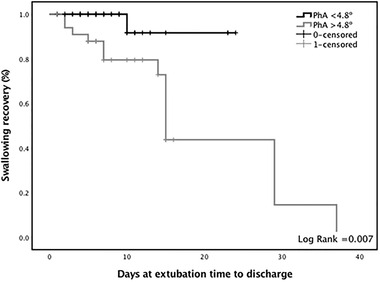
Phase angle (PhA) related to the length of stay and restitution of oral intake. The black line represents those with a PhA lower than 4.8°, whereas the gray line represents those with a PhA over 4.8°
DISCUSSION
This work describes the frequency of post‐extubation dysphagia and factors associated with patients diagnosed with COVID‐19 who were discharged from an ICU. As we expected, a high prevalence of dysphagia was found; 41% of the patients had swallowing impairment at the time of extubation. Although there are few data on post‐extubation dysphagia in patients with COVID‐19, we can compare the findings to some results found in the related literature. 7 , 17 Dawson et al found a prevalence of oropharyngeal dysphagia of 50% in post‐ICU hospitalized patients who were admitted because of COVID‐19. 7 A higher incidence of dysphagia is seen in patients with severe COVID‐19 than in patients with other conditions at the ICU, probably related to a higher requirement for MV, as Zuercher et al found when evaluating dysphagia risk factors after MV. 18 Our study found that patients with dysphagia had a higher MV requirement than the nondysphagia group. Age has always been an essential factor associated with changes in the swallowing process. 19 , 20 , 21 In concordance, we found that age was associated with the presence of post‐extubation dysphagia.
Nutrition status and swallowing function are usually related, especially when there is a loss in muscle mass. 22 For this reason, we were interested in analyzing the body composition by BIA to assess whether lower muscle mass index could be associated with the presence of dysphagia. However, given that there was a significant difference between groups in the relation between ECW and TBW, the evaluation and measurement of the muscle mass index could not be determined because of the high risk of bias on the muscle mass results. Our results of ECW/TBW ratio are slightly less than those reported by Moonen et al, who found an overall ECW/TBW ratio of 0.40 (0.39–0.40), but this could be because our measurements were performed after patients were discharged from the ICU, whereas Moonen et al performed measurements at the time of admission. 15 To our knowledge, this is one of the first studies that showed by segments a body composition analysis of ECW/TBW ratio and PhA. In the case of ECW/TBW ratio, we could identify that patients without dysphagia were slightly overhydrated in the legs and trunk but with normal ECW/TBW ratio in the arms; by contrast, in those with dysphagia, both arms were slightly overhydrated, whereas the trunk and legs were overhydrated.
Furthermore, and as an important variable, we observed a lower PhA in the dysphagia group than in the nondysphagia group, highlighting the relevance of this parameter as a prognosis survival factor. 23 These results are similar to what we previously reported when we evaluated the impact of a modified diet on patients with dysphagia for whom the baseline PhA was 4.2° and those results found by Moonen et al and Cornejo‐Pareja et al in recently admitted patients with COVID‐19 who had PhAs of 4.5° and 4.4°, respectively. 15 , 24 , 25 Interestingly, when segmental PhA analysis was performed, we did find a difference between patients with or without dysphagia, in which all segments of the dysphagia group were under 4.8°, but there was no difference between segments in any of the groups.
Additionally, our results show that a lower PhA (<4.8°) was a predictor of swallowing impairment at the discharge time. Interestingly, a high percentage of patients with low (<4.8°) PhA still had swallowing impairment at the time of hospital discharge compared with patients with a higher PhA (≥4.8°). These findings suggest that the severity and some factors related to the body composition before admission are important to recovery and the prognosis of patients with severe COVID‐19.
This study has some limitations; first, the evaluation of body composition at the time of admission was not possible because of the condition of the patients. Another limitation was that the protection equipment available to the healthcare providers was limited at the time, making it difficult to assess more patients. Finally, because of the loss in muscle strength of many patients after an extended stay in the ICU, some patients could not be weighed and therefore were not included in this study. Further studies that include more patients are needed to analyze other factors associated with post‐extubation dysphagia adjusted with other variables, such as comorbidities, previous body composition, or previous muscle mass index.
This study highlights the importance of swallowing evaluation at extubation time in patients with COVID‐19. A high incidence of post‐extubation dysphagia is found in patients with severe COVID‐19 compared with patients with other conditions at the ICU. Specific interventions such as nutrition support and rehabilitation are highly recommended in patients with swallowing impairment due to COVID‐19. These interventions are necessary to improve prognosis as restitution to total oral intake and improve the COVID‐19 survivors' quality of life.
CONFLICT OF INTEREST
None declared.
FUNDING INFORMATION
None declared.
AUTHOR CONTRIBUTIONS
Carlos A. Reyes‐Torres and Aurora E. Serralde‐Zúñiga equally contributed to the conception and design of the research; Adriana Flores‐López contributed to the design of the research; Carlos A. Reyes‐Torres, Adriana Flores‐López, Ivan A. Osuna‐Padilla, and Carmen Margarita Hernández‐Cárdenas contributed to the acquisition and analysis of the data; Carlos A. Reyes‐Torres and Adriana Flores‐López contributed to the interpretation of the data; and Carlos A. Reyes‐Torres, Adriana Flores‐López, and Aurora E. Serralde‐Zúñiga drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
ACKNOWLEDGMENT
The authors thank Lilia Castillo‐Martínez, MD, for her comments through the manuscript writing.
