VITT is a potential complication of ChAdOx1‐nCov‐19 vaccination — early recognition is key to improved outcomes
A syndrome of thrombosis and thrombocytopenia has been described in a small proportion of patients vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The thrombosis can be severe and, on occasion, fatal. The syndrome has been described in patients receiving adenovirus vector ChAdOx1 nCov‐19 (AstraZeneca) or Ad26.COV2.S (Johnson & Johnson–Janssen) encoding SARS‐CoV‐2 spike protein. 1 , 2 , 3 , 4
This novel thrombosis syndrome is known as vaccine‐induced immune thrombotic thrombocytopenia (VITT), vaccine‐induced prothrombotic immune thrombocytopenia, or thrombosis with thrombocytopenia syndrome. For consistency with the prevailing literature, we refer to this syndrome as VITT.
Reporting rates of VITT currently vary. A Norwegian case series identified five patients from a population of 132 686 receiving ChAdOx1 nCov‐19; the United Kingdom Medicines and Healthcare products Regulatory Agency received 209 reports after 22 million first doses of ChAdOx1 nCoV‐19 by 28 April 2021; 2 , 4 , 5 and at time of writing, 24 cases have been confirmed in Australia by the Thrombosis and Haemostasis Society of Australia and New Zealand (THSANZ) VITT advisory group, with 2.1 million ChAdOx1 nCov‐19 vaccines given.
Antibodies against platelet factor 4 (PF4) or PF4–polyanion complexes have been identified in VITT. Serum and plasma from these patients directly and strongly activate platelets (functional assays). An immunological basis for the syndrome is further supported by the ability of both pooled human immunoglobulins and a monoclonal antibody (IV.3) which blocks the immune complex receptors on platelets (FcγRIIa) to abrogate platelet activation. 1 , 3
The syndrome is analogous to but distinct from another thrombotic thrombocytopenic syndrome: spontaneous or autoimmune heparin‐induced thrombocytopenia (HIT). Similar to autoimmune HIT, in VITT the serological activation of platelets is abolished with high concentrations of heparin and enhanced by PF4. 6 In contrast to HIT, VITT cases are not associated with antecedent exposure to heparin, and a heparin independent hyperactive platelet response is seen in in vitro assays. VITT is a distinct syndrome from HIT and standard HIT diagnostic pathways are not appropriate for the diagnostic work‐up.
Thrombosis driven by classical factors of Virchow’s triad (vessel wall injury, hypercoagulable state, stasis) is not uncommonly coincidental to vaccination. VITT is rare; however, it requires an alternative, immediately instituted management pathway. Understanding of the syndrome is rapidly evolving and currently only observational studies are available to formulate expert consensus guidance (evidence grading: low). Against this background, we present our current strategy for the Australian context.
Australia and New Zealand experts in thromboembolic disorders and laboratory haemostasis drafted an approach to VITT investigation and management appropriate for the Australian context in March 2021. All publications and pre‐publications on VITT, information from regulatory authorities and available guidance from international societies were reviewed and discussed in a series of online meetings. Revisions were made via email, and the consensus was published in a living online document on 1 April 2021, which has been endorsed by the THSANZ and the Haematology Society of Australia and New Zealand. Additional published evidence continues to be curated and circulated before a weekly update meeting in which clinical and laboratory data of all confirmed Australasian VITT cases are reviewed. Updates are uploaded after consensus at https://www.thanz.org.au/documents/item/591. 7
When to suspect VITT
Patients who present with symptom onset suggestive of thrombosis or thrombocytopenia 4–42 days after vaccination with ChAdOx1 nCov‐19 or Ad26.COV2.S merit urgent clinical assessment to exclude VITT.
Although cases of well controlled thrombosis have been encountered, the tempo of disease can be catastrophic within hours and we strongly advise careful clinical review of persistent symptoms with repeat screening blood tests in patients with a high index of suspicion. Thrombosis has occurred in the cerebral venous sinus system (CVST) and the splanchnic (portal, mesenteric, hepatic), deep vein, pulmonary and arterial circulation.
While early reports showed an over‐representation of females (80%) aged 22–54 years, 1 , 2 the Australian experience does not suggest a strong gender bias and vigilance irrespective of age and gender is strongly recommended.
How to investigate for VITT
The THSANZ criteria for VITT diagnosis are summarised in Box 1, and a diagnostic algorithm is provided in Box 2.
