Abstract
In this study, we attempted to record the breakthrough cases reported through passive and voluntary reporting at various healthcare facilities from different districts of Odisha, their clinical presentation, requirement of hospitalization postinfection, and antibody titer against spike antigen. Nasopharyngeal swab and serum samples alongwith demographic, clinical presentation and requirement of hospitalization postinfection were collected from vaccinated individuals through passive and voluntary reporting to various healthcare facilities of Odisha state to detect the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) virus infection and quantitative estimation of antibody titers. A total of 274 samples were found to be positive after 14 days of receiving complete doses of the vaccines. More than 83.2% of the individuals were found to be symptomatic with 9.9% of those required hospitalization. The seropositivity in individuals receiving Covishield (96.7%) was significantly higher than in Covaxin (77.1%). Hospitalized patients were having less median antibody titers than individuals in home isolation. The median age for breakthrough infection among the referred cases was 47.0 years (interquartile range [IQR]: 28.0) with a significantly older age group among Covishield recipients. The median spike receptor binding domain IgG titer values for Covaxin and Covishield recipients were 213.5 AU/ml (IQR: 537.5) and 647.5 AU/ml (IQR: 1645.1), respectively. The results reported here highlight the need for systematic data capture for the breakthrough infections to monitor the emergence of any vaccine escape variants and to plan the next steps in the coronavirus disease‐19 (COVID‐19) vaccine development by understanding the link between clinical protection and measured immunity against SARS‐CoV‐2 infection.
Keywords: breakthrough, COVAXIN®, COVID‐19, COVISHIELDTM , vaccination
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which emerged in December 2019 has caused tremendous panic around the globe for the past one and a half years. During a health crisis wherein the scientific community is trying to contain the effects of the first wave of coronavirus disease‐19 (COVID‐19), the world was recently struck by a second wave. 1 As of June 20, 2021, more than 178 million individuals were infected with SARS‐CoV‐2 and 3.86 million SARS‐CoV‐2‐associated deaths were reported. 2 United States, India, and Brazil account for most of the cases worldwide with India recording about 29.88 million cases and 3.86 million deaths. 2 , 3
To tackle the ongoing pandemic, the Government of India initiated the world's largest vaccination drive since January 16, 2021, in a phased manner with healthcare workers (HCWs) getting inoculated initially with either of the two vaccines named BBV‐152 (COVAXIN®) and AZD1222 (COVISHIELDTM) after the requisite approval for emergency use in the country. The vaccination of frontline workers (FLWs) was initiated on February 2. Subsequently, the Gam‐COVID‐Vac (Sputnik V) got approval and was launched in India on May 14, 2021. 4 , 5 India has administered 245.9 million citizens with at least a single dose of either BBV‐152 or AZD1222 vaccine and among them, 52.1 million people got both the dosages (complete vaccination) and Odisha has vaccinated 9 million citizens with at least a single dose of either BBV‐152 or AZD1222 vaccine and among them, 1.9 million people got both the dosages (complete vaccination) as of mid‐June. 6
During the early stages of the pandemic, scientists hypothesized that SARS‐CoV‐2 transmission would be slowed by herd immunity resulting from spontaneous infection, vaccination, or both. 7 Although several studies have demonstrated that SARS‐CoV‐2 infection in vaccinated individuals presents clinically mild symptoms, it is critical to determine whether severe symptoms can arise in others despite vaccination, as the development of variants is a continuous process. 8 A vaccine breakthrough infection is defined as the detection of SARS‐CoV‐2 RNA or antigen in a respiratory specimen collected from a person ≥14 days after receipt of all recommended doses of an approved COVID‐19 vaccine. 9
In the present study, we attempted to record the breakthrough cases reported through passive and voluntary reporting at various healthcare facilities from different districts of Odisha with their clinical presentation, the requirement of hospitalization postinfection, and antibody titer against spike antigen.
2. METHODOLOGY
2.1. Study design
From March 1 to June 10, 2021, nasopharyngeal swab and serum samples were collected from vaccinated individuals who reported to the various healthcare facilities of Odisha state with COVID‐19 symptoms and sent to ICMR‐Regional Medical Research Centre, Bhubaneswar, for testing and confirmation. Individuals who tested positive by real time polymerase chain reaction (RT‐PCR) after ≥14 days of complete doses of either BBV‐152 (Covaxin) or AZD1222 (Covishield) vaccine were considered as breakthrough cases and included in the study. Covaxin (BBV152), developed by Bharat Biotech, India, in collaboration with the Indian Council of Medical Research, is an inactivated severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) vaccine, whereas Covishield is manufactured by Serum Institute of India under license from Astra Zeneca (adenovirus vectored ChAdOx1 nCoV‐19 vaccine–AZD1222). Demographic characteristics, symptoms present, medical history, vaccination details, and duration of hospital/home isolation of the individuals were collected using a questionnaire. The data for the study were part of the diagnostic service provided to the participants as part of the routine procedure and available in the laboratory and were analyzed and hence no additional written consent was obtained. The Institutional Human Ethics Committee of ICMR‐Regional Medical Research Centre, Bhubaneswar, approved the study.
