ABSTRACT
Objective
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vertical transmission has been investigated extensively. Recently, the World Health Organization (WHO) published strict criteria to classify the timing of mother‐to‐child transmission of SARS‐CoV‐2 into different categories. The aim of this study was to investigate the possibility of vertical transmission in asymptomatic SARS‐CoV‐2‐positive women.
Methods
Pregnant women attending for delivery at a perinatology center in Mexico City, Mexico, who had a SARS‐CoV‐2‐positive nasopharyngeal swab 24–48 h before delivery, were asymptomatic at the time of the test and had an obstetric indication for Cesarean section were eligible for inclusion in this study. Amniotic fluid was collected during Cesarean delivery, and neonatal oral and rectal swabs were collected at birth and at 24 h after birth. SARS‐CoV‐2 detection was carried out using real‐time reverse‐transcription polymerase chain reaction in all samples. Relevant medical information was retrieved from clinical records. The WHO criteria for classifying the timing of mother‐to‐child transmission of SARS‐CoV‐2 were applied to the study population.
Results
Forty‐two SARS‐CoV‐2‐positive asymptomatic pregnant women fulfilled the inclusion criteria. Twenty‐five (59%) women developed mild disease after discharge. Neonatal death occurred in three (7%) cases, of which one had a positive SARS‐CoV‐2 test at birth and none had coronavirus disease 2019‐related symptoms. There were five (12%) cases with strong evidence of intrauterine transmission of SARS‐CoV‐2, according to the WHO criteria, as amniotic fluid samples and neonatal samples at birth and at 24 h after birth were positive for SARS‐CoV‐2. Our results also showed that 40–60% of infected neonates would have been undetected if only one swab (oral or rectal) was tested.
Conclusion
This study contributes evidence to reinforce the potential for vertical transmission of SARS‐CoV‐2 even in asymptomatic women and highlights the importance of testing more than one neonatal sample in order to increase the detection rate of SARS‐CoV‐2 in affected cases. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: COVID‐19, newborn testing, pregnancy, SARS‐CoV‐2, vertical transmission
CONTRIBUTION —
What are the novel findings of this work?
The findings of this study provide evidence of possible vertical transmission of severe acute respiratory sydrome coronavirus 2 (SARS‐CoV‐2) in asymptomatic pregnant women, using the novel World Health Organizaton criteria for the establishment of mother‐to‐child transmission of SARS‐CoV‐2. The presence of SARS‐CoV‐2 RNA in amniotic fluid and its persistence in the neonate 24 h after birth was demonstrated.
What are the clinical implications of this work?
This work highlights the importance of testing more than one sample for SARS‐CoV‐2 in neonates of asymptomatic infected women in order to increase the detection rate in positive cases. Identification of neonatal SARS‐CoV‐2 infection might help clinicians to understand better the long‐term effects or complications of the disease.
INTRODUCTION
In December 2019, an outbreak of a novel coronavirus occurred in Wuhan, China. Since then, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has infected over 160 million people worldwide, causing more than 3.3 million deaths 1 . Several groups have been identified as being vulnerable to developing serious complications after SARS‐CoV‐2 infection, but data on pregnant women are still sparse 2 . Pregnant women are considered more susceptible to coronavirus disease 2019 (COVID‐19) due to the immunological and physiological adaptations inherent to pregnancy 3 . Clinical manifestations in pregnant women with COVID‐19 range from asymptomatic to severe. Although SARS‐CoV‐2 has been identified in the cytoplasm of perivillous trophoblastic cells, indicating placental infection 4 , 5 , only a few cases of vertical transmission have been reported 6 , 7 , 8 . Most cases lack analysis of adequate biological samples to rule out neonatal infection 9 ; therefore, the potential for SARS‐CoV‐2 vertical transmission remains controversial.
Presence of SARS‐CoV‐2 immunoglobulin (Ig) M antibodies in the peripheral blood of neonates suggests fetal exposure to the virus. However, this test alone is not sufficient for diagnosing intrauterine infection, and analysis of IgM is useful only in patients with clinical manifestation of the infection for more than 11 days 10 , 11 . Criteria to define vertical transmission of SARS‐CoV‐2, based on the presence of the virus in biological samples at different timepoints, have been proposed 12 . According to these criteria, intrauterine transmission is considered to have likely occurred if the mother, even if asymptomatic, is positive for SARS‐CoV‐2 from 14 days before until 2 days after delivery, the virus is detected in either the amniotic fluid (AF), the placenta or the neonate within the first 24 h postpartum, and there is viral persistence in the neonate beyond 24 h. Criteria have also been provided for intrapartum transmission and superficial exposure 12 . Recently, the World Health Organization (WHO) classified the timing of mother‐to‐child transmission (intrauterine, intrapartum or early postnatal) into the following categories: confirmed, possible, unlikely or indeterminate 13 . The different tests and samples required to determine the occurrence of vertical transmission have also been described by the WHO 13 .
The objective of this study was to investigate the presence of SARS‐CoV‐2 in asymptomatic pregnant women and the presence and persistence of the virus in their neonates, in order to explore the possibility of vertical transmission, according to the strict criteria of the WHO.
METHODS
Ethics statement
Participation in this study was voluntary and all women signed an informed consent form. The study was approved by the ethics and research institutional review board of the National Institute of Perinatology, Mexico City, Mexico (Registration number 2020‐1‐32).
