Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors globally; it is valuable to predict its prognosis after treatment. Aspartate aminotransferase-to-platelet index (APRI), a non-invasive biomarker consists of two routine test parameters easily available in all the patients. Our study aimed to investigate whether APRI can serve as an independent prognostic marker in the patients with HCC.
Methods: We extensively searched PubMed, Embase, and Web of Science databases on June 20, 2021 to determine all relevant literature. The studies that explored the association between the APRI levels and prognosis of patients with HCC and reported risk estimate data were included. The Newcastle-Ottawa Scale was used to assess the quality of the included studies.
Results: A total of 1,097 articles were initially identified, of which 28 studies involving 11,041 patients met the eligibility criteria for the meta-analysis. The pooled hazard ratios (HRs) for overall survival (OS) and disease-free survival (DFS) were 1.77 (95% CI: 1.53–2.05, P < 0.001) and 1.59 (95% CI: 1.47–1.71, P < 0.001), respectively, suggesting a significant correlation between the increased APRI levels and poor prognosis in the patients with HCC. In the subgroup analyses, statistical significance of the correlation disappeared in the Korean and Japanese population and in the patients undergoing transarterial chemoembolization (TACE). Of note, the current results may be overestimated due to publication bias, but the conclusion remained unchanged when the bias was adjusted.
Conclusion: High APRI levels are associated with poor OS and DFS in the patients with HCC. In most cases, pretreatment APRI can be used as an independent prognostic factor, but it is necessary to incorporate other predictive prognostic systems to ensure accuracy. Further studies are needed to determine the specific beneficiary population and the optimal cutoff value.
Keywords: hepatocellular carcinoma, aspartate aminotransferase-to-platelet ratio index, APRI, noninvasive biomarker, prognosis, overall survival, disease-free survival, meta-analaysis
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide with a poor 5-year survival rate (1). Despite the development of effective anti-viral therapeutics, incidence of HCC is continuing to rise, in part driven by the epidemic of non-alcoholic fatty liver disease (2). At present, in addition to liver transplantation, the surgical resection and chemotherapy are still the effective and routine options for HCC; accurate prognostic judgment is helpful for patient management and formulation of the medication strategies. Although the Tumor, Node, Metastasis (TNM) staging system is an effective independent prognostic factor for HCC, its predictive ability is lagging and limited. Therefore, it is necessary to combine new indices to improve the accuracy and timeliness of predicting survival in the patients with HCC.
Several inflammation-based markers and biomarkers of liver fibrosis and cirrhosis have been explored to predict prognosis (3). The previous studies have found that liver fibrosis and cirrhosis are important risk factors for postoperative recurrence of liver cancer (4–6). Liver biopsy is the gold standard for grading liver fibrosis or cirrhosis, but the invasive and expensive properties limited its clinical application (7). In 2003, aspartate aminotransferase (AST)-to-platelet (PLT) index (APRI) was first proposed by Wai et al. as a substitute index for the diagnosis of advanced fibrosis and cirrhosis in the patients with chronic hepatitis C; compared with liver biopsy, APRI is a simple, feasible, and non-invasive predictor and is derived from routine laboratory data calculated as the ratio of [(aspartate aminotransferase/upper limit of normal value)/platelet counts (× 109/L)] × 100 (8–10). Meta-analysis has revealed that increased APRI levels can predict the risk of HCC development in the patients with chronic hepatitis (11). Furthermore, the preoperative APRI scores are associated with the postoperative complications of HCC and can predict the occurrence of hepatic failure after liver resection (12–15). As there is a strong correlation between APRI and liver histopathology, and liver fibrosis and cirrhosis are the key factors in the development of HCC; multiple studies attempt to explore the accuracy of APRI in predicting the prognosis of HCC.
The studies that explored the association between the APRI levels and the prognosis of HCC have shown inconsistent findings. In addition, these studies tend to come from a single center and are limited to specific populations, such as the patients with hepatitis B virus (HBV)-associated HCC or after hepatectomy. Therefore, to examine the prognostic potential of APRI comprehensively and systematically in the patients with HCC, we extensively reviewed all the cohort studies that explored the association of APRI levels with overall survival (OS) and disease-free survival (DFS) in the patients with HCC. Furthermore, we investigated the effects of multiple host and external factors on the predictive strength, which can help the clinicians fully utilize clinical data for risk stratification and develop personalized monitoring strategies.
Methods and Materials
This meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA 2020) statement (16). The protocol for this study is not registered.
Search Strategy
Two reviewers independently conducted an extensive search of PubMed, Embase, and Web of Science databases to determine all relevant literature on November 15, 2020 and updated on June 20, 2021. The search query was formed by combining the subject words with free words, specifically, that include the following terms: “Carcinoma, Hepatocellular,” “Hepatocellular Carcinoma(s),” “Liver Cancer(s),” “Cell Carcinoma(s), Liver,” “Carcinoma(s), Liver Cell,” “Liver Cell Carcinoma(s),” “Adult Liver Cancer(s),” “Cancer(s), Adult Liver,” “Liver Cancer(s), Adult,” “Hepatoma(s),” “APRI,” “Aspartate aminotransferase-to-platelet ratio index,” and “AST/platelet ratio index.” There were no language restrictions or filters for the search. We also checked the reference lists of relevant reviews and included articles and performed a forward search. The search results discrepancies were resolved by consulting a library search professional.
The complete search strategies for each database are available in the Appendix.
Eligibility Criteria
The included studies must meet all the following inclusion criteria: (1) the population was patients with HCC; (2) the subjects were divided into low and high APRI level groups; (3) the study investigated the hazard ratio (HR), risk ratio (RR), or odds ratio (OR) of OS or DFS between the different APRI levels or provided sufficient data to calculate theses risk effects; (4) the study type was a cohort study. The studies were excluded if it met any of the following criteria: (1) pathological type was not HCC; (2) not relevant to the prognosis of HCC; (3) the risk effects and corresponding 95% CI were not provided and available data did not enough to calculate them; (4) studies without original clinical data, such as the reviews, systematic reviews, meta-analysis, expert opinions, editorial, or comment. (5) The articles were not written in full text or the full text was not available, such as the conference abstract. If a patient cohort was included in multiple publications, we selected the study with the largest sample size among the eligible studies to avoid data overlap.
Study Selection and Data Extract
Two authors independently reviewed the titles and abstracts of literature according to the eligibility criteria, initially excluding obvious irrelevant literature, and then re-evaluated the full text of the remaining articles to identify the relevant studies; the reasons for exclusion were recorded. Any disagreements were resolved through the discussion among all the authors. Two reviewers independently extracted the following data from the included literature: first author, publication year, sample size, gender, study region, median/mean age, follow-up time, treatment method, cutoff value of APRI, method of APRI selection, study design, proportion of patients with cirrhosis, vascular invasion, tumor size, number, and stage, hepatitis virus status, and the risk effects of OS and DFS along with corresponding 95% CI between high and low APRI groups. Considering that DFS and recurrence-free survival are often similar in clinical practice, we did not distinguish their HRs. Two reviewers cross-check the extracted data. If the study did not directly provide the risk effect and corresponding 95% CI but presented it as a figure, we extracted them using Engauge Digitizer software (version 4.1). In studies that provided both adjusted and unadjusted HRs, adjusted HR was preferred.
Statistical Analysis
All data analysis was performed using Stata 14 (StataCorp LLC, TX, USA). We calculated the pooled HRs and corresponding 95% CI. Cochran's Q-test and Higgins' I2 statistics were used to assess the heterogeneity between the studies. When I2 < 50% and Pheterogeneity > 0.05, we considered that the heterogeneity was acceptable, and the fixed effects model was used to pool the results; otherwise, significant heterogeneity existed, and the random-effects model was selected (17). A univariate meta-regression analysis was performed to identify the potential sources of heterogeneity. The subgroup analyses were performed for the region, age (mean/median year <55 or ≥55), treatment, disease stage (CTP A, BCLC stage 0-A, Edmonson grade I–II, TNM stage I classified as early stage, and otherwise as advanced stage), hepatitis virus status, APRI level (cutoff value <1 or ≥1), method of APRI selection, proportion of cirrhosis (<50% or ≥50%), analysis mode, sample size (<300 or ≥300), and tumor size (median/mean size <3 cm or ≥3 cm). Hepatitis viral status and tumor stage were grouped according to the main patient characteristics of each study. The sensitivity analysis was performed by excluding one study at a time to evaluate the stability of the results. Potential publication bias was examined by visually inspecting the funnel plot and further confirmed by the Begg's and Egger's tests. No potential publication bias existed if both the P-values were >0.05 and the funnel diagram was symmetrical (17). When publication bias occurs, we use the trim-and-fill method to evaluate the influence of potentially unpublished studies on the pooled results (18). If HR < 1, APRI was considered a protective predictor of OS and DFS in the patients with HCC, while HR > 1 was considered a risk predictor. The Poveralleffect value <0.05 was considered statistically significant. The Newcastle-Ottawa Scale (NOS) scale (cohort study) was used to assess the quality of the included studies that mainly included selection, comparability, and outcome. When the NOS score ≥ 7, the study was classified as high quality, otherwise considered to be at high risk of bias (19).
