Abstract
Introduction
The various cutaneous manifestations have lately appeared in the setting of COVID‐19. Psoriasis flare‐ups have been reported during a COVID‐19 infection.
Case presentation
We present a case of a 32‐year‐old woman with COVID‐19 who presented with generalized pustular psoriasis. She received oral prednisolone, hydroxyzine, and topical clobetasol. The patient received follow‐up two weeks later and found that her lesions were favorably desquamating.
Methods
The PubMed, SCOPUS, and ISI Web of Science databases were thoroughly searched for English studies reporting psoriasis flare‐ups following SARS‐CoV‐2 infection. Ten case reports/series were included after screening.
Conclusions
Our case report brings awareness to clinicians for the possible cutaneous manifestation of COVID‐19, which should be considered part of the differential diagnoses.
Keywords: Azithromycin, COVID‐19, Hydroxychloroquine, Pustular psoriasis, SARS‐CoV‐2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), 1 has influenced a significant impact on public health, with approximately 175 million confirmed cases and more than 3 million deaths worldwide as of June 12, 2021. 2 Predominant pulmonary characteristics are the hallmark of this infection. Extrapulmonary symptoms have been reported worldwide in patients with COVID‐19, including cardiovascular, 3 central nervous system (CNS), 4 renal, 5 gastrointestinal, 6 and hematologic manifestations. 7 In addition, various cutaneous manifestations have lately appeared in the setting of COVID‐19 due to adverse effects of medications or increased immune response, one of which is the psoriasis flare‐up. 8 , 27 Here, we present an unusual case of pustular psoriasis in a 32‐year‐old woman with a newly diagnosed COVID‐19 infection.
2. CASE REPORT
A 32‐year‐old woman with a positive family history of psoriasis was admitted with progressive annular skin lesions with small sterile pustules overlying painful, erythematous skin and urticarial eruptions, myalgia, and arthralgia for 10 days. She had 2 episodes of such lesion in the past 10 years but was in remission before getting the COVID‐19 infection. The lesions were generalized and involved almost every body part of hers, including face, trunk, upper and lower limbs, forearm, palms, and soles (Figure 1). She reported no fever, dyspnea, and cough and received azithromycin and hydroxychloroquine (HCQ) 40 days earlier for a documented COVID‐19 infection. At the onset of her cutaneous eruptions, she started retaking another course of azithromycin without a prescription.
FIGURE 1.
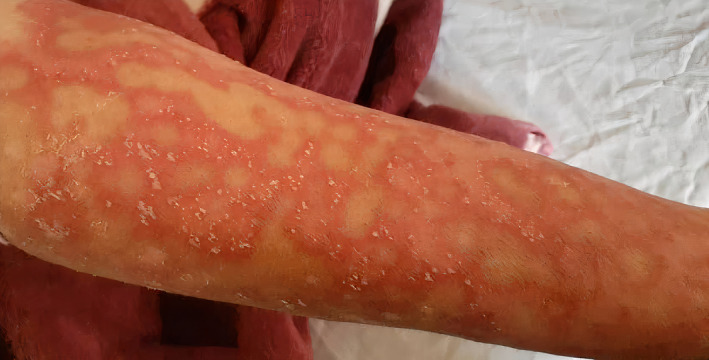
Involvement of lesions in the forearm
Skin examination revealed diffuse tender erythematous plaques with diffuse pustules, scale, and crust formation over 75% of her body. The pustules were at the edges of expanding erythematous plaques or over erythematous skin. Pitting in some fingernails was evident. No lymphadenopathy or organomegaly was detected. Initial laboratory tests were not significant except for a neutrophilia (white blood cell (WBC) count, 16,800/µL, 7% lymphocytes, and 90% neutrophils) [Normal range: 4,000–10,000], and elevated inflammatory markers: lactate dehydrogenase (LDH), 815 U/L [Normal range: <480]; C‐reactive protein (CRP), 89 mg/L [Normal range: <10]; and erythrocyte sedimentation rate (ESR), 50 mm/h [Normal range: <20]. The reverse transcriptase‐polymerase chain reaction (RT‐PCR) of the nasopharyngeal swab tested for SARS‐CoV‐2 was positive, and the lung computed tomography (CT) scan was indicative of COVID‐19. Although drug eruption, especially acute generalized exanthematous pustulosis (AGEP), was the most probable diagnosis, due to constitutional symptoms and elevated inflammatory markers, rheumatologic and immunologic disorders were also suspected regarding her age. Therefore, comprehensive diagnostic workup, including antinuclear (ANA), anti‐double‐stranded DNA (anti‐dsDNA), anti‐Smith (anti‐Sm), anti‐ribonuclear protein (anti‐RNP), and anti‐Ro/La, anti‐histone (AHAs) antibodies, and HLA‐B27 were performed, with all were negative.