2.2. Laboratory testing
All nasopharyngeal swab samples were tested in an automated machine Cobas 6800 (Roche Molecular Systems), which targets two genes, that is, the ORF1 gene (target 1) and E gene (target 2). Spike receptor binding domain IgG antibodies against SARS‐CoV‐2 were quantitatively estimated in an automated chemiluminescence electro assay (CLIA)‐based platform, ARCHITECT i1000SR (Abbott Diagnostics) using a commercial quantitative kit ARCH SARS‐CoV‐2 IgG II Quant. The cut‐off value for this kit was 50 AU/ml.
2.3. Statistical analysis
Statistical Analyses were performed using SPSS version 24 (IBM) and GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla). Qualitative data were described using frequencies and percentages and analyzed using the χ 2 test. Quantitative data were described using median and interquartile range (IQR) and analyzed using Mann–Whitney U test. A p value of less than 0.05 was considered statistically significant.
2.4. Ethics approval
The study was ethically approved by the institutional human ethical committee of ICMR‐Regional Medical Research Centre, Bhubaneswar.
3. RESULTS
A total of 361 samples from vaccinated individuals were referred to our center for confirmation of infection and quantitative antibody estimation. All these 361 individuals were found to be RT‐PCR positive; however, 87 individuals were excluded as they were either not fully vaccinated or were found positive within 14 days of completing the second dose of their vaccination. Out of the 274 samples with confirmed breakthrough infection, 35 (12.8%) individuals received Covaxin and 239 (87.2%) individuals received Covishield. The median age for breakthrough infection among referred cases was 47.0 years (IQR: 28.0) with a significantly older age group among Covishield recipients (Table 1). Around 43% of the Covaxin recipients with breakthrough infection and reported to the facility were HCWs whereas it was around 10% in Covishield recipients and the difference was statistically significant. The duration between the second dose and confirmed SARS‐CoV‐2 infection by RT‐PCR was higher in Covishield (45 days; IQR: 36) than in Covaxin (33 days; IQR: 42) recipients but was statistically insignificant. About 83.2% of individuals were found to be symptomatic in nature and which was almost similar in both the vaccine groups. Around 9.9% of the individuals were hospitalized with no significant difference between Covaxin and Covishield recipient groups. Only one individual (Covishield recipient) died postinfection during the study period. The median duration of hospitalization among the reported cases was 11.0 days (IQR: 6.0) with a single person still hospitalized to date. The median cycle threshold (c t) value of the individuals who tested positive in RT‐PCR was 21.2 (IQR: 7.0) with no significant differences in both the groups.
Table 1.
Demographic and medical characteristics of breakthrough cases receiving both the doses of Covaxin and Covishield
| Variable | Total (n = 274) | Covaxin (n = 35) | Covishield (n = 239) | p value |
|---|---|---|---|---|
| Age (median, IQR) | 47.0 (28.0) | 31.0 (20.0) | 48.0 (25) | 0.000* |
| Sex | ||||
| Male | 186 (67.9%) | 20 (57.1%) | 166 (69.5%) | 0.145 |
| Female | 88 (32.1%) | 15 (42.9%) | 73 (30.5%) | |
| Occupation | ||||
| HWCs | 40 (14.6%) | 15 (42.9%) | 25 (10.5%) | 0.000* |
| Non‐HWCs | 234 (85.4%) | 20 (57.1%) | 214 (89.5%) | |
| Duration of vaccination and RT‐PCR positivity (median, IQR) | 44.5 (36.0) | 33.0 (42.0) | 45.0 (36.0) | 0.318 |
| Symptomatic | ||||
| Yes | 228 (83.2%) | 29 (82.9%) | 199 (83.3%) | 0.952 |
| No | 46 (16.8%) | 6 (17.1%) | 40 (16.7%) | |
| Hospitalization | ||||
| Yes | 27 (9.9%) | 3 (8.6%) | 24 (10.0%) | 0.537 |
| No | 247 (90.1%) | 32 (91.4%) | 215 (90.0%) | |
| Duration of hospitalization (median, IQR) | 11.0 (6.0) | 12.0 (–) | 10.5 (7.0) | 0.533 |
| C t value (median, IQR) | 21.2 (7.0) | 21.0 (6.0) | 22.0 (7.0) | 0.812 |
| Antibody positive against S protein | ||||
| Yes | 258 (94.2%) | 27 (77.1%) | 231 (96.7) | 0.000* |
| No | 16 (5.8%) | 8 (22.9%) | 8 (3.3%) | |
| Antibody titer (median, IQR) | 564.4 (1516.4) | 213.5 (537.5) | 647.5 (1645.1) | 0.000* |
| Comorbidities | ||||
| Yes | 64 (23.4%) | 5 (14.3%) | 59 (24.7%) | 0.174 |
| No | 210 (76.6%) | 30 (85.7%) | 180 (75.3%) | |
Abbreviations: HWCs, healthcare workers; IQR, interquartile range; RT‐PCR, real time polymerase chain reaction.