Results
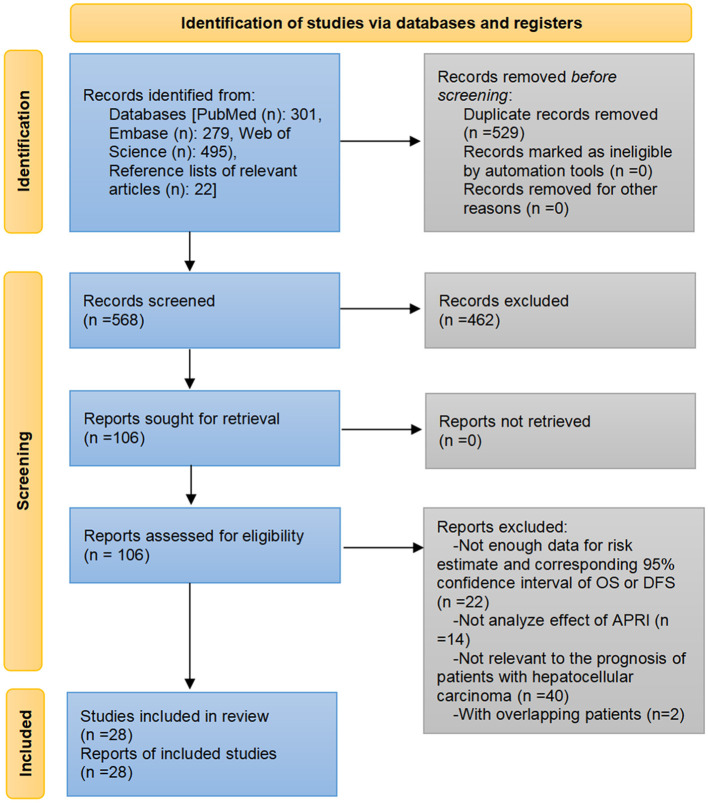
Through the pre-established search strategies, a total of 1,097 records were identified from Embase, PubMed, Web of Science, and reference lists of related papers. Eliminating duplicate literature and screening the titles and/or abstracts resulted in 991 records being excluded. We read the full text of the remaining 106 articles. Among them, 22 articles did not provide the risk effects along with corresponding 95% CI for OS or DFS and the data were insufficient to calculate them, 14 articles did not investigate the effect of APRI levels or analyze APRI separately, 40 articles were not relevant to the prognosis of HCC, two articles included the patients who overlapped with other larger cohorts. The list of articles excluded after a full-text reading is available in the Appendix. Finally, a total of 32 cohorts from 28 studies were included in the meta-analysis (20–47) (Figure 1).
Figure 1.
Flow diagram of the article selection process.
Description of the Included Studies
All the included 28 studies were retrospective cohort studies with a sample size ranging from 72 to 1,669, such as a total of 11,041 patients consisting of 8,776 men, 2,159 women, and 106 with unknown gender. Men account for the main proportion in all the studies. The median/mean age of patients in 11 studies was <55 years, 15 studies were >55 years, and the remaining two were unclear. The patients in 18 cohorts were treated with surgery, and the other cohorts included the patients with transarterial chemoembolization (TACE) (n = 4), radiotherapy (n = 1), radiofrequency ablation (RFA) (n = 3), or mixed therapy (n = 6), with a median/mean follow-up of 19.8–77.9 months. Some proportions or the entirety of patients in all the studies that reported hepatitis virus status, except one, were complicated with HBV (15.3–100%) and/or HCV (0.9–100%). Similarly, a certain percentage of patients in each evaluated study had cirrhosis (17.9–100%). Most of the studies were dominated by the patients with an early-stage and solitary number of tumors, and the proportion of patients with tumor vascular invasion ranged from 9.8 to 76.3%. The mean/median tumor size in 14 studies exceeded 3 cm. Thirteen studies were grouped with the APRI cutoff values <1, and 12 were ≥1. The APRI test timing in all the studies was before HCC treatment. The determination of the cutoff value was mainly based on an ROC analysis, as well as X-tile plots and other methods, such as experience or reference to the previous research. More detailed characteristics of the included studies are shown in Table 1. The NOS scores of the included studies ranged from 7 to 9, and the main risk of bias was short follow-up time (Table 2).
Table 1.
The characteristics of the included studies.
| References | Region | No. (M/F) | Age (years) | Cutoff value | Cutoff value determination | Endpoint | Vascular invasion | Tumor size (cm) | Tumor number (multiple) | Proportion of cirrhosis | Hepatitis virus status | Tumor stage | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. (20) | China | 405(356/49) | Median 52 | 0.45 | ROC analysis | OS | NA | Median 4.8 (0.8–26) | 14.8% | 79.8% | HBV 100% | CTP: A (96.0%)–B (4.0%) | Surgery | Median 60.7 (36–117.8) |
| Lai et al. (21) | China | 72(61/11) | Median 57 | 0.47 | ROC analysis | OS, DFS | NA | Median 3.4 (1.5–5.0) | NA | NA | HBV 100% | CTP: A (87.5%)–B (12.5%) | Radiotherapy | Median 66.3 (7.6–125.8) |
| Zhao et al. (22) | China | 429 (392/37) | Median 54 | 1.37 | ROC analysis | OS | NA | NA | NA | NA | NA | CTP: A (59.0%)–B (32.9%)–C (8.1%) | Mixed | 1–82.2 |
| Zhao et al. (22) | China | 169 (151/18) | Median 52 | 1.37 | ROC analysis | OS, | NA | NA | NA | NA | NA | CTP: A (52.7%)–B (33.7%)–C (13.6%) | Mixed | 1–82.2 |
| Zhao et al. (22) | China | 150 (131/19) | Median 48 | 1.37 | ROC analysis | OS | NA | NA | NA | NA | NA | CTP: A (88.7%)–B (10.7%)–C (0.6%) | Surgery | 1–82.2 |
| Lee et al. (23) | Korea | 184 (147/37) | Mean 52.3 | 1.5 | NA | OS | 36.4% | Median <3 | 7.1% | 100% | HBV 100% | BCLC stage 0–A | Surgery | Mean 77.9 |
| Li et al. (24) | China | 628 (526/102) | Median 49.2 | 0.5 | NA | OS, DFS | 20.7% | Median 5.0 | 3.2% | 75.3% | HBV 84.1%; HCV 2.1% | BCLC stage 0–A | Surgery | Mean 51.1 ± 31.8 |
| Maegawa et al. (25) | USA | 475 (361/8/ missing) | Mean 65.6 | 1.5 | NA | OS | NA | NA | NA | NA | HCV 64.8% | CTP: A (72.8%)–B (27.2%) | Surgery | Mean 56.4 ± 45.6 |
| Sonohara et al. (26) | Japan | 305 (245/60) | Median 67 | 1.5 | NA | OS, DFS | 27.9% | Median 3.5 (0.1–21) | 22.3% | 41.2% | HBV 27.2%; HCV 45.6% | CTP: A (87.5%)–B (12.5%) | Surgery | Median 44 (0–188) |
| Yang et al. (27) | China | 661 (574/87) | Mean: 47.45 | 0.25 | ROC analysis | OS, DFS | NP | Median >5 | 29.7% | 83.2% | HBV 85.5% | BCLC stage 0–A (35.7%)/B–C (64.3%) | Surgery | 1–60 |
| Matsumoto et al. (28) | Japan | 162 (138/24) | Median 63 | 0.45 | ROC analysis | OS, DFS | 24.7% | Median 3.5 | 18.5% | 43.2% | HBV: 27.8%; HCV:40.7% | CTP: A (92.6%)–B (7.4%) | Surgery | 1–120 |
| Sarkar et al. (29) | USA | 94 (71/23) | Mean 62 | 0.5 | NA | OS* | NA | Mean 2.3 ± 0.5 | NA | 91.5% | HBV: 30.9%; HCV: 55.3% | Single HCC ≤ 3.0 cm | Mixed | 1–60 |
| Allenson et al. (30) | USA | 829 (645/184) | Mean 55.9 | NA | NA | OS | NA | NA | NA | NA | HBV: 16.2%; HCV: 66.7% | Stage I (18.3%)–II (16.4%)–III (26.5%)–IV (33.2%)–unknown (5.4%) | Mixed | NA |
| Ji et al. (31) | China | 321 (285/36) | Mean 51 | 1.68 | NA | OS, DFS | NA | Median size > 5 | 30.0% | 78.8% | HBV: 87.5% | Edmonson grade I–II (77.3%)/III–IV (22.7%) | Surgery | 1–96 |
| Zhu et al. (32) | China | 351 (299/52) | Median age <65 | 0.5 | X-tile plots | DFS | NA | Median size <5 | 13.7% | 76.4% | HBV: 82.6%; HCV:1.4% | BCLC stage 0 (54.4%)–A (35.0%)–B (10.5%) | Surgery | Median 40.5 |
| Tang et al. (33) | China | 158 (136/22) | Median age ≤ 50 | 0.4 | X-tile plots | OS* | NA | Median >5 | >80.4% | NA | HBV: 93.7% | BCLC stage B | TACE | 1–40 |