Box 1. Thrombosis and Haemostasis Society of Australia and New Zealand diagnostic criteria for vaccine‐induced immune thrombotic thrombocytopenia*.
|
* Further details available at https://www.thanz.org.au/documents/item/591. 7
Box 2. Management of vaccine‐induced immune thrombotic thrombocytopenia (VITT) in patients who have received ChAdOx1 nCov‐19*.
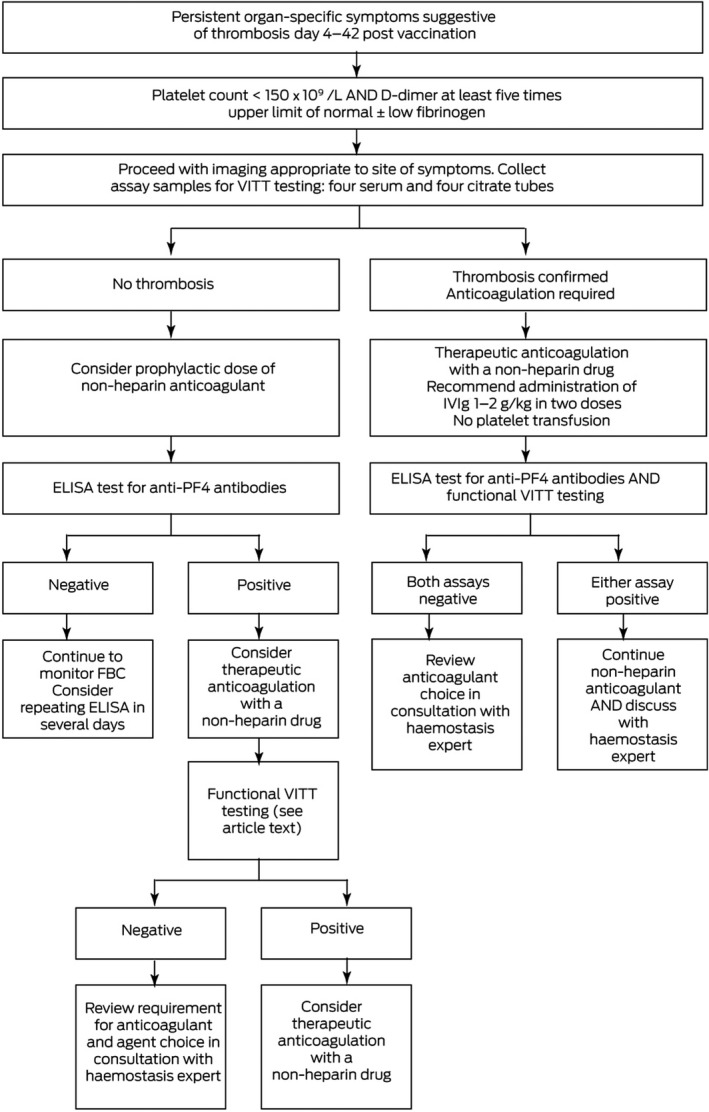
ELISA = enzyme‐linked immunosorbent assay; FBC = full blood count; IVIg = intravenous immunoglobulin; PF4 = platelet factor 4; ULN = upper limit of normal.
For ongoing updates, refer to https://www.thanz.org.au/documents/item/591. 7
In patients presenting with symptoms suggestive of either thrombosis or thrombocytopenia within 4–42 days of COVID‐19 vaccination, appropriate investigations should always be initiated based on the severity of presenting symptoms after clinical assessment. Neurological symptoms of CVST can include visual changes, seizures, focal neurological deficits, and symptoms of encephalopathy. Symptoms of splanchnic thrombosis may be subtle. It may be necessary to transfer urgently to a facility where laboratory investigations and appropriate radiology are readily available.
While the suspicion of VITT is being explored, avoid platelet transfusions and do not begin heparin‐based anticoagulation.
Investigation for VITT in Australia occurs in two stages: screen and confirm.
Screen
Screening involves blood samples marked “urgent”, to assess the full blood count, and D‐dimer and fibrinogen levels.
VITT is suspected in patients who present in the appropriate timeframe from ChAdOx1 nCov‐19 vaccination with symptoms of thrombosis if (i) the platelet count is < 150 × 109/L or falling in serial counts, and either (ii) D‐dimer levels are elevated (five times the upper limit of normal) or (iii) fibrinogen levels are reduced to < 2 g/L. Further serum and plasma samples must be taken (at least four citrate tubes and four serum clot tubes), specialist haemostasis haematologist consultation obtained, and radiology performed and reported urgently to investigate for relevant organ‐specific thrombosis (eg, computed tomography of the brain with or without a venogram for CVST, abdominal computed tomography for splanchnic vein thrombosis).