p < 0.05
A total of 258 (94.2%) individuals were found to be positive for SARS‐CoV‐2 IgG antibodies against spike protein. There were significant differences in seropositivity between Covaxin (77.1%) and Covishield (96.7%). Recipients of Covishield showed higher median titer values than Covaxin among the reported cases, which were statistically significant. The median antibody titer values for Covaxin and Covishield were 213.5 AU/ml (IQR: 537.5) and 647.5 AU/ml (IQR: 1645.1), respectively. The titer values ranged from 4.0 AU/ml to 19835.2 AU/ml in Covaxin, whereas it ranged from 0.4 AU/ml to 40 000.0 AU/ml in the case of Covishield recipients. The median antibody titer among hospitalized individuals (344.0 AU/ml) was lower than individuals advised for home isolation (593.4 AU/ml) (Figure 1). There was a marginal increase in the median titer values of symptomatic patients (564.4 AU/ml) than asymptomatic (527.9 AU/ml) individuals. There was no significant difference between the antibody titers in terms of gender, occupation, hospitalization, and symptom status. The most common symptoms found were fever (88.5%) followed by cough (77.6%) and sore throat (59.6%). Comorbidities, such as diabetes, hypertension, hypothyroidism, and asthma were present in about 64 (23.3%) individuals.
Figure 1.
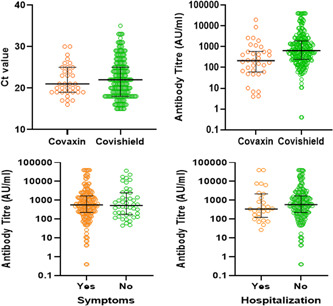
Median antibody titer values of demographic and medical characteristics of breakthrough cases
4. DISCUSSION
A total of 274 cases were found to be infected with the SARS‐CoV‐2 virus ≥14 days after receiving a second COVID‐19 vaccine (Covaxin or Covishield) dose and were defined as breakthrough infections. Only 16.8% of individuals were asymptomatic with no difference in the c t values between the symptomatic and asymptomatic. Out of the 27 individuals with breakthrough infections and requiring hospitalizations, one died. Odisha administered more than 10.07 million doses of vaccine out of which 1.09 million doses are of Covaxin and 8.98 million doses are of Covishield till mid‐June. A total of 379 505 persons were fully vaccinated with Covaxin whereas around 1 508 294 persons were fully vaccinated with Covishield. A higher number of fully vaccinated individuals with Covishield could have led to more breakthrough cases in individuals who received Covishield in comparison to Covaxin in the state. As COVID‐19 vaccines do not provide 100% protection, postvaccination breakthrough infection is possible but rare. 10 , 11 Earlier studies have suggested that COVID‐19 vaccines might protect against the occurrence of severe illness and might help in preventing infection. 12 A study on HCWs had found symptomatic breakthrough infections occurring in 15 persons (13.3%), out of which one required hospitalization. 13 The study on the variants of the breakthrough cases would help further to enlighten knowledge toward various mutations and their effect on the vaccine's capacity in preventing severe illness. 14 The mean c t value of the infections in our study was in line with another study in Cleveland, which showed a mean c t value of 20 in about 5 (12%) fully vaccinated individuals detected positive postvaccination. 15
The study has three limitations; first, the number of documented COVID‐19 vaccination breakthrough cases may be significantly underestimated of all SARS‐CoV‐2 infections among fully vaccinated persons; second, these data are based on passive and voluntary reporting of individuals and might not be a complete representation of breakthrough cases as most of the individuals being asymptomatic or with mild illness postvaccination might have not got tested; and finally, sequencing of the samples was not conducted, which could have identified the virus lineage that had caused these breakthrough cases. It is anticipated that even with the administration of effective authorized vaccines, breakthrough cases are expected to happen, till the immunity reaches sufficient levels among the population to further decrease transmission. 9