Study population and specimens
All pregnant women attending for delivery at the National Institute of Perinatology in Mexico City, Mexico, from March 2020 to March 2021 were tested routinely for SARS‐CoV‐2 infection using real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) of nasopharyngeal swabs at least 24 to 48 h before delivery, according to the Centers for Disease Control and Prevention (CDC) recommendations 14 . The participants were selected according to the following inclusion criteria: absence of COVID‐19 symptoms; RT‐PCR‐confirmed SARS‐CoV‐2 infection; third trimester of gestation; a viable fetus; and obstetric indication for Cesarean delivery, such as previous Cesarean delivery, cephalopelvic disproportion and maternal comorbidity. Only patients with planned Cesarean delivery were invited to participate since we needed to collect AF before membrane rupture and umbilical cord blood before the umbilical cord was cut. Exclusion criteria were contaminated AF sample, incomplete clinical data for the woman or fetus or inability to obtain AF or neonatal samples. Clinical and demographic data were obtained from medical records. In addition, a follow‐up phone call was made 2 weeks after discharge in order to evaluate the presence of COVID‐19 symptoms in the mother or the child.
Mild COVID‐19 was defined, according to the National Institutes of Health (NIH) COVID‐19 treatment guideline criteria, as an individual who had any of the various signs and symptoms of COVID‐19 (e.g. fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea and loss of taste or smell), but without shortness of breath, dyspnea or abnormal chest imaging 15 .
AF was collected during Cesarean delivery by direct puncture of the intact fetal membranes and aspiration using a sterile needle and syringe. Neonatal oral and rectal swabs were obtained immediately after birth, taking special care to avoid contact with any maternal blood or tissue. Additional neonatal oral and rectal swabs were collected 24 h after birth.
RT‐PCR for detection of SARS‐CoV‐2
Isolation of RNA from maternal and neonatal swabs was carried out using the Quick‐RNA‐Viral Kit (ZYMO Research, Irvine, CA, USA) and RNA isolation from AF (140 µL) was carried out using the QIAamp Viral RNA Midi Kit (QIAGEN, GmbH, Hilden, Germany), according to the manufacturer's instructions.
For SARS‐CoV‐2 detection by real‐time RT‐PCR, the protocol described by Corman et al. was employed 16 . In this protocol, the RdRP and E viral genes, as well as the RNaseP human gene as a control, are amplified. The RT‐PCR reactions were run on a StepOne plus instrument (Thermo Fisher Scientific, Waltham, MA, USA).
The presence of specific SARS‐CoV‐2 IgG antibodies was determined at birth in maternal and umbilical cord serum by chemiluminescent microparticle immunoassay using the SARS‐CoV‐2 IgG kit (Abbott Laboratories, Chicago, IL, USA) and the Architect instrument (Abbott Laboratories).
The SARS‐CoV‐2 cycle threshold (Ct) value on RT‐PCR has an inverse correlation with viral load 17 . We analyzed Ct values in maternal nasopharyngeal swabs, neonatal swabs and AF samples. Depending on the results of the test carried out on AF samples and neonatal oral and rectal swabs at birth, women were subdivided into five categories: (1) all women; (2) women with a positive neonate (according to either oral or rectal swabs or both) and negative AF; (3) women with positive AF and a negative neonate; (4) women with a positive neonate and positive AF; and (5) women with both a negative neonate and negative AF.
Analysis of timing of mother‐to‐child transmission of SARS‐CoV‐2
Classification of mother‐to‐child transmission was assigned according to the WHO criteria 13 . For intrauterine SARS‐CoV‐2 infection, the WHO recommendations assess the following three criteria: (1) evidence of maternal SARS‐CoV‐2 infection during pregnancy at any time, demonstrated through accepted standard methods; (2) in‐utero fetal exposure to SARS‐CoV‐2, demonstrated by positive RT‐PCR of a sterile or non‐sterile sample (such as AF) or placental tissue, or detection of IgA/IgM antibodies in umbilical cord blood; and (3) persistence of infection or immune response in the neonate, proved by viral detection in a sterile or non‐sterile sample using RT‐PCR in the 24–48 h after birth or detection of IgA/IgM antibodies in neonatal blood at 24 h to < 7 days. Intrauterine transmission can occur through the hematogenous route, when the virus crosses the placental barrier and reaches the fetus to cause infection. Depending on the findings, in‐utero transmission can be categorized as: confirmed, possible, unlikely or indeterminate 13 . If all three of the above criteria for in‐utero transmission are not fulfilled, then neonatal infection can be considered as being potentially due to intrapartum transmission (when there is evidence of lack of in‐utero exposure) or early postnatal contact (when the neonatal age at infection is > 48 h to 28 days and there is evidence of lack of in‐utero and intrapartum exposure).
Statistical analysis
Qualitative variables are presented as n (%) and were compared using χ2 or Fisher's exact test. Quantitative variables are presented as mean with SD or median with interquartile range. Analysis of quantitative variables was performed using parametric (Student's t‐test) or non‐parametric (Mann–Whitney U‐test) tests, depending on their distribution. A univariate logistic regression model was used to determine the association between covariables and the outcome of having a neonate with a positive result for SARS‐CoV‐2 infection. All statistical analyses were performed using the Statistical Package for Social Sciences version 25 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
RESULTS
Characteristics of study population
Of the 190 pregnant women who tested positive for SARS‐CoV‐2 from March 2020 to March 2021, 148 did not meet the inclusion criteria. Therefore, results are presented for 42 mother–child dyads. Maternal and neonatal clinical data are summarized in Table 1. Type‐1 or ‐2 diabetes mellitus, obesity or high blood pressure was observed in 10 (23.8%) women. A pregnancy complication, such as gestational diabetes mellitus or placenta accreta spectrum disorder, was diagnosed in four (9.5%) women. There were three (7.1%) cases of neonatal death, of which one tested positive for SARS‐CoV‐2 at birth. Two of the neonatal deaths were related to a congenital heart defect and the third case died on the second day postpartum, with no obvious external defects, respiratory failure or COVID‐19‐related symptoms. The cause of death could not be determined in the third case because the parents declined postmortem examination. As per the inclusion criteria, symptoms related to COVID‐19 were absent in all women at the time of the SARS‐CoV‐2 test. However, the follow‐up phone call revealed that 25 (59.5%) women subsequently developed mild disease, with headache being the most common symptom (Table 2).