| Tang et al. (33) | China | 157 (135/22) | Median age ≤ 50 | 0.4 | X-tile plots | OS* | NA | Median >5 | >79.0% | NA | HBV: 92.4% | BCLC stage B | TACE | 1–35 |
| Chen et al. (34) | China | 349(209/140) | Mean 66.9 | 1 | NA | OS, DFS*, # | NA | Mean 1.8 ± 0.6‡ | 26.9%‡ | NA | HBV: 37.0%; HCV: 59.6% | BCLC stage: 0 (54.2%)–A (45.8%) | RFA | Median 36.2 |
| Jaruvongvanich et al. (35) | USA | 900(660/240) | Mean 63.2 | 0.5, 1.5 | NA | OS, DFS | NA | Median 2–5 | 31.6% | 66.6% | HBV: 25.9%; HCV: 40.6% | BCLC stage: 0 (4.4%)–A (37.2%)–B (41.4%)–C (10.7%) -D (6.2%) | Mixed | Median 19.8 |
| Shen et al. (36) | China | 332 (292/40) | Mean 49.82 | 0.62 | ROC analysis | OS, DFS | 25.6% | Median >5 | 22.0% | 76.2% | HBV: 85.5% | Edmonson grade I–II (77.4%)/III–IV (22.6%) | Surgery | 1–80 |
| Hung et al. (37) | China | 76 (64/12) | Median 57 | 0.47 | ROC analysis | OS, DFS* | 76.3% | Median 2.5 | NA | NA | HBV 100% | CTP: A (90.8%)–B (9.2%) | Surgery | Median 77.0 (4.7–226.6) |
| Toyoda et al. (38) | Japan | 1,669 (1,181/488) | Mean 68.7 | 1.2 | ROC analysis | OS, DFS* | 16.90% | Median 2–5 | 38.60% | NA | HBV: 15.3%; HCV: 67.4% | BCLC stage 0 (15.6%)–A (43.8%)–B (12.2%)–C (18.4%)–D (9.2%) | Mixed | 1–240 |
| Choi et al. (39) | Korea | 303 (246/57) | Median 55 | NA | NA | OS*, DFS* | NA | 3.7 (2.5–5.2 | 13.9% | 49.8% | HBV | AJCC: I (64.0%)–II (32.3%)–IIIA (3.6%) | Surgery | Median 56.0 |
| Okamura et al. (40) |
Japan | 140 (115/25) | Median 71 | 0.544 | ROC analysis | OS*, DFS* | 20.0% | Median 5.0 (1.0–17.5) | 17.1% | 17.9% | Non-HBV/HCV | CTP: A (97.9%)–B (2.1%) | Surgery | Median 38.9 (2.4–120) |
| Chung et al. (41) | Korea | 98 (70/28) | Mean 60.5 | 1.38 | ROC analysis | DFS | NA | Mean 1.9 | NA | 91.8% | HBV: 72.4%; HCV: 11.2% | CTP: A (81.6%)–B (18.4%) | RFA | Median 40 (4–95) |
| Kao et al. (42) | China | 190 (121/69) | Mean 67.4 | 1 | NA | OS, DFS | NA | Mean 2.4 ± 0.92 | 20.0% | NA | HBV: 47.6%; HCV: 45.7% | BCLC stage: 0 (15.3%)–A (75.8%)–B (8.9%) | RFA | Median 30.7 ± 17.5 |
| Huang et al. (43) | China | 451 (383/68) | Median age <60 | 0.9 | X-tile plots | OS, DFS | 30.4% | Median <5 | 2.9% | 57.0% | HBV: 89.8% | BCLC stage: 0 (10.9%)–A (89.1%) | Surgery | 3–86 |
| Pang et al. (44) | China | 172 (139/33) | Mean 53.5 | 1.23 | ROC analysis | OS, DFS | 31.4% | Median >5 | 18.6% | 34.3% | HBV: 70.3%; HCV: 4.7% | CTP: A/B (93.0%)–C (7.0%) | Surgery | Median 46 |
| Pang et al. (44) | China | 191 (159/32) | Mean 54.1 | 1.79 | ROC analysis | OS, DFS | 49.7% | Median >5 | 28.8% | 44.0% | HBV: 77.5%; HCV: 3.1% | CTP: A/B (86.4%)–C (13.6%) | TACE | Median 40 |
| Liu et al. (45) | China | 223 (189/34) | Median 54 | 0.23 | ROC analysis | DFS¶ | 36.3% | Median >5 | 24.7% | 89.2% | HBV: 78.0%; HCV: 0.9% | BCLC stage: 0/A (56.5%)-B/C (43.5%) | Surgery | Median 26.1 (1.9–72.6) |
| Peng et al. (46) | China | 244 (213/31) | Mean 50 | 1 | ROC analysis | OS, DFS | 18.9% | Median 3–5 | 14.8% | 85.7% | HBV: 96.3% | CTP A | Surgery | Median 36.3 (3–85.9) |
| Teng et al. (47) | China | 153 (82/71) | Median 64.1 | 2 | NA | OS, DFS | 9.8% | Median 2.7 (1.9–3.8) | NA | NA | HCV: 100% | BCLC stage 0 (4.6%)–A (30.7%)–B (31.4%)–C (33.3%) | TACE | 1–60 |
M/F, male/female; CTP, Child-Turcotte-Pugh; AJCC, American Joint Committee on Cancer TNM staging system; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; BCLC, Barcelona Clinic Liver Cancer; DFS, disease-free survival; HBV, hepatitis B virus; HCV, hepatitis C virus; NA, not available; ROC, receiver operating characteristic curve.
Univariate analysis.
Second recurrence.
Recurrent.
Including early recurrence and late recurrence analysis.
Table 2.
The quality assessment of the included studies.
| References | Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Outcome not present before study | Comparability | Assessment of outcome | Follow-up long enough¶ | Adequacy of follow up | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. (20) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Lai et al. (21) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Zhao et al. (22) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Lee et al. (23) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Li et al. (24) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Maegawa et al. (25) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Sonohara et al. (26) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Yang et al. (27) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Matsumoto et al. (28) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | ⋆ | 9 |
| Sarkar et al. (29) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 | |
| Allenson et al. (30) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | 7 | ||
| Ji et al. (31) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Zhu et al. (32) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Tang et al. (33) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 | |
| Chen et al. (34) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 | |
| Jaruvongvanich et al. (35) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Shen et al. (36) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Hung et al. (37) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Toyoda et al. (38) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Choi et al. (39) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 | |
| Okamura et al. (40) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Chung et al. (41) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Kao et al. (42) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Huang et al. (43) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Pang et al. (44) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Liu et al. (45) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Peng et al. (46) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 | |
| Teng et al. (47) | ⋆ | ⋆ | ⋆ | ⋆ | ⋆⋆ | ⋆ | ⋆ | 8 |
The median/mean follow-up time ≥60 months or the longest follow-up time ≥120 months is acceptable.