The importance of the ongoing battle between immunization and natural selection of potential viral escape mutants is highlighted by our research. SARS‐CoV‐2 variants will likely be evolved continuously, driven by selection for increased transmissibility and evasion of the host immune response. These results, which are the first of its kind from India, highlight the importance of national surveillance to identify the breakthrough infection and detect the circulating viruses. This will help to monitor the emergence of vaccine escape variants if any. This study urges the need for a larger study, which would help to define correlates of protection to determine whether there is a need to produce modified vaccines or doses and to development of monoclonal antibodies, targeting the conserved spike protein epitopes that are difficult to mutate.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Debdutta Bhattacharya and Sanghamitra Pati designed the study. Girish C. Dash, Subhra Subhadra, Jyotirmayee Turuk, Debaprasad Parai, Sonalika Rath, Jyotsnamayee Sabat, Usha K. Rout, and Rashmi R. Nanda were involved in the testing and analysis of data. Girish C. Dash, Srikanta Kanungo, Hari R. Choudhary, and Matrujyoti Pattnaik were responsible for data collection and analysis. Girish C. Dash, Debaprasad Parai, Debdutta Bhattacharya, and Subhra Subhadra wrote the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENT
The authors gratefully acknowledge all the healthcare workers for their tireless dedication at each level to fight COVID‐19 and for voluntarily participating in this cohort study. The authors are thankful to the Indian Council of Medical Research, New Delhi, and the Department of Health & Family Welfare, Government of Odisha for providing financial support for the study.
Dash GC, Subhadra S, Turuk J, et al. Breakthrough SARS‐CoV‐2 infections among BBV‐152 (COVAXIN®) and AZD1222 (COVISHIELDTM) recipients: report from the eastern state of India. J Med Virol. 2022;94:1201‐1205. 10.1002/jmv.27382
Girish Chandra Dash, Subhra Subhadra, Jyotirmayee Turuk, Debaprasad Parai, Sonalika Rath, and Jyotsnamayee Sabat contributed equally to this study.
Contributor Information
Sanghamitra Pati, Email: drsanghamitra12@gmail.com.
Debdutta Bhattacharya, Email: drdebduttab.rmrc-od@gov.in.
DATA AVAILABILITY STATEMENT
All data and statistical code to reproduce the tables and figures in the manuscript are available on request to the corresponding author.
REFERENCES
- 1. Madiha A, Misbahud D. The expected second wave of COVID‐19. Int J Clin Virol. 2020;4:109‐110. [Google Scholar]
- 2. WHO Coronavirus Disease (COVID‐19) Dashboard. Accessed April 12, 2021. https://covid19.who.int/
- 3. Ministry of Health and Family Welfare , Government of India (COVID‐19) Dashboard. Accessed April 12, 2021. https://www.mohfw.gov.in/
- 4. Gupta R, Kumar VM, Tripathi M, et al. Guidelines of the Indian Society for Sleep Research (ISSR) for practice of sleep medicine during COVID‐19. Sleep Vigil. 2020;4:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagcchi S. The world's largest COVID‐19 vaccination campaign. Lancet Infect Dis. 2021;21:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaccination Dashboard . MoHFW, Government of India. 2021. Accessed June 24, 2021. https://dashboard.cowin.gov.in/
- 7. Fontanet A, Cauchemez S. COVID‐19 herd immunity: Where are we? Nat Rev Immunol. 2020;20:583‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CDC COVID‐19 Vaccine Breakthrough Case Investigations Team . COVID‐19 Vaccine Breakthrough Infections Reported to CDC—United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:792‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CDC . COVID‐19 Vaccine Effectiveness Research. 2021. Accessed April 12, 2021. https://www.cdc.gov/vaccines/covid-19/effectiveness-research/protocols.html
- 10. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bagchi S, Mak J, Li Q, et al. Rates of COVID‐19 Among Residents and Staff Members in Nursing Homes—United States, May 25–November 22, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tyagi K, Ghosh A, Nair D, et al. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr. 2021;15:1007‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS‐CoV‐2 variants. N Engl J Med. 2021;384:2212‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redmond SN, Jones LD, Sadri N, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in vaccinated and unvaccinated healthcare personnel in a Veterans Affairs healthcare system. Infect Control Hosp Epidemiol. 2021;27:1‐2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8185409/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and statistical code to reproduce the tables and figures in the manuscript are available on request to the corresponding author.