Table 1.
Clinical and demographic data in 42 asymptomatic SARS‐CoV‐2‐positive pregnant women and their neonates
| Case | Maternal age (years) | Developed symptoms after discharge | Maternal comorbidity/pregnancy complication | Neonatal sex | Birth weight (g) | Birth length (cm) | GA at birth (weeks) | 1‐/5‐min Apgar score | Neonatal outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | Yes | DM2 | F | 2815 | 47.5 | 38.0 | 8/9 | A&W |
| 2 | 40 | Yes | GDM | M | 3645 | 52.0 | 39.0 | 8/9 | A&W |
| 3 | 30 | Yes | Cholestasis | F | 2815 | 48.0 | 37.3 | 8/9 | NND |
| 4 | 28 | Yes | Obesity | M | 3250 | 50.0 | 38.4 | 8/9 | A&W |
| 5 | 25 | Yes | Obesity, metabolic syndrome | F | 2615 | 47.0 | 39.6 | 7/9 | Umbilical hernia, patent urachus |
| 6 | 23 | Yes | DM1, hypothyroidism | M | 3025 | 49.0 | 37.6 | 9/9 | A&W |
| 7 | 33 | Yes | None | M | 3580 | 53.0 | 38.4 | 8/9 | A&W |
| 8 | 27 | Yes | None | M | 3010 | 49.0 | 38.1 | 9/9 | A&W |
| 9 | 33 | Yes | Obesity | M | 2960 | 49.0 | 38.1 | 8/9 | A&W |
| 10 | 33 | No | Rheumatoid arthritis | M | 1965 | 43.5 | 36.2 | 8/9 | Low birth weight |
| 11 | 45 | Yes | None | F | 3060 | 50.0 | 38.2 | 8/9 | A&W |
| 12 | 37 | No | None | M | 2835 | 48.0 | 37.2 | 8/9 | Ventriculomegaly |
| 13 | 23 | No | None | M | 2790 | 48.0 | 38.4 | 8/9 | A&W |
| 14 | 37 | No | None | M | 3215 | 49.0 | 39.2 | 8/9 | A&W |
| 15 | 35 | Yes | DM2, hypothyroidism | M | 3505 | 48.0 | 38.0 | 8/9 | A&W |
| 16 | 30 | Yes | None | F | 3305 | 50.0 | 38.1 | 9/9 | A&W |
| 17 | 36 | Yes | Hypothyroidism | M | 3245 | 51.0 | 38.0 | 8/9 | A&W |
| 18 | 37 | No | None | F | 2685 | 48.0 | 39.5 | 8/9 | A&W |
| 19 | 22 | No | None | M | 3020 | 51.0 | 38.6 | 8/9 | A&W |
| 20 | 24 | No | None | F | 3125 | 49.0 | 40.0 | 9/9 | A&W |
| 21 | 33 | No | PAS | F | 2730 | 48.0 | 37.2 | 7/9 | A&W |
| 22 | 43 | No | High BP, obesity | M | 3680 | 50.0 | 38.6 | 8/9 | A&W |
| 23 | 37 | Yes | PAS | F | 3790 | 45.0 | 38.0 | 8/9 | Dysmorphic syndrome |
| 24 | 28 | Yes | DM2 | F | 3225 | 51.0 | 38.4 | 8/9 | A&W |
| 25 | 24 | Yes | None | M | 3165 | 50.5 | 38.5 | 8/9 | A&W |
| 26 | 38 | No | None | M | 3090 | 50.0 | 38.4 | 8/10 | A&W |
| 27 | 32 | Yes | None | M | 3930 | 53.0 | 39.5 | 8/9 | A&W |
| 28 | 23 | Yes | Anorexia, hydronephrosis | F | 3245 | 51.0 | 39.4 | 8/9 | A&W |
| 29 | 28 | Yes | None | M | 3360 | 49.0 | 37.1 | 9/9 | Tuberous sclerosis |
| 30 | 27 | Yes | None | M | 3320 | 52.0 | 37.1 | 8/9 | A&W |
| 31 | 22 | Yes | HIV | F | 3005 | 49.0 | 39.0 | 8/9 | A&W |
| 32 | 24 | No | None | M | 2710 | 49.5 | 40.5 | 8/9 | A&W |
| 33 | 35 | Yes | None | F | 2745 | 46.0 | 38.5 | 8/9 | Complex CHD, NND |
| 34 | 35 | No | Obesity | F | 3525 | 52.5 | 39.4 | 8/9 | A&W |
| 35 | 25 | No | GDM | M | 2985 | 51.0 | 37.6 | 9/9 | A&W |
| 36 | 35 | No | None | M | 2765 | 49.0 | 38.1 | 8/9 | A&W |
| 37 | 33 | No | None | F | 2970 | 49.5 | 39.6 | 8/9 | A&W |
| 38 | 36 | Yes | HIV | M | 3170 | 49.0 | 39.0 | 8/9 | A&W |
| 39 | 37 | Yes | DM2, hypothyroidism, high BP | M | 3690 | 52.0 | 38.3 | 8/9 | A&W |
| 40 | 24 | No | None | M | 2980 | 49.0 | 40.1 | 8/9 | A&W |
| 41 | 29 | No | None | M | 2535 | 48.0 | 40.2 | 8/9 | HLHS, chromosomal abnormality, NND |
| 42 | 26 | Yes | Pyelonephritis, pyelectasis | F | 2905 | 50.0 | 39.5 | 8/9 | A&W |
| Mean ± SD | 30.88 ± 6.09 | — | — | — | 3094.88 ± 382.08 | 49.38 ± 1.99 | 38.52 ± 0.95 | — | — |
A&W, alive and well at discharge; BP, blood pressure; CHD, congenital heart disease; DM1, Type‐1 diabetes mellitus; DM2, Type‐2 diabetes mellitus; F, female; GA, gestational age; GDM, gestational diabetes mellitus; HIV, human immunodeficiency virus; HLHS, hypoplastic left heart syndrome; M, male; NND, neonatal death; PAS, placenta accreta spectrum disorder.