Association Between the APRI Levels and OS
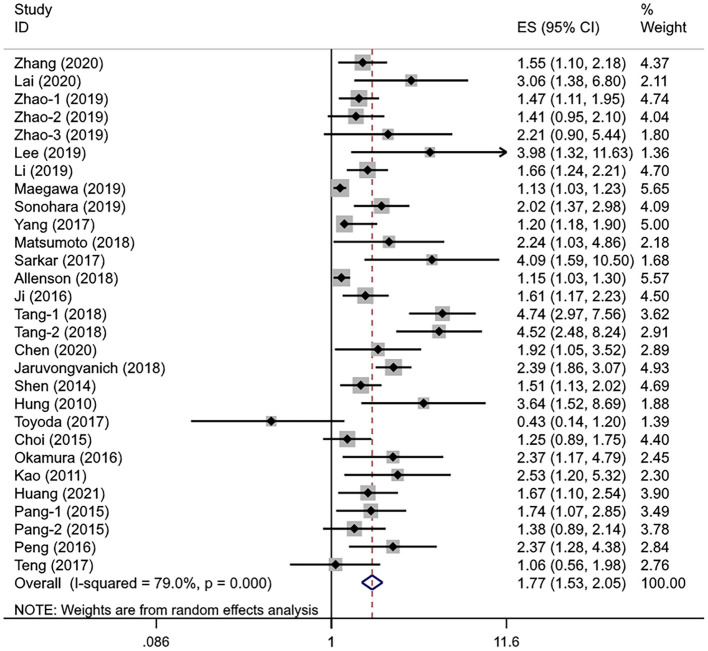
A total of 25 studies involving 10,369 patients with HCC evaluated the association between different APRI levels and OS. The heterogeneity test showed significant heterogeneity (I2 = 79.0%, Pheterogeneity < 0.001), so the random-effects model was used for effect size analysis. The pooled HR was 1.77 (95% CI: 1.53–2.05, Poverall effect < 0.001), indicating that the APRI index had a significant predictive ability for the OS of patients with HCC (Figure 2). The patients with high APRI levels had an increased risk of death by 77% compared with the patients with low APRI levels.
Figure 2.
The forest plot of pooled hazard ratio (HR) for overall survival (OS).
In the subgroup analysis and regression analysis, we found that the sample size and analysis mode were the possible source of heterogeneity (Pinteraction = 0.003 and 0.017, respectively); the studies with fewer than 300 subjects and the univariate analysis reported a higher HR. Furthermore, APRI cutoff value (Pinteraction = 0.083) and cutoff determination method (Pinteraction = 0.094) may also be the sources of heterogeneity, while age, treatment, study region, disease stage, hepatitis virus status, cirrhosis, and tumor size were not related to observed substantial heterogeneity. A multivariate analysis showed that APRI could be an independent predictive factor for the OS (HR =1.60, 95% CI: 1.40–1.83, Poverall effect < 0.001). Of note, the pooled results from the studies in Japan (HR = 1.67, 95% CI: 0.95–2.93, Poverall effect = 0.076) and Korea (HR = 1.98, 95% CI: 0.65–6.00, Poverall effect = 0.229) showed no significant correlation between APRI and OS. In other subgroups, higher APRI levels were consistently associated with poorer OS (Table 3). In addition, we performed a univariate meta-regression analysis based on the sex ratio of the patients in each study, and no statistical significance was observed (P = 0.202).
Table 3.
The results of the subgroup analysis for overall survival (OS).
| Subgroups | No. of studies | Pooled HR (95%CI) | P overall effect | Heterogeneity(I2, PQ) | P interaction |
|---|---|---|---|---|---|
| Median/mean age | |||||
| <55 years | 10 | 1.83 (1.50–2.23) | <0.001 | 70.4%, <0.001 | 0.695 |
| ≥55 years | 14 | 1.70 (1.37–2.12) | <0.001 | 81.4%, <0.001 | |
| Treatment | |||||
| Surgery | 16 | 1.63 (1.39–1.90) | <0.001 | 65.4%, <0.001 | 0.362 |
| RFA | 2 | 2.14 (1.34–3.43) | 0.001 | 0.0%, 0.573 | |
| TACE | 3 | 2.38 (1.11–5.07) | 0.025 | 88.0%, <0.001 | |
| Radiotherapy | 1 | 3.06 (1.38–6.80) | 0.006 | - | |
| Mixed | 5 | 1.53 (1.06–2.20) | 0.023 | 86.6%, <0.001 | |
| Region | |||||
| China | 15 | 1.83 (1.55–2.17) | <0.001 | 64.2%, <0.001 | 0.571 |
| Korea | 2 | 1.98 (0.65–6.00) | 0.229 | 74.8%, 0.046 | |
| USA | 4 | 1.55 (1.13–2.12) | 0.006 | 92.1%, <0.001 | |
| Japan | 4 | 1.67 (0.95–2.93) | 0.076 | 62.4%, 0.046 | |
| Sample size | |||||
| <300 | 12 | 2.39 (1.83–3.21) | <0.001 | 61.2%, 0.001 | 0.003 |
| ≥300 | 14 | 1.47 (1.27–1.69) | <0.001 | 76.8%, <0.001 | |
| Disease stage | |||||
| Early | 19 | 1.72 (1.46–2.01) | <0.001 | 66.9%, <0.001 | 0.646 |
| Advanced | 5 | 2.00 (1.28–3.11) | 0.002 | 93.0%, <0.001 | |
| Hepatitis virus status | |||||
| HBV | 14 | 1.94 (1.59–2.37) | <0.001 | 70.9%, <0.001 | 0.459 |
| HCV | 9 | 1.55 (1.20–1.98) | 0.001 | 84.8%, <0.001 | |
| Non-HBV/HCV | 1 | 2.37 (1.17–4.79) | 0.016 | - | |
| APRI level | |||||
| <1 | 11 | 2.22 (1.69–2.91) | <0.001 | 76.7%, <0.001 | 0.083 |
| ≥1 | 11 | 1.56 (1.29–1.89) | <0.001 | 64.1%, 0.001 | |
| APRI selection | |||||
| ROC analysis | 11 | 1.51 (1.35–1.69) | <0.001 | 40.2%, 0.060 | 0.094 |
| X-tile plots | 2 | 3.24 (1.58–6.67) | 0.001 | 84.6%, 0.002 | |
| Other | 12 | 1.63 (1.34–1.99) | <0.001 | 81.8%, <0.001 | |
| Proportion of cirrhosis | |||||
| <50% | 5 | 1.62 (1.34–1.95) | <0.001 | 15.0%, 0.318 | 0.798 |
| ≥50% | 10 | 1.76 (1.45–2.12) | <0.001 | 61.0%, 0.006 | |
| Analysis modes | |||||
| Univariate analysis | 4 | 2.96 (1.54–5.71) | 0.001 | 71.8%, <0.001 | 0.017 |
| Multivariate analysis | 21 | 1.60 (1.40–1.83) | <0.001 | 85.7%, <0.001 | |
| Median/mean tumor size | |||||
| <3 cm | 6 | 2.18 (1.60–2.98) | <0.001 | 47.6%, 0.089 | 0.401 |
| ≥3 cm | 13 | 1.89 (1.55–2.30) | <0.001 | 70.4%, <0.001 | |
RFA, radiofrequency ablation; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus; ROC, receiver operating characteristic curve; APRI, aspartate aminotransferase-to-platelet ratio index.
