Abstract
The WTO was formed in 1995 and since then countries have abide by Trade Related Aspects of Intellectual Property Rights (TRIPS). The agreement provides for comprehensive plan for patenting and protection including those of medical supply units including vaccines and diagnosis. Recently developing countries such as India and South Africa have demanded TRIPS waiver for access to vaccines for all the developing countries The TRIPS waiver demanded, would apply to vaccines, diagnosis, and treatment related to COVID‐19. The waiver is important as it would allow member state in researching, manufacturing, and supplying of vaccines. The proposal by the developing countries for temporary waiver of IP rights argues that IP could impede the supply of COVID‐19 drugs and vaccines. However, there is no near consensus as most of the developed countries opposed this stance and they even argue that waiving TRIPS is not going to ramp up the manufacturing process. The pharmaceutical industry is also against this stance of developing countries, they put their argument forward that waiving of the IP will inhibit research and development of future prospects.
Keywords: developing countries, public Health, TRIPS, vaccines, waiver
1. BACKGROUND
Patents are the exclusive right over the intangible creation of human mind. 1 The rules regarding patent is that it must be available for any invention or process. The owner of the patent exempted others from use of making, using or selling of his/her invention up to the period of 20 years. The first step in introduction of patents is the successful completion of the Uruguay round in 1994. The Trade Related Aspects of Intellectual Property Rights (TRIPS) agreement is the first multilateral treaty to set out the core criteria for patentable subject matter. 2 The governments of developed countries such as United States were strongly promoting global harmonization of intellectual property (IP) as against the strong opposition from developing countries. 3 There was a demand of compulsory extension for patent protection of pharmaceutical products with serious effects on domestic pharmaceutical industries. Nevertheless, the demand for a stronger and more internationally harmonised intellectual property rights (IPRs) lead to the creation of TRIPS which is considered to be the most ambitious IPRs agreement in the history. 4 Article 7 of the TRIPS agreement states the objective of the protection of IPRs in terms of balance of rights and obligations. 5 Article 8 states that WTO member countries may adopt measures necessary for protection of public health provided that such measures are consistent with the TRIPS agreement. 6 The implementation of these measures requires a balance between international and national needs of the country. The Doha declaration (adopted in WTO ministerial conference 2001) provides the objectives mentioned in Article 7 of the agreement. It confirms the objectives of the protection of IPRs with the public health policy. There is no universal way to regard patent it varies from country to country.
The need to introduce patent protection as in 2005 with subsequent protection for 20 years these altered the market‐based conditions for pharmaceuticals in international trade. 7 Under Article IX.3 of the Marrakesh Agreement provides that the conditions given in exceptional circumstances only can ministerial conference waive the conditions imposed on WTO member country. 8 The IPRs system provided under TRIPS that guarantee to companies protection which includes time limited monopoly on marketing of their products. 9 TRIPS gives autonomy to parties as can be seen from Article 1 of the agreement which states that members are free to determine their own appropriate method for implementing the provision within their own domain of legal system. 10 The objective of TRIPS agreement is to liberalize international trade and providing protection to IPRs. 11 As it can be deduced from Article 8 that it purports to the balance between the private and public right in protecting public health and nutrition and also economic and technological development. To sum up it can be said that IP provides for the creation and distribution of innovation and their product provides interest in increasing the number of commercially and serving public interest.
In the wake of COVID‐19 epidemic, India and South Africa asked for the waiver of IPRs provided under the WTO.
1.1. Relationship between TRIPS and access to public health
The Doha declaration recognises the “gravity of the public health problems afflicting many developing and least developed countries, especially those resulting from the HIV/AIDS, Malaria, Tuberculosis and other epidemics.” 12 It was stated within the declaration that this agreement should be act as a means to address both national and international problems occurring as public health problems. It is clearly to be derived from the declaration that the public health supreme and should not prevent members from taking steps to uphold the rights of public health. The agreement should be interpreted in a manner that it provides more leverage to the public health.
Doha declaration provides for Article 31 para 5(b) “states that each WTO member has a right to grant compulsory license and freedom upon which such licenses are granted.” 13 Compulsory licensing is defined as a license that is issued by the government as an authorization to an applicant for making, using or selling a patented product. 14 This acts as an exception to the general principle of TRIPS and through this the waiver can be granted to the respective countries. Article 30 and 31 of the TRIPS agreement stipulates and enable the WTO members to use the subject matter of patent when the public interest is involved. 15 In absence of voluntary licensing, compulsory licensing will significantly reduce the cost of generic medicine and lead to better access of COVID‐19 drugs. The agreement only provides the grounds on which compulsory licensing is given such as in extreme emergency and public noncommercial use. Another condition mentioned is where to supply the “domestic market.” 16
Paragraph 6 of the Doha declaration has its genesis in a proposal submitted by developing countries requesting an interpretation of Article 30 of TRIPS agreement to permit member countries who does not have resources to export and manufacture of the patented medicine to the third parties. This could ensure affordable and access to not only COVID‐19 vaccines but also all the products such as diagnostic kits. 17 Paragraph 6 provides for two temporary kinds of waiver: under the first kind a patent holder must be compensated for compulsory licensing in the importing country and the second there must be a strict national legislation on the patent. As far as the eligibility is concerned, WTO least developed countries (LDCs) are automatically considered in the imports, whereas non‐LDCs have to submit two notifications to TRIPS council. For the non‐LDCs the justification needs to be provided as the “insufficient or no manufacturing capacity.” 18
The patenting of public‐related inventions involved economic and social challenges. The pharmaceutical companies claim that money obtained through patent is used in research and development. However, access to the drugs are protected through patent often are no cost effective and are high in price. 19 There has always been a conflict with regard to access to public health and the patent of the product.