Table 2.
Frequency of COVID‐19‐related symptoms that developed after discharge in 25 SARS‐CoV‐2‐positive pregnant women who were asymptomatic at the time of testing
| Symptom | n (%) |
|---|---|
| Headache | 12 (48) |
| Myalgia | 8 (32) |
| Odynophagia | 6 (24) |
| Chest pain | 5 (20) |
| Rhinorrhea | 5 (20) |
| Anosmia | 5 (20) |
| Arthralgia | 5 (20) |
| Dysgeusia | 5 (20) |
| Dyspnea | 5 (20) |
| Diarrhea | 3 (12) |
| Coughing | 3 (12) |
| Shivering | 3 (12) |
| Fever | 2 (8) |
SARS‐CoV‐2 analysis of neonatal swabs
All 42 neonates were tested for SARS‐CoV‐2 by RT‐PCR of oral and rectal swab samples at birth and 28 were retested at 24 h after birth. Eighteen (42.9%) neonates were positive at birth, 10 (23.8%) showed viral persistence at 24 h after birth, five (11.9%) had a positive test only at 24 h after birth (negative at birth) and six (14.3%) were negative at both timepoints (Figure 1a and Table S1). Of the 14 neonates that were not tested at 24 h after birth, one was positive at birth. SARS‐CoV‐2 RNA amplification was observed more frequently in rectal swab samples than in oral swab samples, regardless of the timing of sampling (Figure 1b,c). Only four neonates were positive for SARS‐CoV‐2 on RT‐PCR of both oral and rectal swabs at birth (Figure 1b), of which three remained positive on RT‐PCR of both oral and rectal swabs at 24 h after birth (Figure 1c).
Figure 1.
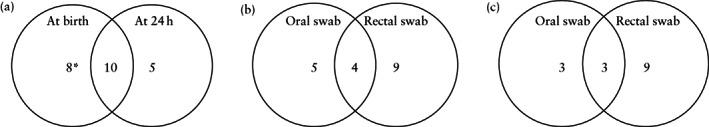
Venn diagrams showing neonatal SARS‐CoV‐2 test results in asymptomatic SARS‐CoV‐2‐positive women. (a) Number of positive neonates at birth and at 24 h after birth. (b,c) Number of positive neonatal oral and rectal swabs at birth (b) and at 24 h after birth (c). *Including one case that did not undergo testing at 24 h.
Vertical transmission according to WHO criteria
In order to identify instances of vertical transmission and classify peripartum transmission according to the classification proposed by Blumberg et al. 12 and modified by the WHO 13 , we analyzed the detection timeline in cases with a positive neonatal SARS‐CoV‐2 test result. The 10 cases with a persistent positive neonatal SARS‐CoV‐2 result at 24 h were considered to correspond to possible intrauterine transmission, of which five (5/42; 11.9%) presented strong evidence of vertical transmission as the AF also tested positive for SARS‐CoV‐2 (Figure 2a and Table S1). Additionally, seven neonates were classified as having transient viremia (unlikely intrauterine transmission) because SARS‐CoV‐2 RNA amplification was not persistent at 24 h after birth (Figure 2b). The five neonates who were negative at birth but positive 24 h after birth were categorized as having early postnatal transmission (intrapartum transmission) (Figure 2c and Table S1). In the one neonate who was positive at birth but in whom a test was not performed at 24 h after birth, the timing of mother‐to‐child transmission was classified as indeterminate.
Figure 2.
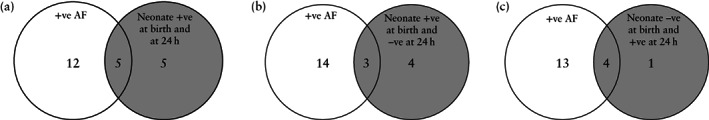
Venn diagrams showing peripartum SARS‐CoV‐2 test results in asymptomatic SARS‐CoV‐2‐positive women, classified according to the World Health Organization criteria 13 for mother‐to‐child SARS‐CoV‐2 transmission: (a) possible intrauterine transmission (n = 10); (b) unlikely intrauterine transmission or transient viremia (n = 7); and (c) intrapartum transmission (n = 5). AF, amniotic fluid.
Antibody analysis after infection
IgG antibodies were detected in 10 (33.3%) of the 30 women who were tested (Figure 3 and Table S1); however, passive immunity was transferred to the neonate in only five (50%) of these cases, of which two were in the possible‐intrauterine‐transmission
Figure 3.