Reyes‐Torres CA, Flores‐López A, Osuna‐Padilla IA, Hernández‐Cárdenas CM, Serralde‐Zúñiga AE. Phase angle and overhydration are associated with post‐extubating dysphagia in patients with COVID‐19 discharged from the ICU. Nutr Clin Pract. 2022;37:110–116. 10.1002/ncp.10781
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrando C, Suarez‐Sipmann F, Mellado‐Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID‐19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang R, Elhusseiny KM, Yeh Y‐C, Sun W‐Z. COVID‐19 ICU and mechanical ventilation patient characteristics and outcomes—A systematic review and meta‐analysis. PLoS One. 2021;16(2):e0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frajkova Z, Tedla M, Tedlova E, Suchankova M, Geneid A. Postintubation dysphagia during COVID‐19 outbreak‐contemporary review. Dysphagia. 2020;35(4):549‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brodsky MB, Huang M, Shanholtz C, et al. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5‐year longitudinal study. Ann Am Thorac Soc. 2017;14(3):376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawson C, Capewell R, Ellis S, et al. Dysphagia presentation and management following coronavirus disease 2019: an acute care tertiary centre experience. J Laryngol Otol. 2020;134(11):981‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schindler A, Baijens LWJ, Clave P, et al. ESSD commentary on dysphagia management during COVID pandemia. Dysphagia. 2021;36(4):764‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim M, Kim H. Accuracy of segmental multi‐frequency bioelectrical impedance analysis for assessing whole‐body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr. 2013;67(4):395‐400. [DOI] [PubMed] [Google Scholar]
- 10. Sun G, French CR, Martin GR, et al. Comparison of multifrequency bioelectrical impedance analysis with dual‐energy X‐ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81(1):74‐78. [DOI] [PubMed] [Google Scholar]
- 11. Di Vincenzo O, Marra M, Di Gregorio A, Pasanisi F, Scalfi L. Bioelectrical impedance analysis (BIA) ‐derived phase angle in sarcopenia: a systematic review. Clin Nutr. 2021;40(5):3052‐3061. [DOI] [PubMed] [Google Scholar]
- 12. Belarmino G, Gonzalez MC, Torrinhas RS, et al. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9(7):401‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ward M, Skelley‐Ashford M, Brown K, Ashford J, Suiter D. Validation of the yale swallow protocol in post‐acute care: a prospective, double‐blind, multirater study. Am J Speech Lang Pathol. 2020;29(4):1937‐1943. [DOI] [PubMed] [Google Scholar]
- 14. Clave P, Arreola V, Romea M, Medina L, Palomera E, Serra‐Prat M. Accuracy of the volume‐viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr. 2008;27(6):806‐815. [DOI] [PubMed] [Google Scholar]
- 15. Moonen HPFX, van Zanten FJL, Driessen L, et al. Association of bioelectric impedance analysis body composition and disease severity in COVID‐19 hospital ward and ICU patients: the BIAC‐19 study. Clin Nutr. 2021;40(4):2328‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stapel SN, Looijaard WGPM, Dekker IM, Girbes ARJ, Weijs PJM, Oudemans‐van Straaten HM. Bioelectrical impedance analysis‐derived phase angle at admission as a predictor of 90‐day mortality in intensive care patients. Eur J Clin Nutr. 2018;72(7):1019‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ajemian MS, Nirmul GB, Anderson MT, Zirlen DM, Kwasnik EM. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg. 2001;136(4):434‐437. [DOI] [PubMed] [Google Scholar]
- 18. Zuercher P, Schenk NV, Moret C, Berger D, Abegglen R, Schefold JC. Risk factors for dysphagia in ICU patients after invasive mechanical ventilation. Chest. 2020;158(5):1983‐1991. [DOI] [PubMed] [Google Scholar]
- 19. Nakao Y, Uchiyama Y, Honda K, Yamashita T, Saito S, Domen K. Age‐related composition changes in swallowing‑related muscles: a Dixon MRI study. Aging Clin Exp Res. Accepted manuscript. Published online April 26, 2021. doi:10.1007/s40520‐021‐01859‐2 [DOI] [PubMed] [Google Scholar]
- 20. Nishikubo K, Mise K, Ameya M, Hirose K, Kobayashi T, Hyodo M. Quantitative evaluation of age‐related alteration of swallowing function: videofluoroscopic and manometric studies. Auris Nasus Larynx. 2015;42(2):134‐138. [DOI] [PubMed] [Google Scholar]
- 21. McIntyre M, Chimunda T, Koppa M, Dalton N, Reinders H, Doeltgen S. Risk factors for postextubation dysphagia: a systematic review and meta‐analysis. Laryngoscope. Accepted manuscript. Published online December 15, 2020. doi:10.1002/lary.29311 [DOI] [PubMed] [Google Scholar]
- 22. Yoshimi K, Hara K, Tohara H, et al. Relationship between swallowing muscles and trunk muscle mass in healthy elderly individuals: a cross‐sectional study. Arch Gerontol Geriatr. 2018;79:21‐26. [DOI] [PubMed] [Google Scholar]
- 23. Norman K, Stobäus N, Pirlich M, Bosy‐Westphal A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31(6):854‐861. [DOI] [PubMed] [Google Scholar]
- 24. Reyes‐Torres CA, Castillo‐Martínez L, Reyes‐Guerrero R, et al. Design and implementation of modified‐texture diet in older adults with oropharyngeal dysphagia: a randomized controlled trial. Eur J Clin Nutr. 2019;73(7):989‐996. [DOI] [PubMed] [Google Scholar]
- 25. Cornejo‐Pareja I, Vegas‐Aguilar IM, García‐Almeida JM, et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID‐19: a longitudinal cohort study. Clin Nutr. Accepted manuscript. Published online February 17, 2021. doi:10.1016/j.clnu.2021.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]