Acute retroviral syndrome and serum sickness were also among the differential diagnoses, which were ruled out by negative test results of hepatitis B (HBV), hepatitis C (HCV), and human immunodeficiency viruses (HIV). According to the consultant dermatologist, oral prednisolone 50 mg daily, cyclosporine 200 mg daily in divided doses, topical clobetasol 0.05% were started for the patient, and a skin biopsy was suggested. Histologic examination demonstrated skin tissue with intermittent parakeratosis and exaggerated spongiform pustules of Kogoj and sub‐corneal micro‐abscesses of Munro, epidermis with mild acanthosis, and neutrophilic exocytosis. Moreover, the upper dermis showed perivascular lymphocytes and neutrophils whiteout eosinophils, which were compatible with pustular psoriasis. The patient was then recommended to be followed up as an outpatient. At her 2‐week follow‐up, her lesions began to desquamate, and she was in a more favorable condition toward healing at this time.
3. DISCUSSION
Psoriasis is a cutaneous disorder triggered by various factors, such as medications and infections. Table 1 summarized previous reported cases of psoriasis associated with COVID‐19. This pandemic can increase the psoriasis cases by limiting patients’ access to required healthcare settings in many countries, 9 increasing the emotional stress in patients, which itself could act as a catalyst for the onset and aggravation of psoriasis. 10 , 11 Thus, maximizing the psychosocial support available to patients with this condition during the current pandemic may significantly influence the disease activity. 12 Moreover, SARS‐CoV‐2 infection causes a hyperinflammatory response in patients, often known as COVID‐19 cytokine storm syndrome (COVID‐CSS). 13 Thus, a surge in the secretion of pro‐inflammatory cytokines, such as tumor necrosis factor‐α (TNF‐α), interleukin‐1α/β (IL‐1α/β), 13 and IL‐17, 14 which are all upregulated in the COVID‐CSS, can be another pathophysiology of psoriasis in these patients. 12 , 15 Another important etiology of this condition in COVID‐19 patients is believed to be mediated via COVID‐19‐related medications. 12
TABLE 1.
Summary of the reported cases of psoriasis in patients with COVID‐19
| Reference | Age (years) | Gender | Previous psoriasis type | Current psoriasis type | Location in involvement | Other potential psoriasis exacerbation factors | Treatment for psoriasis |
|---|---|---|---|---|---|---|---|
| Carugno et al. 13 | 69 | Male | Plaque psoriasis | Plaque psoriasis | Trunk, back, and extremities | ‒ | Conservative management |
| Gananandan et al. 14 | 38 | Male | Plaque psoriasis | Guttate psoriasis | Lateral aspect of the right ankle | ‒ | Betamethasone 0.025% cream twice daily |
| Ghalamkarpour et al. 15 | 45 | Male | Psoriatic erythroderma | Psoriatic erythroderma | Generalized | ‒ | Initially acitretin 35 mg daily, later cyclosporine A 100 mg twice a day combined with oral prednisolone 10 mg daily |
| Kutlu et al. 12 | 71 | Female | Plaque psoriasis | Silver‐scaled plaque psoriasis | Generalized | HCQ administration for COVID−19 | Not reported |
| Mathieu et al. 16 | 62 | Female | None | Pustular psoriasis | Scalp, trunk, and extremities | ‒ | Triamcinolone 0.1% ointment |
| Nasiri et al. 8 | 73 | Male | Plaque psoriasis | Plaque psoriasis | Not reported | HCQ administration for COVID−19 | Cyclosporine A 100 mg daily |
| Ozaras et al. 17 | 48 | Female | Plaque psoriasis | Plaque psoriasis | Scalp, trunk, and extremities | HCQ administration for COVID−19 | No specific treatment as the lesions resolved spontaneously |
| Samotij et al. 18 | 72 | Female | Acrodermatitis continua of Hallopeau | Generalized pustular psoriasis | Generalized | ‒ | Infliximab 5 mg/kg and acitretin 35 mg daily |
| Shahidi Dadras et al. 19 | 60 | Male | Plaque psoriasis | Pustular psoriasis | Generalized | HCQ administration for COVID−19 | Acitretin 25 mg daily and oral prednisolone |
| Shakoei et al. 20 | 47 | Female | Pustular psoriasis | Pustular psoriasis | Trunk and extremities | HCQ administration for COVID−19 | Not reported |
Abbreviation: HCQ, Hydroxychloroquine.