If thrombosis is found, VITT is probable, and treatment must be urgently initiated with non‐heparin anticoagulation and intravenous immunoglobulin (IVIg). 8
If no thrombosis is found, VITT remains possible.
VITT is less likely if the platelet count is > 150 × 109/L but D‐dimer levels are elevated or fibrinogen levels are reduced.
VITT is much less likely if the platelet count is stable and > 150 × 109/L, D‐dimer levels are not elevated and fibrinogen levels are within normal range. Patients can be treated for non‐VITT thrombosis.
It is important to recognise that not all thrombocytopenia following vaccination is VITT. Secondary immune thrombocytopenia from immunisation has been seen with BNT162b2 (Pfizer–BioNTech), mRNA‐1273 (Moderna) and ChAdOx1 nCov‐19 (AstraZeneca) vaccines. An alternative diagnosis of immune thrombocytopenia should always be considered in patients with thrombocytopenia, with or without raised D‐dimer levels and normal fibrinogen levels, without evidence of thrombosis. Immune thrombocytopenia may manifest a bleeding phenotype and patients are managed with usual first line therapies including corticosteroids and IVIg. 9
Likewise, the majority of deep vein thrombosis and pulmonary embolism cases following vaccination are statistically unlikely to be VITT. Once VITT is excluded, such patients can be treated for deep vein thrombosis and pulmonary embolism via the usual venous thromboembolism management pathways.
Confirm
Patients with thrombosis (probable VITT) or no thrombosis (possible VITT) or thrombosis with D‐dimer levels > 5 times the upper limit of normal should be further investigated for:
presence of PF4 or PF4–polyanion antibodies using an enzyme‐linked immunosorbent assay (ELISA) platform; and
the ability for serum/plasma to activate platelets in vitro — platelet‐activating antibodies on functional testing are considered pathological, and are requisite for confirming the diagnosis of VITT.
Antigen‐based VITT immune assay. Antibodies against PF4 or PF4–polyanion complexes using ELISA are present in the majority of VITT cases. Other platforms used for HIT antibody detection (eg, automated chemiluminescent assay, lateral flow immunoassay and particle gel immunoassay) do not reliably detect VITT antibodies and are not appropriate for use in this setting.
Functional antibody testing. In vitro assessments of platelet‐activating antibodies are available in centralised laboratories (serotonin release assay, flow cytometry procoagulant assay and whole blood aggregation assays). These should be performed in all probable or possible VITT cases or in less likely cases with a positive ELISA result, to confirm the diagnosis (see Box 3 for case vignettes).
Box 3. Case vignettes*.
|
Case 1 A 44‐year‐old man presented with fevers, fatigue, head fogginess and abdominal discomfort on day 8 after ChAdOx1 nCov‐19 vaccination. His platelet count was 70 × 109/L (reduction to 17 × 109/L within 12 hours; reference interval, 150–400 × 109/L), D‐dimer level was 114 mg/L (upper limit of normal [ULN], 0.5 mg/L) and fibrinogen level was normal. Radiology showed portal splenic and mesenteric thrombosis. The patient was treated as probable vaccine‐induced immune thrombotic thrombocytopenia (VITT). Treatment was immediately initiated with intravenous immunoglobulin (IVIg) and anticoagulation with fondaparinux. Anticoagulation changed to bivalirudin when surgery was required. Confirmatory investigations supported a diagnosis of VITT with a positive enzyme‐linked immunosorbent assay (ELISA) result and serum/plasma induced platelet activation on functional assay testing. VITT was confirmed. 10 Case 2 A 46‐year‐old woman with a history of quiescent systemic lupus erythematosus presented 4 days after ChAdOx1 nCov‐19 vaccination with investigations consistent with proximal deep vein thrombosis and bilateral pulmonary emboli without evidence of right heart strain. Her platelet count was 97 × 109/L and D‐dimer level was 1.35 mg/L (ULN, 0.25 mg/L); her fibrinogen level was normal. She was treated as probable VITT, immediately anticoagulated with fondaparinux and received one dose of IVIg 1 mg/kg. Her platelet count was stable during hospitalisation. Confirmatory investigations returned negative ELISA and functional assay results and a positive lupus anticoagulant result. She was deemed to have venous thromboembolism secondary to antiphospholipid syndrome, not VITT, and was changed to standard low molecular weight heparin anticoagulation. VITT was not supported. Case 3 A 60‐year‐old man smoker presented with chest pain and dyspnoea on day 15 after