Association Between the APRI Levels and DFS
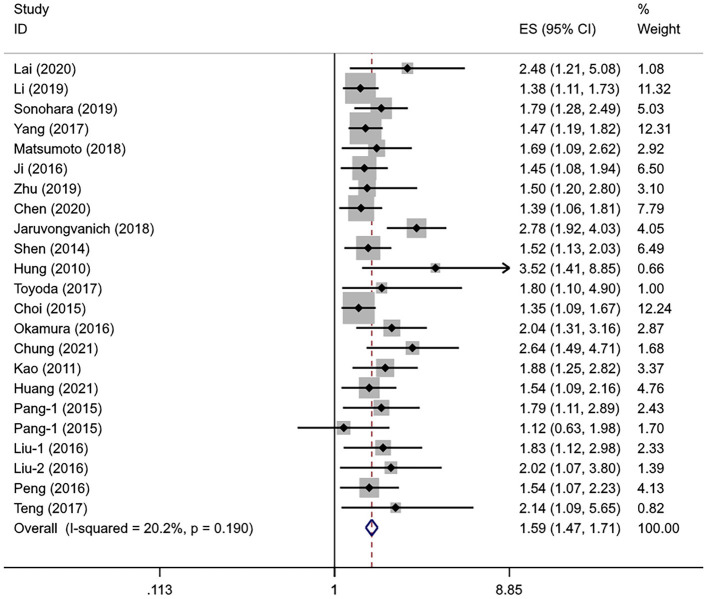
A total of 21 studies with 7,991 patients with HCC evaluated the association between the APRI levels and DFS. The heterogeneity level was subtle (I2 = 20.2%, Pheterogeneity = 0.190), so the fixed-effects model was used. Similar to OS, the pooled HR showed a significant association between the higher APRI levels and poorer DFS (HR = 1.59, 95% CI: 1.47–1.71, Poverall effect < 0.001) (Figure 3).
Figure 3.
The forest plot of pooled HR for DFS.
The sample size remained the potential source of heterogeneity (Pinteraction = 0.037); the studies with the small sample sizes tended to report a higher HR than the large sample sizes. Age (Pinteraction = 0.084) and hepatitis virus status (Pinteraction = 0.081) may also be the sources of heterogeneity. A subgroup analysis showed no statistical correlation between the APRI levels and DFS in the Korean population (HR = 1.79, 95% CI: 0.93–3.42, Poverall effect = 0.079) and those who received TACE (HR = 1.38, 95% CI: 0.86–2.21, Poverall effect = 0.176). The statistical significance could be consistently observed in other subgroups. The pooled results of the multivariate analysis indicated that APRI could serve as an independent predictor of DFS in the patients with HCC (HR = 1.62, 95% CI: 1.49–1.77, Poverall effect < 0.001) (Table 4). The regression analysis showed that the change of HR with sex ratio was not statistically significant (P = 0.168).
Table 4.
The results of the subgroup analysis for disease-free survival (DFS).
| Subgroups | No. of studies | Pooled HR (95%CI) | P overall effect | Heterogeneity (I2, PQ) | P interaction |
|---|---|---|---|---|---|
| Median/Mean age | |||||
| <55 | 7 | 1.49 (1.34–1.66) | <0.001 | 0.0%, 0.888 | 0.084 |
| ≥55 | 12 | 1.87 (1.58–2.22) | <0.001 | 46.1%, 0.040 | |
| Treatment | |||||
| Surgery | 14 | 1.53 (1.41–1.67) | <0.001 | 0.0%, 0.766 | 0.268 |
| RFA | 3 | 1.78 (1.25–2.51) | 0.001 | 55.9%, 0.104 | |
| TACE | 2 | 1.38 (0.86–2.21) | 0.176 | 37.6%, 0.205 | |
| Radiotherapy | 1 | 2.48 (1.21–5.08) | 0.013 | - | |
| Mixed | 2 | 2.55 (1.83–3.56) | <0.001 | 4.2%, 0.307 | |
| Region | |||||
| China | 14 | 1.53 (1.40–1.67) | <0.001 | 0.0%, 0.757 | 0.357 |
| Korea | 2 | 1.79 (0.93–3.42) | 0.079 | 78.2%, 0.032 | |
| USA | 1 | 2.78 (1.92–4.03) | <0.001 | - | |
| Japan | 4 | 1.82 (1.47-2.26) | <0.001 | 0.0%, 0.945 | |
| Sample size | |||||
| <300 | 10 | 1.85 (1.59–2.14) | <0.001 | 0.0%, 0.670 | 0.037 |
| ≥300 | 11 | 1.51 (1.38–1.65) | <0.001 | 27.7%, 0.180 | |
| Disease stage | |||||
| Early | 17 | 1.56 (1.44–1.70) | <0.001 | 0.0%, 0.524 | 0.321 |
| Advanced | 3 | 2.01 (1.23–3.27) | 0.005 | 77.3%, 0.012 | |
| Hepatitis virus status | |||||
| HBV | 14 | 1.52 (1.40–1.66) | <0.001 | 0.0%, 0.483 | 0.081 |
| HCV | 6 | 1.77 (1.51–2.08) | <0.001 | 45.0%, 0.106 | |
| Non-HBV/HCV | 1 | 2.04 (1.31–3.16) | 0.002 | - | |
| APRI level | |||||
| <1 | 10 | 1.57 (1.41–1.75) | <0.001 | 0.0%, 0.556 | 0.802 |
| ≥1 | 9 | 1.60 (1.41–1.82) | <0.001 | 0.0%, 0.558 | |
| APRI selection | |||||
| ROC analysis | 11 | 1.66 (1.48–1.87) | <0.001 | 0.0%, 0.498 | 0.394 |
| X-tile plots | 2 | 1.52 (1.17–1.99) | 0.002 | 0.0%, 0.925 | |
| Other | 8 | 1.62 (1.37–1.90) | <0.001 | 54.0%, 0.033 | |
| Proportion of cirrhosis | |||||
| <50% | 5 | 1.54 (1.34–1.78) | <0.001 | 10.0%, 0.352 | 0.800 |
| ≥50% | 10 | 1.59 (1.44–1.75) | <0.001 | 34.5%, 0.123 | |
| Analysis mode | |||||
| Univariate analysis | 5 | 1.48 (1.28–1.73) | <0.001 | 39.8%, 0.156 | 0.483 |
| Multivariate analysis | 16 | 1.62 (1.49–1.77) | <0.001 | 14.6%, 0.280 | |
| Median/mean tumor size | |||||
| <3 cm | 5 | 1.72 (1.41–2.10) | <0.001 | 45.6%, 0.118 | 0.273 |
| ≥3 cm | 12 | 1.52 (1.39–1.66) | <0.001 | 0.0%, 0.723 | |
RFA, radiofrequency ablation; TACE, transarterial chemoembolization; HBV, hepatitis B virus; HCV, hepatitis C virus; ROC, receiver operating characteristic curve; APRI, aspartate aminotransferase-to-platelet ratio index.
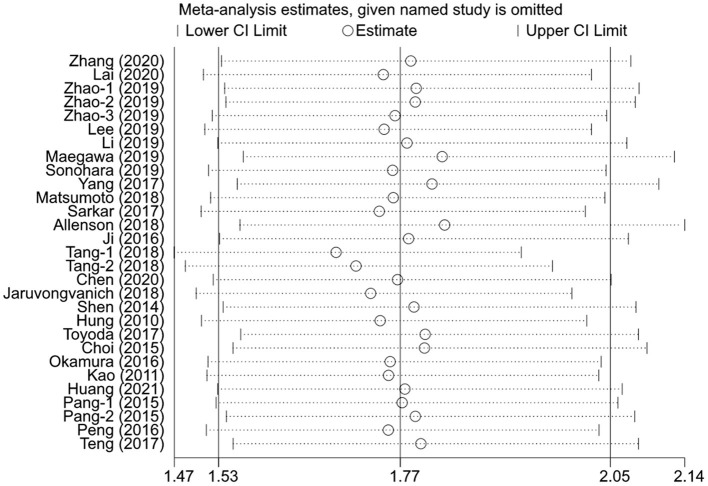
Sensitivity Analysis
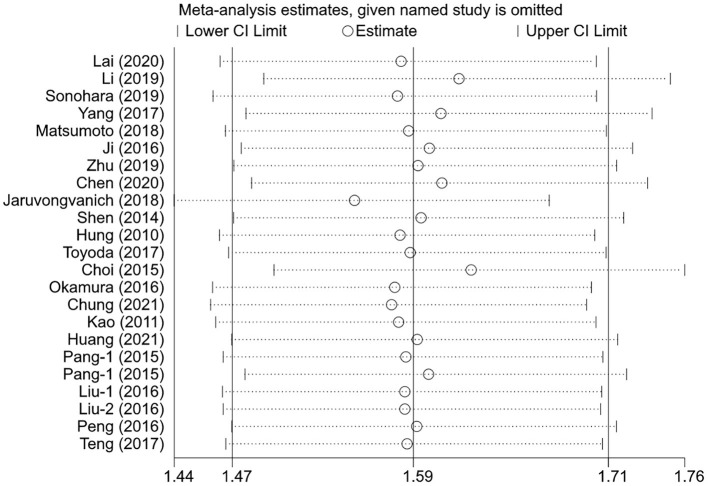
The sensitivity analysis was performed for the pooled results by excluding one study at a time. The results showed that the pooled effect sizes were stable, and each individual study had little effect on the pooled HRs for OS and DFS (Figures 4, 5).