Antimalarial agents were the most widely used drugs in COVID‐19 treatment early in the pandemic. The cutaneous side effects of such drugs, such as HCQ, include skin eruptions such as xerosis, generalized pustulosis, hives, and most important of all, psoriasis exacerbation. 16 The exacerbation of psoriasis by chloroquine (CQ) occurs more frequently than HCQ. Other factors responsible for this complication include drug dosage, duration, and underlying photosensitivity conditions. Sometimes, cutaneous involvements are not typical for psoriasis. Therefore, they may be mentioned as psoriasiform. The difference is the less erythema and scaling of psoriasiform lesions and sparing of knees and elbows than true psoriasis. 17 HCQ is also a well‐known triggering factor in psoriasis flare‐ups. The underlying mechanism contributing to this condition is the inhibitory effect of HCQ on epidermal trans‐glutaminase, leading to the epidermal cells collection and its promotion effects on interleukin‐17 (IL‐17) production resulting in the overgrowth of keratinocytes. 18 , 19 These eruptions could be attributed to azithromycin and HCQ, considering our patient's drug history. Azithromycin has been shown to cause some cutaneous features, such as acne, diffuse red or purple skin eruptions, blistering, rosacea, drug rash with eosinophilia, systemic symptoms (DRESS) syndrome, and psoriasis‐like patches. 16
Nevertheless, our patient developed the eruptions at least one month post‐drug consumption, making azithromycin unlikely to be responsible for this event. On the other hand, HCQ might be responsible for various types of skin involvement. The most common cutaneous side effects of this agent have been listed to be drug eruption or rash, skin discoloration, and pruritus, while the most critical ones include Stevens‐Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN). 20 In addition, psoriasis exacerbations and exfoliating dermatitis are the most prevalent cutaneous lesions observed recently, perhaps due to the widespread use of HCQ along with COVID‐19 epidemics. Pre‐existing psoriasis flare‐up appears to occur most commonly, but there have been cases such as the current patient presenting unmasking new cutaneous disease. In addition, some studies reported that the median period between newly consumed HCQ and the first appearance of an eruption is 1–2 weeks. 21 Thus, the appearance of rashes at least one month after discontinuation of HCQ in the current case is rare.
Other causes of new skin eruptions in a young female should have been ruled out, such as connective tissue disorders, paraneoplastic syndromes, and sexually transmitted disorders. However, this patient's diagnostic challenge was present due to her age and accompanying symptoms such as myalgia and arthralgia. In a woman's fourth decade of life, rheumatologic disorders such as systemic lupus erythematosus (SLE) should be the first differential diagnosis. It is important to note, due to suspicion of psoriasis, its type should be considered, such as a drug‐induced pustular type, Von Zumbusch type of generalized pustular psoriasis (GPP), the idiopathic annular type, and HCQ‐induced type. The vital issue is that usually, a pustular drug eruption subsides spontaneously or responds to corticosteroids. Our patient did partially respond to this first‐line therapy, but eruptions reappeared immediately after treatment cessation. Because of the relatively long period between drug consumption and skin eruption onset, pustular psoriasis induced by HCQ seems unlikely. The von Zumbusch type of generalized pustular psoriasis manifests by extensive pustules on erythematous skin, leading to diffuse scaling after rupture. 22 It is usually accompanied by fever and burning feeling, and if septicemia occurs, it may result in death. Therefore, the von Zumbusch type of generalized pustular psoriasis or the idiopathic annular type might have happened. Besides, in any psoriasis‐like lesion, subacute cutaneous lupus erythematosus (SCLE) is yet a significant impression. The primary diagnostic markers for this disorder are Anti‐Ro ⁄SSA antibodies, both of which were negative in our patient.