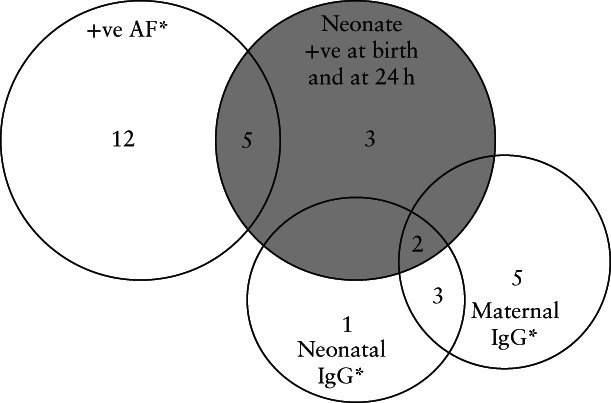
Venn diagram showing peripartum SARS‐CoV‐2 test results and immunoglobulin G (IgG) detection in asymptomatic pregnant women classified according to the World Health Organization criteria for mother‐to‐child SARS‐CoV‐2 transmission as having possible intrauterine transmission (n = 10). Two neonates classified as having possible intrauterine transmission presented passive immunity specific for SARS‐CoV‐2. *Four out of 10 cases with positive maternal IgG had positive amniotic fluid (AF) and two out of six cases with positive neonatal IgG had positive AF, none of which was classified as having possible intrauterine transmission. For simplicity, these intersections are not shown.
group (Figure 3), two tested positive for SARS‐CoV‐2 only at 24 h after birth (not at birth), fulfilling the intrapartum transmission criteria, and one neonate was classified as indeterminate.
Ct values on RT‐PCR as an indicator of viral load
Considering the Ct value as an indicator of the viral load, we compared median maternal Ct values between the five groups, defined according to the results of RT‐PCR of AF samples and neonatal oral and rectal swabs at birth, and found no statistically significant difference (Figure 4). On comparison of median Ct values between maternal nasopharyngeal swabs, neonatal swabs (at birth and at 24 h) and AF samples, the only statistically significant difference observed was between maternal nasopharyngeal swabs and AF samples (P = 0.005) (Figure 5). In addition, we evaluated whether SARS‐CoV‐2 detection in AF samples was associated with the SARS‐CoV‐2 status of the neonate at birth and did not find an association (P > 0.999).
Figure 4.
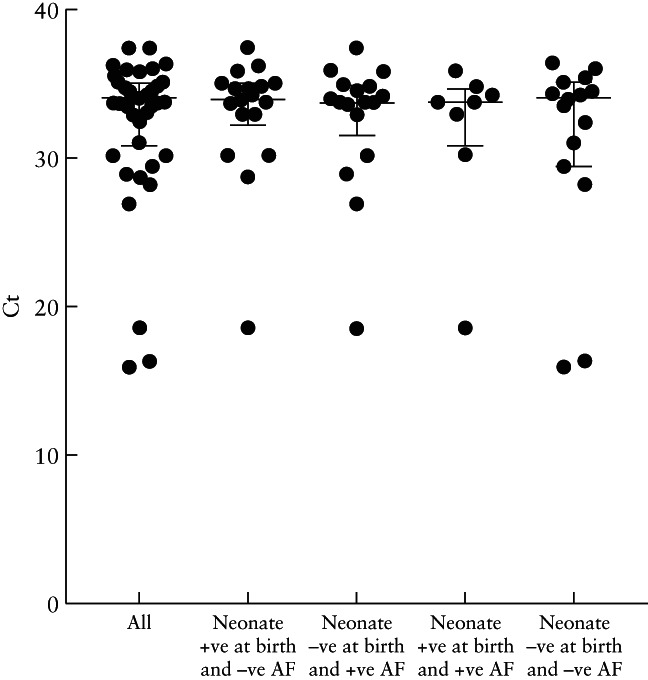
Cycle threshold (Ct) values in asymptomatic SARS‐CoV‐2‐positive pregnant women, overall and in those with a positive neonate at birth and negative amniotic fluid (AF), those with positive AF and a negative neonate at birth, those with a positive neonate at birth and positive AF and those with a negative neonate at birth and negative AF. No significant differences were observed between the groups. Lines represent median and interquartile range.
Figure 5.
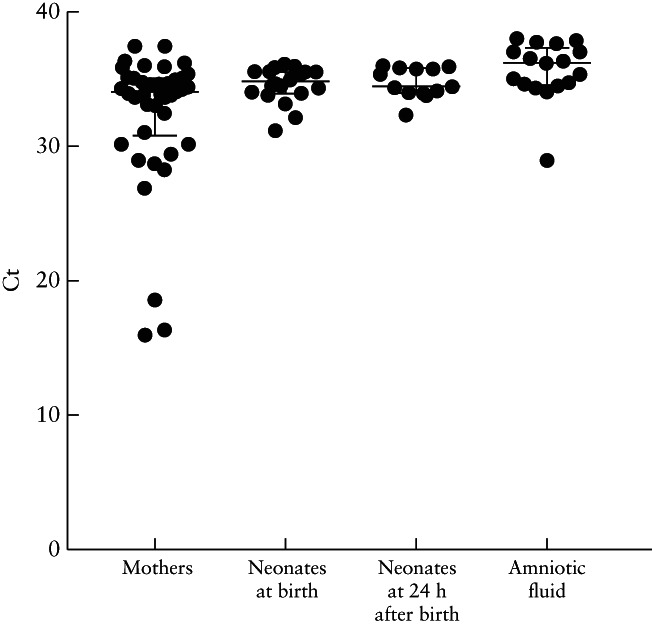
Cycle threshold (Ct) values in SARS‐CoV‐2‐positive samples from asymptomatic pregnant women, their neonates at birth and at 24 h after birth, and amniotic fluid. The only statistically significant difference observed was between positive maternal samples and positive amniotic fluid samples (P = 0.005). Lines represent median and interquartile range.