receiving ChAdOx1 nCov‐19, and was found to have bilateral pulmonary emboli without haemodynamic compromise or right heart strain. His platelet count was 228 × 109/L and D‐dimer level was 0.6 mg/L (ULN, 0.5 mg/L); his fibrinogen level was normal, and his baseline platelet count was 235 × 109/L. He was treated as much less likely to have VITT (based on normal platelet count), and apixaban was commenced at standard dosing. A repeat platelet count 3 days later was stable and confirmatory testing for VITT did not proceed. VITT was not supported. Case 4 A 50‐year‐old woman presented with extensive petechiae and oral mucosal wet purpura 4 days after ChAdOx1 nCov‐19 vaccination. No symptoms of thrombosis were present on system review. She had no previous history of idiopathic thrombocytopenic purpura or autoimmune disease. Her platelet count was 3 × 109/L and D‐dimer level was 1.25 mg/L (ULN, 0.25 mg/L); her fibrinogen level was normal. She was treated as less likely VITT based on normal D‐dimer levels and lack of thrombosis. She was diagnosed with idiopathic thrombocytopenic purpura, likely vaccine associated, and commenced IVIg 2 g/kg divided over 2 days, with dexamethasone 40 mg daily for 4 days. Her platelet count improved to 50 × 109/L on day 2. Non‐urgent VITT testing supported a diagnosis of not VITT with a negative ELISA result. VITT was not supported. Case 5 An 82‐year‐old man with prostate cancer presented with dyspnoea 17 days after receiving ChAdOx1 nCov‐19. He had a background of inflammatory bowel disease and was found to have sub‐massive pulmonary emboli. His platelet count was 198 × 109/L, D‐dimer level was 9.8 mg/L (ULN, 0.25 mg/L) and fibrinogen level was normal. He was treated as less likely to have VITT (normal platelet count, markedly raised D‐dimer), and commenced therapeutic fondaparinux. Confirmatory investigations did not support a diagnosis of VITT, with a negative ELISA immunoassay, and he did not progress to functional testing. His treatment was changed to low molecular weight heparin and he was discharged on a direct oral anticoagulant when he was stable. VITT was not supported. |
* Case 1 summarises a published case: Hocking et al. 10 Other vignettes are hypothetical and do not refer to specific individuals.
Currently, there is a coordinated national effort to ensure timely investigation: complete the specific request form available at https://www.thanz.org.au/documents/item/579, contact the nearest local haemostasis expert, and send samples to the appropriate listed location.
How to treat suspected VITT
Suspected VITT will require treatment before results of PF4–polyanion ELISA are available. Specialist consultation with a haemostasis haematologist is recommended.
Probable VITT 8
We recommend that probable VITT (suspected with thrombosis) be treated with non‐heparin anticoagulation.
IVIg (1–2 g/kg over at least two divisions) is recommended initially, or high dose steroids if IVIg is unavailable. This is particularly important in patients at high risk from deterioration (including presentation platelets < 30 × 109/L, fibrinogen levels < 1.5 g/L, severe thrombosis). The haematologist may consider the addition of high dose methylprednisolone and/or plasma exchange in the appropriate context (eg, progressive thrombosis or rapid deterioration).
Anticoagulant treatment options are as per local therapeutic practice for HIT: bivalirudin, argatroban, danaparoid, fondaparinux, rivaroxaban, apixaban, dabigatran, and (after initial treatment with another agent) warfarin.
Avoid platelet transfusion.
Hospitalisation is considered safest until there is a reduction of in vivo platelet activation and thrombin generation (normalised platelet count, falling D‐dimer levels, normal fibrinogen levels). We currently suggest retesting for presence of VITT antibodies before cessation of anticoagulation in confirmed cases.
Possible VITT
We recommend that possible VITT (suspected without thrombosis) be monitored closely with repeat full blood count, D‐dimer and fibrinogen testing about every 3 days.
Anticoagulation with a non‐heparin anticoagulant should be considered — particularly with very high D‐dimer levels and a positive immunoassay result. We currently recommend consideration of fondaparinux or a direct oral anticoagulant at prophylactic dosing until platelet counts normalise, D‐dimer levels fall, and normal fibrinogen levels are achieved. IVIg may be considered if there are any signs to suggest progression. 11 Anticoagulation duration should be time limited or until HIT ELISA and functional testing results are negative.