In adults, first‐line treatment options for pustular psoriasis include systemic retinoids (acitretin), cyclosporine, and methotrexate. Although retinoids have the highest efficacy among the first‐line treatment options, they have a high‐risk potential for severe teratogenic effects that may last up to 3 years. 23 Hence, they are not good options in women of childbearing ages except in life‐threatening situations. On the contrary, cyclosporine is a highly effective treatment for the severe manifestations of psoriasis with a relatively quick onset of action with improvement observed as early as 2 weeks from the initiation of treatment. 24 As there was some earlier evidence available in the literature about the effect of cyclosporine on coronaviruses replication in vitro, we hypothesize that the patient may benefit from its antiviral effects. Also, she will probably be at a lower risk of developing severe symptoms related to COVID‐19. On the other hand, methotrexate has a slower onset of action and potential hepatotoxicity and hematologic toxicity. 25 Thus, our recommendation is concomitant use of cyclosporine with prednisolone, but the patient did not continue therapy in the hospital.
The last important issue is that some infectious agents are documented to be responsible for psoriasis exacerbations, such as HCV, rhinovirus, and coronavirus. However, other viruses such as Epstein‐Barr Virus (EBV) have been precipitating factors for other skin disorders such as pustular DRESS Syndrome. 26 Therefore, the potential of any infection to induce psoriasis, particularly the significant role of COVID‐19 in giving rise to the pustular form, should not be neglected. The reason might be the inflammatory cytokine release, which is the main factor in this dermatologic condition's pathogenesis, and the immunologic reactions induced by SARS‐CoV2. The current report will bring awareness to several cutaneous presentations of COVID‐19, allowing clinicians and researchers to confirm this issue further.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Ronak Miladi and Alireza Janbakhsh have given substantial contributions to the conception and the design of the manuscript. Arefeh Babazadeh, Zeinab Aryanian, Soheil Ebrahimpour, and Zeinab Mohseni Afshar contributed to the data's acquisition, analysis, and interpretation. All authors have participated in drafting the manuscript. Mohammad Barary, Terence T. Sio, Uwe Wollina, and Mohammad Goldust critically revised the first draft of the manuscript and contributed to the analysis and interpretation of the clinical data. All authors read and approved the final version of the manuscript.
ETHICAL STATEMENT
As required by the Kermanshah University of Medical Sciences ethics committee, an informed consent form was taken from the patient to report the case.
Miladi R, Janbakhsh A, Babazadeh A, et al. Pustular psoriasis flare‐up in a patient with COVID‐19. J Cosmet Dermatol. 2021;20:3364–3368. 10.1111/jocd.14508
Funding information
None
[Correction added on October 14, 2021 after first online publication: The institution referred to in the Ethical Statement has been modified]
Contributor Information
Soheil Ebrahimpour, Email: drsoheil1503@yahoo.com.
Mohammad Barary, Email: m.barary@mubabol.ac.ir.