DISCUSSION
SARS‐CoV‐2 vertical transmission remains controversial, particularly in asymptomatic patients 18 . We studied 42 SARS‐CoV‐2‐positive pregnant women who were asymptomatic for COVID‐19 and their neonates. Our main finding is that five (11.9%) cases had strong evidence of possible vertical transmission according to the WHO criteria for classification of the timing of mother‐to‐child transmission of SARS‐CoV‐2. Additionally, IgG transfer from mother to neonate by passive immunity was observed in 50% of cases in which maternal IgG was detected.
Our findings are in agreement with reports showing that most SARS‐CoV‐2‐positive pregnant women are asymptomatic or have mild disease 19 , 20 , 21 . The most frequently reported symptom in our group after discharge was headache, which has been associated with COVID‐19 22 , but is also a common postpartum symptom. No neonate was found to have COVID‐19 symptoms. A meta‐analysis found only mild symptoms in neonates born to SARS‐CoV‐2‐infected mothers. However, the authors acknowledged that it is difficult to rule out that symptoms could be related to other conditions 23 . We observed three (7.1%) neonatal deaths unrelated to SARS‐CoV‐2. The reason for this relatively high mortality rate is that our institute is a national referral center for high‐risk pregnancies.
Rectal swabs for SARS‐CoV‐2 detection in neonates have been reported on previously 24 . The duration of persistence of the virus in anal/rectal swabs and stools ranges from 5 to 35 days in pediatric patients, suggesting gastrointestinal shedding 25 . Interestingly, we observed a higher proportion of positive rectal swabs than positive oral swabs. Furthermore, 40–60% of infected neonates would have been undetected if only one swab had been tested, which represents a silent transmission risk for other individuals 26 . We propose that neonatal diagnosis of SARS‐CoV‐2 infection should be performed using both rectal and oral swabs, in order to improve the detection rate at birth.
Not all AF samples were positive among SARS‐CoV‐2‐infected women. There are only a few studies reporting cases with a positive AF sample in which the neonate was positive and symptomatic, suggesting vertical transmission 27 , 28 . Nevertheless, the presence of SARS‐CoV‐2 in AF is not sufficient to suggest vertical transmission 13 .
Establishing diagnostic criteria for SARS‐CoV‐2 vertical transmission has been challenging. Based on the strict WHO criteria, we observed five (11.9%) cases that were considered as having strong evidence of possible intrauterine transmission. In these cases, maternal and AF samples tested positive, and the neonate was positive both at birth and at 24 h. One of these neonates had persistent SARS‐CoV‐2 identification for 10 days. Despite the evidence, these five cases remained considered as having only possible vertical transmission, since the new WHO criteria 13 for confirmed intrauterine transmission require viral presence in neonatal sterile samples, which were not collected.
The time needed for SARS‐CoV‐2 to cross the placenta into the AF and cause intrauterine infection remains unknown. For other viruses, such as herpes simplex virus, neonates born to mothers infected from 5 days before to 2 days after delivery might develop infection 29 . Although SARS‐CoV‐2 tests were performed a few days before delivery in this study population, this does not provide information on the timing of maternal infection. Accurate determination of the timing of maternal infection is not always possible, especially in asymptomatic women 30 . Data from animal models show that cytomegalovirus reaches the placenta and infects the fetus 7 and 14 days after infection, respectively 31 . For arboviruses, viral RNA is detected in the placenta and fetus 4 days after infection 32 . Hsu et al. 33 reported the first case of placental infection with SARS‐CoV‐2 in a mildly symptomatic woman with a positive test 2 days before delivery; the neonate was negative, but the virus was detected in the placenta. Some studies have reported the presence of SARS‐CoV‐2 in the placenta of women infected from 4–17 days before delivery 28 , 34 . It is worth mentioning that most of the women remained asymptomatic in this study population, making it difficult to determine the timing of infection. Despite the controversy, evidence supports that SARS‐CoV‐2 can replicate in the placenta and reach the AF; however, the timeline for this event is not clear 35 . Prospective studies are needed to determine the length of time that the virus needs to cross the placenta and infect the fetus.
When IgG was measured in maternal and umbilical cord blood, only 10 (33%) women and five of their neonates tested positive, demonstrating passive immunity transferred from the mother to the child in 50% of these cases. Of these women, eight developed symptoms. Previous reports showed that seroconversion occurs more than 22 days after symptom onset 36 in cases of mild COVID‐19. Unfortunately, we could not follow‐up the mothers or neonates to monitor seroconversion after discharge. It has been proposed that seroconversion should be investigated separately in mild and severe COVID‐19 cases, as IgG determination in mild cases is poor and delayed 37 .
Numerous studies have investigated whether viral load is related to disease severity or infectiousness 17 , with a higher viral load found in critically ill patients compared to asymptomatic patients or those with mild disease 38 . We investigated whether maternal Ct value (as an indicator of viral load) could be a factor that influences intrauterine transmission, even in asymptomatic patients. No differences were observed when assessing maternal Ct values according to whether the neonatal swabs and AF samples were positive or negative. However, Ct values were significantly lower in maternal swabs compared to AF samples, meaning that viral load was higher in the upper respiratory tract than in the AF. As we were unable to demonstrate an association between maternal viral load or AF status and the risk of vertical transmission, other maternal, fetal and environmental factors should be investigated.