Figure 4.
The effects of the individual studies on the pooled HR of OS.
Figure 5.
The effects of the individual studies on the pooled HR of DFS.
Publication Bias
We performed the funnel plots to evaluate the publication bias of included 28 studies, and the results showed obvious asymmetry (Figures 6, 7). The Begg's and Egger's tests were performed to validate the results, and both the P-values for OS and DFS were <0.05, demonstrating the risk of publication bias in the current published studies. In OS analysis, after adjusting with the trim-and-fill method, 12 unpublished studies were added. The re-pooled results showed that the HRs of the fixed-effects model and the random-effects model were 1.25 (95% CI: 1.19–1.31, P < 0.001) and 1.32 (95% CI: 1.13–1.55, P < 0.001), respectively. In DFS analysis, eight unpublished studies were added according to the trim-and-fill method. The re-pooled results showed that the HRs of the fixed-effects model and the random-effects model were 1.48 (95% CI: 1.38–1.59, P < 0.001) and 1.50 (95% CI: 1.35–1.66, P < 0.001), respectively.
Figure 6.
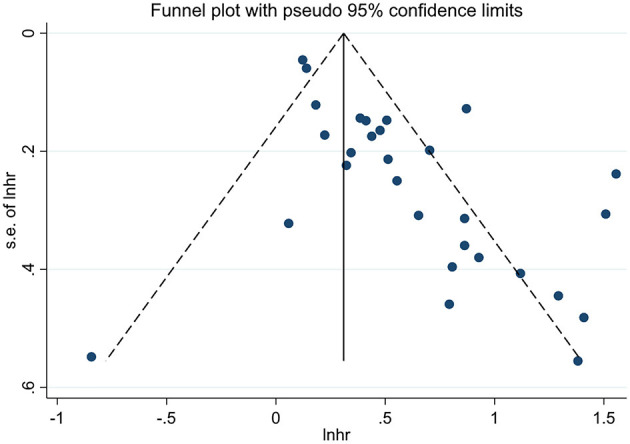
The funnel plot for OS.
Figure 7.
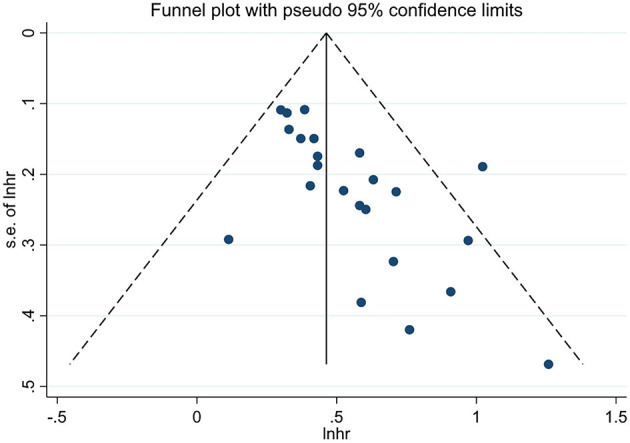
The funnel plot for DFS.
Discussion
In this study, OS and DFS were used as the endpoints to evaluate whether the APRI levels could predict the prognosis of HCC. The overall pooled results suggested that the patients with HCC with high APRI levels had a significantly increased risk of death and recurrence than the patients with low APRI levels. Although the pooled results may be influenced by publication bias, the significant correlation between the APRI levels and prognosis of HCC remained after adding potentially unpublished literature. Furthermore, we analyzed the impacts of age, treatment, region, sample size, disease stage, hepatitis virus status, APRI cutoff value, cutoff value selection, cirrhosis, analysis mode, and tumor size on the results. Among them, the pooled results from Korea, Japan, and the patients treated with TACE showed no statistical association between the pretreatment APRI levels and prognosis (OS and/or DFS), while a stable correlation could be observed in other subgroups. The results from the multivariate analysis suggested that APRI levels seem to serve as an independent predictive factor for the patients with HCC.
In addition to predicting the prognosis of patients with HCC throughout the follow-up period, Liu et al. found that the APRI levels could be an independent risk factor for early recurrence <1 year in the background of cirrhosis/fibrosis, and the study conducted by Allenson et al. showed that a high APRI level was associated with OS <30 days (30, 45). Furthermore, Chen et al. found that a high APRI level predicted a higher risk of second recurrence (34). These findings can broaden the prediction range of APRI.
Lee et al. and Chung et al. also found that the patients who developed HCC death or relapses had persistently higher APRI values than the non-relapsing patients during follow-up (23, 41). Moreover, the study conducted by Peng et al. indicated that the difference value between postoperative APRI and preoperative APRI could serve as an independent prognostic factor for OS and DFS; change of APRI ≥ 0.02 was associated with the poorer outcomes (46). A similar result was found in the study conducted by Lee et al. (23). In addition, Chung et al. observed that the APRI value decreased significantly during follow-up in the patients who remained relapse-free, suggesting that it may be feasible to use APRI as a dynamic monitoring indicator during follow-up (41). However, there was a lack of data on dynamic AST and/or PLT, and no detailed description of the specific fluctuations of APRI over time.
APRI is a non-invasive serum predictor that can be calculated at the bedside using the routine laboratory test parameters. The exact mechanism between APRI and poor prognosis in the patients with HCC after treatment remains unclear. It may be explained by the following reasons. First, the PLT count can promote hepatocyte regeneration and reflect the degree of liver function; thrombopenia is considered a risk factor for cirrhosis, and the patients with cirrhosis generally have decreased the PLT levels due to reduced thrombopoietin and PLT capture by the liver (48–53). PLT is also an important regulator of tumor vascular homeostasis and can facilitate and regulate tumor angiogenesis (54). Second, AST not only represents the degree of cirrhosis, but is also a biological indicator of liver inflammation; as AST exists in the mitochondria of liver cells, it can be released into the serum when mitochondria are damaged, and therefore AST is used as a reliable and sensitive biomarker to indicate the damage of liver function (55, 56). Liver cirrhosis and hepatocyte damage were closely associated with hepatocarcinogenesis (57–59). As a result, APRI that combined AST and PLT count is expected to reflect the prognosis of HCC from liver reserve and inflammation. Several studies have suggested that the low preoperative PLT or the high AST levels are associated with poor survival after therapy (60–63).
Of note, although current data show that the APRI level can generally reflect the prognosis of patients with HCC, it cannot determine which populations can achieve the best sensitivity and specificity, so it is necessary to combine with other prognostic prediction systems in clinical practice. On the one hand, it can be combined with currently used clinical assessment methods, such as CT, PET-CT, MRI, DSA, ultrasonography, TNM staging system, AFP, or Model for End-Stage Liver Disease score. On the other hand, it is possible to combine other serum markers with prognostic value, such as AST/lymphocyte ratio, neutrophil/lymphocyte ratio, and platelet/lymphocyte ratio, to establish a novel prognostic scoring system. For instance, Zhao et al. established a prognostic nomogram involving APRI, AST/lymphocyte ratio, and systemic immune-inflammation index, and Ji et al. found that APRI combined with neutrophil/lymphocyte ratio improved the predictive accuracy (22, 23).