Zeinab Mohseni Afshar, Email: baboldr2019@gmail.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Wollina U, Karadağ AS, Rowland‐Payne C, et al. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020;33:e13549. 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (2021) WHO Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/
- 3. Afshar ZM, Babazadeh A, Javanian M, et al. A review of cardiac involvement in COVID‐19 infection. Cor et Vasa. 2020;62:610–615. 10.33678/cor.2020.102 [DOI] [Google Scholar]
- 4. Mohseni Afshar Z, Babazadeh A, Alizadeh Khatir A, et al. Neurological manifestations in COVID‐19: an overview. Minerva Respiratory Medicine. 2021;60. 10.23736/s2784-8477.20.01895-7 [DOI] [Google Scholar]
- 5. Armaly Z, Kinaneh S, Skorecki K. Renal Manifestations of Covid‐19: Physiology and Pathophysiology. J Clin Med. 2021;10:1216. 10.3390/jcm10061216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kariyawasam JC, Jayarajah U, Riza R, et al. Gastrointestinal manifestations in COVID‐19. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2021;trab042. 10.1093/trstmh/trab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahi MS, Jindal V, Reyes S‐P, et al. Hematologic disorders associated with COVID‐19: a review. Ann Hematol. 2021;100:309‐320. 10.1007/s00277-020-04366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nasiri S, Araghi F, Tabary M, et al. A challenging case of psoriasis flare‐up after COVID‐19 infection. J Dermatolog Treat. 2020;31:448‐449. 10.1080/09546634.2020.1764904 [DOI] [PubMed] [Google Scholar]
- 9. Kutlu Ö, Güneş R, Coerdt K, et al. The effect of the “stay‐at‐home” policy on requests for dermatology outpatient clinic visits after the COVID‐19 outbreak. Dermatol Ther. 2020;33:e13581. 10.1111/dth.13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sathyanarayana Rao T, Basavaraj K, Das K. Psychosomatic paradigms in psoriasis: Psoriasis, stress and mental health. Indian J Psychiatry. 2013;55:313. 10.4103/0019-5545.120531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kutlu Ö, Metin A. Dermatological diseases presented before COVID‐19: Are patients with psoriasis and superficial fungal infections more vulnerable to the COVID‐19? Dermatol Ther. 2020;33:e13509. 10.1111/dth.13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elmas ÖF, Demirbaş A, Kutlu Ö, et al. Psoriasis and COVID‐19: A narrative review with treatment considerations. Dermatol Ther. 2020;33:e13858. 10.1111/dth.13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the Cytokine Storm’ in COVID‐19. J Infect. 2020;80:607‐613. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shibabaw T. Inflammatory cytokine: Il‐17a signaling pathway in patients present with covid‐19 and current treatment strategy. J Inflamm Res. 2020;13:673‐680. 10.2147/JIR.S278335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342‐350. 10.1016/j.cyto.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Türsen Ü, Türsen B, Lotti T. Cutaneous sıde‐effects of the potential COVID‐19 drugs. Dermatol Ther. 2020;33:e13476. 10.1111/dth.13476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gencler OS, Gencler B, Altunel CT, Arslan N. Levetiracetam induced psoriasiform drug eruption: a rare case report. Saudi Pharm J. 2015;23:720‐722. 10.1016/j.jsps.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Öncü INS, Güler D, Gürel G. Exacerbation of psoriasis following hydroxychloroquine in a patient with suspected COVID‐19. Dermatol Ther. 2021;34:e14806. 10.1111/dth.14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kutlu Ö, Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID‐19: Will cases of psoriasis increase after COVID‐19 pandemic? Dermatol Ther. 2020;33:e13383. 10.1111/dth.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davoodi L, Jafarpour H, Kazeminejad A, et al. Hydroxychloroquine‐induced Stevens–Johnson syndrome in COVID‐19: a rare case report. Oxford Medical Case Reports. 2020;2020. 10.1093/omcr/omaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salido M, Joven B, D’Cruz DP, et al. Increased cutaneous reactions to hydroxychloroquine (Plaquenil) possibly associated with formulation change: Comment on the letter by Alarcón. Arthritis Rheum. 2002;46:3392‐3396. 10.1002/art.10565 [DOI] [PubMed] [Google Scholar]
- 22. Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405‐425. 10.1016/j.det.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919. 10.1080/1744666X.2019.1648209 [DOI] [PubMed] [Google Scholar]
- 24. Rudnicka L, Glowacka P, Goldust M, et al. Cyclosporine therapy during the COVID‐19 pandemic. J Am Acad Dermatol. 2020;83:e151‐e152. 10.1016/j.jaad.2020.04.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boehner A, Navarini AA, Eyerich K. Generalized pustular psoriasis ‐ a model disease for specific targeted immunotherapy, systematic review. Exp Dermatol. 2018;27:1067‐1077. 10.1111/exd.13699 [DOI] [PubMed] [Google Scholar]
- 26. Jörg L, Helbling A, Yerly D, Pichler WJ. Drug‐related relapses in drug reaction with eosinophilia and systemic symptoms (DRESS). Clin Transl Allergy. 2020;10:52. 10.1186/s13601-020-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohseni Afshar Z, Babazadeh A, Hasanpour A, et al. Dermatological manifestations associated with COVID‐19: A comprehensive review of the current knowledge. Journal of Medical Virology. 2021;93:5756–5767. 10.1002/jmv.27187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