A strength of this study is the improved neonatal detection rate of SARS‐CoV‐2 by collecting two different samples. Possible limitations of this study are the inability to determine the timing of maternal infection and lack of assessment of the presence of IgM in the neonates. Moreover, we were unable to test other tissues or sterile neonatal samples.
In conclusion, our data support that intrauterine transmission of SARS‐CoV‐2 is possible even in asymptomatic women. Testing for SARS‐CoV‐2 in both oral and rectal neonatal swabs improves considerably the detection rate, as compared with when only one swab is tested. Studies including a larger number of subjects are necessary to determine the biological mechanisms involved in vertical transmission of SARS‐CoV‐2. This knowledge is critical when dictating health policies for clinical management of women and neonates infected with SARS‐CoV‐2.
Supporting information
Table S1 Classification of SARS‐CoV‐2 vertical transmission based on peripartum reverse‐transcription polymerase chain reaction (RT‐PCR) results and the World Health Organization (WHO) criteria
ACKNOWLEDGMENTS
This work would not have been possible without the effort of every healthcare worker in the COVID‐19 areas at the National Institute of Perinatology, Mexico City, Mexico. We especially thank the nursery team and medical residents Erandi Vela, Mauricio Rejón, Filiberto Martínez, Angélica Pedraza and Alberth Challapa for sample collection, Dr J. Villanueva‐Calleja for technical assistance and the molecular diagnosis team.
This research was funded by the National Institute of Perinatology, Mexico City, Mexico (grant 2020‐1‐32).
Contributor Information
A. C. Helguera‐Repetto, Email: ceciliahelguera@yahoo.com.mx.
Collaborators:
L. D. Gonzalez‐García, C. D. Mora‐Vargas, P. Mateu‐Rogell, M. Rodriguez‐Bosch, I. Coronado‐Zarco, S. Acevedo‐Gallegos, M. Aguinaga‐Ríos, V. H. Ramirez‐Santes, M. A. Ortiz‐Ramirez, M. Valdes‐Flores, and M. Cortes‐Bonilla
DATA AVAILABILITY STATEMENT
Data available in article supplementary material
REFERENCES
- 1. Johns Hopkins University . Johns Hopkins University Coronavirus COVID‐19 Global Cases . https://coronavirus.jhu.edu/map.html.
- 2. Narang K, Enninga EAL, Gunaratne MDSK, Ibirogba ER, Trad ATA, Elrefaei A, Theiler RN, Ruano R, Szymanski LM, Chakraborty R, Garovic VD. SARS‐CoV‐2 Infection and COVID‐19 During Pregnancy: A Multidisciplinary Review. Mayo Clin Proc 2020; 95: 1750–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu H, Wang LL, Zhao SJ, Kwak‐Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID‐19? An immunological viewpoint. J Reprod Immunol 2020; 139: 103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID‐19. Am J Clin Pathol 2020; 154: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mongula JE, Frenken MWE, van Lijnschoten G, Arents NLA, de Wit‐Zuurendonk LD, Schimmel‐de Kok APA, van Runnard Heimel PJ, Porath MM, Goossens SMTA. COVID‐19 during pregnancy: non‐reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet Gynecol 2020; 56: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Facchetti F, Bugatti M, Drera E, Tripodo C, Sartori E, Cancila V, Papaccio M, Castellani R, Casola S, Boniotti MB, Cavadini P, Lavazza A. SARS‐CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine 2020; 59: 102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghema K, Lehlimi M, Toumi H, Badre A, Chemsi M, Habzi A, Benomar S. Outcomes of newborns to mothers with COVID‐19. Infect Dis Now 2021; 51: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, Gismondo MR, Perotti F, Callegari C, Mancon A, Cammarata S, Beretta I, Nebuloni M, Trabattoni D, Clerici M, Savasi V. Analysis of SARS‐CoV‐2 vertical transmission during pregnancy. Nat Commun 2020; 11: 5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettirosso E, Giles M, Cole S, Rees M. COVID‐19 and pregnancy: A review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol 2020; 60: 640–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crovetto F, Crispi F, Llurba E, Figueras F, Gómez‐Roig MD, Gratacós E. Seroprevalence and presentation of SARS‐CoV‐2 in pregnancy. Lancet 2020; 396: 530–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in Infants Born to Mothers With COVID‐19 Pneumonia. JAMA 2020; 323: 1848–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blumberg DA, Underwood MA, Hedriana HL, Lakshminrusimha S. Vertical Transmission of SARS‐CoV‐2: What is the Optimal Definition? Am J Perinatol 2020; 37: 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . COVID‐19 Scientific brief. Definition and categorization of the timing of mother‐to‐child transmission of SARS‐CoV‐2 . https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐mother‐to‐child‐transmission‐2021.1.
- 14. Centers for Disease Prevention and Control . CDC 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel. https://www.fda.gov/media/134922/download.
- 15. National Institutes of Health . COVID‐19 Treatment Guidelines. Clinical Spectrum of SARS‐CoV‐2 Infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum.