The main limitation of this study was the small number of studies in some subgroups (such as non-surgical treatment group, non-HBV/HCV HCC group, United States, European, and African region group), so it is difficult to draw solid conclusions that are universally applicable to all the regions or all populations. The absence of statistical significance in some subgroups could also be explained by a lack of statistical power due to the small number of included studies. Furthermore, the published studies did not provide sufficient data for a more detailed subgroup analysis to accurately characterize the population that can benefit most from the APRI prediction strategy or is unsuitable for applying APRI scoring. Next, all the included studies were retrospective studies that did not consider scientific research needs in the collection of clinical data and did not guarantee the completeness and homogeneity of data. Third, some factors that may affect the results, such as tumor differentiation, vascular invasion, and tumor number, were not included in the analysis due to a lack of detailed data. In addition, the APRI level was also affected by physical activity, anti-hepatitis virus drugs, and concomitant diseases. Therefore, it will be necessary to proceed with caution when formulating a prognostic risk assessment strategy based on these data. Fourth, although the studies with different APRI cutoff values have shown that high APRI can predict worse OS and DFS, it must be unified before APRI score is widely used in clinical settings. Finally, the current results may be impacted by publication bias. Although the conclusion remains unchanged after adding potentially unpublished studies, the presence of publication bias should be emphasized when determining the APRI optimal cutoff value and evaluating prognostic value in the specific patients.
Conclusion
Although APRI is not yet suitable as a single independent prognostic factor for the patients with HCC, we suggest that the clinicians can use the APRI scores combined with other prognostic assessment systems for post-operative risk stratification, which may help to improve the predictive accuracy, leading to more effective clinical decision-making and prolonged survival of patients. Furthermore, more prospective studies with large samples and long-term follow-up from different regions and populations are needed to evaluate the prognostic value and optimal cutoff value of APRI in the patients with different characteristics.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
QS contributed to the conception and design of the study. MLiv and ZS searched the database. XZ and MLiu extracted data. ZS and YZ performed the statistical analysis. XZ, MLiv, and ZS wrote the manuscript. ZS contributed to critical revision of the manuscript. All the authors approved the submitted version.
Funding
The research was supported by the Talent Introduction and Research Start-up Fund of Southwest Medical University (00040056), the Joint Project of the Southwest Medical University-Affiliated Hospital of Traditional Chinese Medicine of Southwest Medical University (2018 No. 6-51), Sichuan Provincial Administration of Traditional Chinese Medicine Project (2021 No. 13-130), Science & Technology Department of Sichuan Provincial Project (2021YFH0150), and People's Government of Luzhou City-Southwest Medical University High-level Talent Introduction Special Funding Project (2017RC-002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.756210/full#supplementary-material
References
- 1.Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. (2019) 13:125–37. 10.1007/s12072-018-9919-1 [DOI] [PubMed] [Google Scholar]
- 2.Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non-alcoholic fatty liver disease. Hepatol Int. (2019) 13:688–94. 10.1007/s12072-019-09995-8 [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Kobayashi T, Kuroda S, Hamaoka M, Okimoto S, Honmyo N, et al. Verification of inflammation-based prognostic marker as a prognostic indicator in hepatocellular carcinoma. Ann Gastroenterol Surg. (2019) 3:667–75. 10.1002/ags3.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RTP, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinorna. Ann Surg. (2000) 232:10–24. 10.1097/00000658-200007000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53:1020–2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki Y, Imaoka S, Masutani S, Ohashi I, Ishikawa O, Koyama H, et al. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery. (1992) 112:515–21. [PubMed] [Google Scholar]
- 7.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. (2002) 36:S152–60. 10.1053/jhep.2002.36381 [DOI] [PubMed] [Google Scholar]
- 8.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. 10.1053/jhep.2003.50346 [DOI] [PubMed] [Google Scholar]
- 9.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. (2011) 53:726–36. 10.1002/hep.24105 [DOI] [PubMed] [Google Scholar]
- 10.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. (2007) 46:912–21. 10.1002/hep.21835 [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Wu J, Xu J, Xu J, Xian J, Xue S, et al. Association between aspartate aminotransferase-to-platelet ratio index and hepatocellular carcinoma risk in patients with chronic hepatitis: a meta-analysis of cohort study. Dis Markers. (2019) 2019:2046825. 10.1155/2019/2046825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amptoulach S, Gross G, Sturesson C, Rissler P, Kalaitzakis E. Preoperative aspartate aminotransferase-to-platelet ratio index predicts perioperative liver-related complications following liver resection for colorectal cancer metastases. Scand J Surg. (2017) 106:311–7. 10.1177/1457496916683094 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Iimuro Y, Hirano T, Hai S, Suzumura K, Fujimoto J. Prediction of postoperative hepatic failure after liver resection for hepatocellular carcinoma: significance of the aspartate aminotransferase-to-platelet ratio index. Hepatogastroenterology. (2014) 61:755–61. https://pubmed.ncbi.nlm.nih.gov/26176070/ [PubMed] [Google Scholar]
- 14.Cheng JW, Zhao P, Liu JB, Liu X, Wu XL. Preoperative aspartate aminotransferase-toplatelet ratio index (APRI) is a predictor on postoperative outcomes of hepatocellular carcinoma. Med. (2016) 95:e5486. 10.1097/MD.0000000000005486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa T, Uenishi T, Takemura S, Oba K, Ogawa M, Kodai S, et al. A simple, noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. (2009) 16:42–8. 10.1007/s00534-008-0003-4 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. (2021) 18:1003583. 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv. (2019) 2019:1–694. 10.1002/9781119536604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O'Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hosp Res Inst; (2000). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 21, 2021) [Google Scholar]
- 20.Zhang TT, Ye SS, Liang J, Bai L. Prognostic value of non-invasive fibrosis indices post-curative resection in hepatitis-B-associated hepatocellular carcinoma patients. Exp Biol Med. (2020) 245:703–10. 10.1177/1535370220914252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai L, Su T, Liang Z, Lu Y, Hou E, Lian Z, et al. Development and assessment of novel predictive nomograms based on APRI for hepatitis B virus-associated small solitary hepatocellular carcinoma with stereotactic body radiotherapy. J Cancer. (2020) 11:6642–52. 10.7150/jca.47291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao LY, Yang DD, Ma XK, Liu MM, Wu DH, Zhang XP, et al. The prognostic value of aspartate aminotransferase to lymphocyte ratio and systemic immune-inflammation index for overall survival of hepatocellular carcinoma patients treated with palliative treatments. J Cancer. (2019) 10:2299–311. 10.7150/jca.30663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JI, Lee HW, Kim SU, Ahn SH, Lee KS. Follow-up liver stiffness measurements after liver resection influence oncologic outcomes of hepatitis-B-associated hepatocellular carcinoma with liver cirrhosis. Cancers. (2019) 11:425. 10.3390/cancers11030425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Peng W, Zhang XY, Wen TF, Chen LP. The preoperative platelet to albumin ratio predicts the prognosis of hepatocellular carcinoma patients without portal hypertension after liver resection. Medicine. (2019) 98:e17920. 10.1097/MD.0000000000017920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maegawa FB, Shehorn L, Aziz H, Kettelle J, Jie T, Riall TS. Association between noninvasive fibrosis markers and postoperative mortality after hepatectomy for hepatocellular carcinoma. J Am Med Assoc Netw Open. (2019) 2:e187142. 10.1001/jamanetworkopen.2018.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonohara F, Yamada S, Tanaka N, Tashiro M, Sunagawa Y, Morimoto D, et al. Comparison of non-invasive liver reserve and fibrosis models: implications for surgery and prognosis for hepatocellular carcinoma. Hepatol Res. (2019) 49:1305–15. 10.1111/hepr.13400 [DOI] [PubMed] [Google Scholar]