We recommend against second dose ChAdOx1 nCov‐19 in patients with confirmed or strongly suspected VITT. 7 , 12
Conclusion
ChAdOx1 nCov‐19 is a key component of the Australian government vaccination strategy against the coronavirus disease 2019 pandemic. Our current perspectives on this novel complication of ChAdOx1 nCov‐19 are summarised in Box 4.
Box 4. Key points.
|
Diagnostic algorithms and treatment strategies for VITT are available and continue to be refined as local experience increases. The THSANZ and the Haematology Society of Australia and New Zealand have endorsed a living guidance document (https://www.thanz.org.au/documents/item/591), which also provides a list of online resources. 7
Competing interests
No relevant disclosures.
Provenance
Not commissioned; externally peer reviewed.
Acknowledgements
This guidance has been produced with ongoing critical review and support from the entire THSANZ VITT advisory group: Vivien Chen (Concord Hospital, Sydney); Huyen Tran (Alfred Hospital, Melbourne); Philip Choi (The Canberra Hospital, ACT); Jennifer Curnow (Westmead Hospital, Sydney); Sanjeev Chunilal (Monash Medical Centre, Melbourne); Christopher Ward (Royal North Shore Hospital, Sydney); Freda Passam (Royal Prince Alfred Hospital, Sydney); Timothy Brighton (Prince of Wales Hospital, Sydney); Beng Chong (St George Hospital, Sydney); Robert Bird (Princess Alexandra Hospital, Brisbane); Anoop Enjeti (John Hunter Hospital, Newcastle); Leonardo Pasalic (ICPMR, Sydney); Emmanuel Favaloro (ICPMR, Sydney); Chee Wee Tan (Royal Adelaide Hospital, Adelaide); Ross Baker (Perth Blood Institute, Murdoch University, WA); Simon McCrae (Launceston Hospital, Tasmania); Ibrahim Tohidi‐Esfahani (ANZAC Research Institute. Sydney); Elizabeth Gardiner (Australian National University, Canberra); Joanne Joseph (St Vincent’s hospital, Sydney); Danny Hsu (Liverpool hospital, Sydney); Laura Young (Auckland City Hospital, NZ); Claire McClintock (Auckland City Hospital, NZ); and Eileen Merriman (Waitemata, NZ). We also thank Haematology Society of Australia and New Zealand contributors Steven Lane and Leanne Berkhan.
The unedited version of this article was published as a preprint on mja.com.au on 10 June 2021.
References
- 1. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med 2021; 384: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med 2021; 384: 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med 2021; 384: 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021; 384: 1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medicines and Healthcare products Regulatory Agency . Coronavirus vaccine ‐ weekly summary of Yellow Card reporting, updated 28 April 2021. https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting (viewed May 2021).
- 6. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin‐induced thrombocytopenia. J Thromb Haemost 2017; 15: 2099–2114. [DOI] [PubMed] [Google Scholar]
- 7. Thrombosis and Haemostasis Society of Australia and New Zealand Vaccine Thrombocytopenia Working Group . Suspected vaccine induced immune thrombotic thrombocytopenia (VITT): THANZ advisory statement for haematologists. 29 June 2021. https://www.thanz.org.au/documents/item/591 (viewed Aug 2021).
- 8. Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of vaccine‐induced immune thrombotic thrombocytopenia (VITT) for SARS‐CoV‐2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost 2021; 19: 1585–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol 2021; 96: 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hocking J, Chiunilal S, Chen VM, et al. The first known case of vaccine‐induced thrombotic thrombocytopenia in Australia. Med J Aust 2021; 215: 19–20. https://www.mja.com.au/journal/2021/215/1/first‐known‐case‐vaccine‐induced‐thrombotic‐thrombocytopenia‐australia [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost 2021; 19: 1819–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Australian Government Department of Health . Joint statement from ATAGI and THANZ on thrombosis with thrombocytopenia syndrome (TTS) and the use of COVID‐19 vaccine AstraZeneca. 23 May 2021. https://www.health.gov.au/news/joint‐statement‐from‐atagi‐and‐thanz‐on‐thrombosis‐with‐thrombocytopenia‐syndrome‐tts‐and‐the‐use‐of‐covid‐19‐vaccine‐astrazeneca (viewed May 2021).