- 16. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill 2020; 25: 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Widders A, Broom A, Broom J. SARS‐CoV‐2: The viral shedding vs infectivity dilemma. Infect Dis Health 2020; 25: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Zhou YH, Yang HX, Poon LC. Intrauterine vertical transmission of SARS‐CoV‐2: what we know so far. Ultrasound Obstet Gynecol 2020; 55: 724–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zimmermann P, Curtis N. COVID‐19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr Infect Dis J 2020; 39: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cruz‐Lemini M, Ferriols Perez E, de la Cruz Conty ML, Caño Aguilar A, Encinas Pardilla MB, Prats Rodríguez P, Muner Hernando M, Forcen Acebal L, Pintado Recarte P, MDC Medina Mallen, Perez Perez N, Canet Rodriguez J, Villalba Yarza A, Nieto Velasco O, Del Barrio Fernandez PG, Orizales Lago CM, Marcos Puig B, Muñoz Abellana B, Fuentes Ricoy L, Rodriguez Vicente A, Janeiro Freire MJ, Alferez Alvarez‐Mallo M, Casanova Pedraz C, Alomar Mateu O, Lesmes Heredia C, Wizner de Alva JC, Posadas San Juan A, Macia Badia M, Alvarez Colomo C, Sanchez Muñoz A, Pratcorona Alicart L, Alonso Saiz R, Lopez Rodriguez M, Barbancho Lopez MC, Meca Casbas MR, Vaquerizo Ruiz O, Moran Antolin E, Nuñez Valera MJ, Fernandez Fernandez C, Tubau Navarra A, Cano Garcia AM, Soldevilla Perez S, Gattaca Abasolo I, Adanez Garcia J, Puertas Prieto A, Ostos Serna R, MDP Guadix Martin, Catalina Coello M, Espuelas Malon S, Sainz Bueno JA, Granell Escobar MR, Cruz Melguizo S, Martinez Perez O, on behalf of the Spanish Obstetric Emergency Group . Obstetric Outcomes of SARS‐CoV‐2 Infection in Asymptomatic Pregnant Women. Viruses 2021; 13: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernández‐Cruz RG, Sánchez‐Cobo D, Acevedo‐Gallegos S, Helguera‐Repetto AC, Rodriguez‐Bosch MR, Ramirez‐Santes VH, Villegas‐Mota I, Cardona‐Pérez A, Cortes‐Bonilla M, Irles C, Mateu‐Rogell P, Villanueva‐Calleja J, Villavicencio Carrisoza O, Estrada‐Gutiérrez G, Espino‐Y‐Sosa S, Torres‐Torres J, Martinez‐Portilla RJ. Clinical characteristics and risk factors for SARS‐CoV‐2 infection in pregnant women attending a third level reference center in Mexico City. J Matern Fetal Neonatal Med 2021. DOI: 10.1080/14767058.2021.1902500. [DOI] [PubMed] [Google Scholar]
- 22. Tolebeyan AS, Zhang N, Cooper V, Kuruvilla DE. Headache in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Narrative Review. Headache 2020; 60: 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raschetti R, Vivanti AJ, Vauloup‐Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS‐CoV‐2 infections. Nat Commun 2020; 11: 5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacot D, Greub G, Jaton K, Opota O. Viral load of SARS‐CoV‐2 across patients and compared to other respiratory viruses. Microbes Infect 2020; 22: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kipkorir V, Cheruiyot I, Ngure B, Misiani M, Munguti J. Prolonged SARS‐CoV‐2 RNA detection in anal/rectal swabs and stool specimens in COVID‐19 patients after negative conversion in nasopharyngeal RT‐PCR test. J Med Virol 2020; 92: 2328–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao F, Yang Y, Wang Z, Li L, Liu L, Liu Y. The Time Sequences of Respiratory and Rectal Viral Shedding in Patients With Coronavirus Disease 2019. Gastroenterology 2020; 159: 1158–1160.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID‐19 infection. Prenat Diagn 2020; 40: 1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun 2020; 11: 3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol 2015; 73: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017; 21: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffith BP, McCormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol 1985; 55: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Platt DJ, Smith AM, Arora N, Diamond MS, Coyne CB, Miner JJ. Zika virus‐related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med 2018; 10: eaao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu AL, Guan M, Johannesen E, Stephens AJ, Khaleel N, Kagan N, Tuhlei BC, Wan XF. Placental SARS‐CoV‐2 in a pregnant woman with mild COVID‐19 disease. J Med Virol 2021; 93: 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong L, Pei S, Ren Q, Fu S, Yu L, Chen H, Chen X, Yin M. Evaluation of vertical transmission of SARS‐CoV‐2 in utero: Nine pregnant women and their newborns. Placenta 2021; 111: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouachba A, Allias F, Nadaud B, Massardier J, Mekki Y, Bouscambert Duchamp M, Fourniere B, Huissoud C, Trecourt A, Collardeau‐Frachon S. Placental lesions and SARS‐Cov‐2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta 2021; 112: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marklund E, Leach S, Axelsson H, Nyström K, Norder H, Bemark M, Angeletti D, Lundgren A, Nilsson S, Andersson LM, Yilmaz A, Lindh M, Liljeqvist JÅ, Gisslén M. Serum‐IgG responses to SARS‐CoV‐2 after mild and severe COVID‐19 infection and analysis of IgG non‐responders. PLoS One 2020; 15: e0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu WT, Howell JC, Ozturk T, Benameur K, Bassit LC, Ramonell R, Cashman KS, Pirmohammed S, Roback JD, Marconi VC, Yang I, Mac VV, Smith D, Sanz I, Wharton W, Lee FE, Schinazi RF. Antibody Profiles According to Mild or Severe SARS‐CoV‐2 Infection, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 2020; 26: 2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, Zhang Y, Xu Z, Liu X, Li Y. SARS‐CoV‐2 Viral Load in Clinical Samples from Critically Ill Patients. Am J Respir Crit Care Med 2020; 201: 1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Classification of SARS‐CoV‐2 vertical transmission based on peripartum reverse‐transcription polymerase chain reaction (RT‐PCR) results and the World Health Organization (WHO) criteria
Data Availability Statement
Data available in article supplementary material