- 27.Yang HJ, Jiang JH, Yang YT, Guo Z, Li JJ, Liu XH, et al. Stratified aspartate aminotransferase-to-platelet ratio index accurately predicts survival in hepatocellular carcinoma patients undergoing curative liver resection. Tumor Biol. (2017) 39:1010428317695944. 10.1177/1010428317695944 [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Wakiyama S, Shiba H, Haruki K, Futagawa Y, Ishida Y, et al. Usefulness of aspartate aminotransferase to platelet ratio index as a prognostic factor following hepatic resection for hepatocellular carcinoma. Mol Clin Oncol. (2018) 2018:1692. 10.3892/mco.2018.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar J, DeLeon T, Wong LL. MELD score and AST-to-platelet ratio index predict long-term survival in patients with a small hepatocellular carcinoma following non-transplant therapies: a pilot study. Hepatoma Res. (2017) 3:79. 10.20517/2394-5079.2017.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allenson K, Roife D, Kao LS, Ko TC, Wray CJ. Estimation of hepatocellular carcinoma mortality using aspartate aminotransferase to platelet ratio index. J Gastrointest Oncol. (2020) 11:291–7. 10.21037/jgo.2018.11.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji F, Liang Y, Fu SJ, Guo ZY, Shu M, Shen SL, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. (2016) 16:137. 10.1186/s12885-016-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu GQ, Wang K, Wang B, Zhou YJ, Yang Y, Chen EB, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. Cancer Manag Res. (2019) 11:63–79. 10.2147/CMAR.S186150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang T, Qiu JL, Li GW, Huang MP, Li Y, Li YJ, et al. Aspartate aminotransferase-to-platelet ratio predicts response to transarterial chemoembolisation and prognosis in hepatocellular carcinoma patients. Clin Radiol. (2018) 73:259–65. 10.1016/j.crad.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 34.Chen HY, Lu SN, Hung CH, Wang JH, Chen CH, Yen YH, et al. Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS ONE. (2020) 15:242113. 10.1371/journal.pone.0242113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaruvongvanich V, Sempokuya T, Wong L. Is there an optimal staging system or liver reserve model that can predict outcome in hepatocellular carcinoma? J Gastrointest Oncol. (2018) 9:750–61. 10.21037/jgo.2018.05.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen SL, Fu SJ, Chen B, Kuang M, Li SQ, Hua YP, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. (2014) 21:3802–9. 10.1245/s10434-014-3771-x [DOI] [PubMed] [Google Scholar]
- 37.Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. (2010) 4:691–9. 10.1007/s12072-010-9213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyoda H, Kumada T, Tada T, Yama T, Mizuno K, Sone Y, et al. Differences in the impact of prognostic factors for hepatocellular carcinoma over time. Cancer Sci. (2017) 108:2438–44. 10.1111/cas.13406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi WM, Lee JH, Ahn H, Cho H, Cho YY, Lee M, et al. Forns index predicts recurrence and death in patients with hepatitis B-related hepatocellular carcinoma after curative resection. Liver Int. (2015) 35:1992–2000. 10.1111/liv.12776 [DOI] [PubMed] [Google Scholar]
- 40.Okamura Y, Ashida R, Yamamoto Y, Ito T, Sugiura T, Bekku E, et al. The FIB-4 index is a significant prognostic factor in patients with non-B non-C hepatocellular carcinoma after curative surgery. Langenbeck's Arch Surg. (2016) 401:195–203. 10.1007/s00423-016-1389-0 [DOI] [PubMed] [Google Scholar]
- 41.Chung H, Kim JH, Hwang Y, Choi H, Ko S, Choe W, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol. (2016) 22:57–63. 10.4103/1319-3767.173760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao WY, Chiou YY, Hung HH, Chou YH, Su CW, Wu JC, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. (2011) 23:528–36. 10.1097/MEG.0b013e328346d529 [DOI] [PubMed] [Google Scholar]
- 43.Huang J, Yang Y, Xia Y, Liu FC, Liu L, Zhu P, et al. Prediction of patient survival following hepatic resection in early-stage hepatocellular carcinoma with indexed ratios of aspartate aminotransferase to platelets: a retrospective cohort study. Cancer Manag Res. (2021) 13:1733–46. 10.2147/CMAR.S284950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang Q, Zhang JY, Xu X, Song SD, Chen W, Zhou YY, et al. The prognostic values of 12 cirrhosis-relative noninvasive models in patients with hepatocellular carcinoma. Scand J Clin Lab Invest. (2015) 75:73–84. 10.3109/00365513.2014.981759 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Wang ZX, Cao Y, Zhang G, Chen WB, Jiang CP. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis Int. (2016) 15:266–74. 10.1016/S1499-3872(16)60094-2 [DOI] [PubMed] [Google Scholar]
- 46.Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, et al. Postoperative aspartate aminotransferase to platelet ratio index change predicts prognosis for hepatocellular carcinoma. Med. (2016) 95:4160. 10.1097/MD.0000000000004160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng W, Hsieh YC, Lui KW, Chen WT, Hung CF, Huang CH, et al. Eradication of hepatitis C virus profoundly prolongs survival in hepatocellular carcinoma patients receiving transarterial chemoembolization. J Viral Hepat. (2017) 24:1160–7. 10.1111/jvh.12745 [DOI] [PubMed] [Google Scholar]
- 48.Liao R, Li DW, Du CY, Li M. Combined preoperative ALBI and FIB-4 is associated with recurrence of hepatocellular carcinoma after curative hepatectomy. J Gastrointest Surg. (2018) 22:1679–87. 10.1007/s11605-018-3810-1 [DOI] [PubMed] [Google Scholar]
- 49.Tomimaru Y, Eguchi H, Gotoh K, Kawamoto K, Wada H, Asaoka T, et al. Platelet count is more useful for predicting posthepatectomy liver failure at surgery for hepatocellular carcinoma than indocyanine green clearance test. J Surg Oncol. (2016) 113:565–9. 10.1002/jso.24166 [DOI] [PubMed] [Google Scholar]
- 50.Pang Q, Qu K, Zhang JY, Song SD, Liu SS, Tai MH, et al. The prognostic value of platelet count in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Med. (2015) 94:1431. 10.1097/MD.0000000000001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. (2017) 37:778–93. 10.1111/liv.13317 [DOI] [PubMed] [Google Scholar]
- 52.Akuta N, Suzuki F, Kobayashi M, Hara T, Sezaki H, Suzuki Y, et al. Correlation between hepatitis B virus surface antigen level and alpha-fetoprotein in patients free of hepatocellular carcinoma or severe hepatitis. J Med Virol. (2014) 86:131–8. 10.1002/jmv.23790 [DOI] [PubMed] [Google Scholar]
- 53.Kondo R, Yano H, Nakashima O, Tanikawa K, Nomura Y, Kage M. Accumulation of platelets in the liver may be an important contributory factor to thrombocytopenia and liver fibrosis in chronic hepatitis C. J Gastroenterol. (2013) 48:526–34. 10.1007/s00535-012-0656-2 [DOI] [PubMed] [Google Scholar]
- 54.Ho-Tin-Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. (2009) 175:1699–708. 10.2353/ajpath.2009.090460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chrysanthos NV, Papatheodoridis GV, Savvas S, Kafiri G, Petraki K, Manesis EK, et al. Aspartate aminotransferase to platelet ratio index for fibrosis evaluation in chronic viral hepatitis. Eur J Gastroenterol Hepatol. (2006) 18:389–96. 10.1097/00042737-200604000-00012 [DOI] [PubMed] [Google Scholar]
- 56.Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. (2015) 21:711–25. 10.3748/wjg.v21.i3.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, et al. Association of nucleos(T)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B - a nationwide cohort study. Gastroenterology. (2014) 147:48. 10.1053/j.gastro.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 58.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. Br Med J. (2004) 328:983–6. 10.1136/bmj.38050.593634.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, et al. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. (2011) 46:536–44. 10.1007/s00535-010-0349-7 [DOI] [PubMed] [Google Scholar]
- 60.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. (2003) 237:536–43. 10.1097/01.SLA.0000059988.22416.F2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witjes CDM, Ijzermans JNM, van der Eijk AA, Hansen BE, Verhoef C, de Man RA. Quantitative HBV DNA and AST are strong predictors for survival after HCC detection in chronic HBV patients. Neth J Med. (2011) 69:508–13. https://pubmed.ncbi.nlm.nih.gov/22279629/ [PubMed] [Google Scholar]
- 62.Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. (2013) 57:1426–35. 10.1002/hep.25832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amano H, Tashiro H, Oshita A, Kobayashi T, Tanimoto Y, Kuroda S, et al. Significance of platelet count in the outcomes of hepatectomized patients with hepatocellular carcinoma exceeding the milan criteria. J Gastrointest Surg. (2011) 15:1173–81. 10.1007/s11605-011-1538-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.