1.2. The stance of developing countries
The classification into three tier system of so called developed, developing, and least developed is a result of multilateralism. The system of institutionalisation provides a discussion or rather a conflict resolution system in order for nations to function properly without any glitch. For developed countries primarily institutional setting helps them in enforcing collective arguments and cost reduction. 20 As for developing countries one advantage could be reduction of uncertainty, the knowledge of what can be the type of behaviour is accepted. Special and differential treatment that finds throughout WTO law, the preamble itself provides for the special treatment of developing countries. The objective of TRIPS must be understood in accordance in overall objectives of WTO relating to developing countries. 21 Developed nations with greater capital and resources tend to invent more things and have greater degree of patent protection. Developing countries however with less resources and capital lacks domestic political stance for patent protection. 22
The patent precondition are given in TRIPS Article 27–30 which includes that it must be accessible by everyone. The advantage to the least developed countries were given in form that they can delay it to five years after its entry in force as they were not be able to make available all the provisions due to lack of infrastructure. However, an exception is provided under Article 27.2 where members are excluded from patentability of invention for larger public interests to protect human health. 23 Another area of concern for the developed countries is to use without authorization of right holder and is known as compulsory license. 24 This is what developing countries such as India and South Africa is pushing forward. In order for successful implementation of Article 31 there must be completion of certain conditions such as what is the reasonable amount of time to negotiate from right holder and use must be predominantly to domestic markets, what does a national emergency mean, can COVID‐19 be considered as a national emergency.
1.2.1. Lesson from AIDS epidemic
Through the late 1990s the developing countries were struggling to meet their obligations under TRIPS. The treatment of AIDS comes as a result of active containment of it in developed countries that paves as a model for developing countries. 25 Drugs such as antiretrovirals (ARVs) make treatment of disease possible and reduce its spread. The two main constraints in containing HIV virus would be high prices of the drugs and its availability require system with adequate healthcare facilities. 26 As we can see from the example of Brazil that to obtain the required medicine it has to spend 36% of the amount went out for purchase of two or three patented drugs. Even after procurement of drugs some developing and least developed countries still lacks the required health infrastructure to help in effecting efficient drug delivery system. This is exactly what happens in countries like India and South Africa during COVID‐19 outbreak the vaccine procurement system is very low. Only 2% of Indian population has received full vaccination by now which is very low as compared to most developed countries.
In 1999, the prices of drugs of AIDS were very high for developing countries, this being said the citizens of developed countries were able to afford the treatment for AIDS because of their push to medical insurance coverage. The developing countries were lagging way behind and the annual costs for those who are able to afford is about $10,000. 27 The important issue is not whether drug is affordable but whether the supply is stable. HIV is a disease that requires treatment throughout life and in order that drug to be available the supply must be intact. To control any epidemic the important step is to invest in R&D and health infrastructure.
During HIV epidemic in Africa an NGO based in South Africa sued it government over the decision of not allowing cipla an Indian Pharmaceutical company to produce and sell cheaper generics. 28 The costs were reduced significantly to about 97% and this in turn helps in controlling the epidemic. 29 These investments need a substantial amount of resource marshalling from training of healthcare workers, building treatment facilitate and they require commitment and great spending. 30 The question that needs to be addressed is that how developing countries is going to ensure affordability availability at the same time.
1.2.2. Access to medicine
There has been a unimpeded demand of supply of vaccines in recent years. The system of patent provide a leverage for introduction of pharmaceutical products by encouraging research and development. The development of a vaccine is a composite and expensive process and indicating that a wide variety of process might not reach the market. 31 The possibility of patent protection provides momentum for innovation, however a powerful patent system does not always act as sufficient when it comes to availability of medicine or vaccines. If the purchasing power needed does not justify an investment in newly made vaccines or medicines, then private industry does not invest on its own. As a result, when the disease aggravated more in developing country than the developed one, the free market does not support development of new vaccine even with patent protection.
The European Commission even claims that IP is not an impediment to scale up production, even suggesting that it would not immediately speeds up production. 32 It reminds the engagement in making of COVAX leading to promote equitable access to the vaccines. 33 However, if we see the facts the vaccine making facility of COVAX are itself in shortage of 190 million doses. The goal to promote COVAX was ramping up production and providing 20% of the population of with the vaccine but due to shortage of doses the future seems dim. The European Commission should not forget that it is their fiscal incentives that was put up in research for making vaccines and it should not be given away to make profits by pharmaceutical companies.
The role of public‐private partnership is to increase the area of research and development targeted the epidemic. There are basically like four steps in any medicine or vaccine development: basic research, preclinical research, clinical research, and post marketing. 34 The void between these steps needs to be filled either by private investment which can either by product patent. This will help in bridging the gap and make the medicine more accessible to the public. To start at the initial stage of research it is mostly funded by the government and it is clear that developed nations are able to invest more compared to developing countries. Under this regime international organizations such as WHO plays an important role when it is about the distribution and making of vaccines. And the thing that helps in bridging the gap and make the manufacture of vaccines faster is through the Intellectual Property Protection. When the gap does not fill up it is usually due to demand by a large population for the vaccines, the purchasing power reduces and as a result there is vaccine shortage. This can be very well be visible from the scenario that is happening in major parts of world particularly including developing and least developed countries. A global research report by United Nations Conference on Trade and Development (UNCTAD) displays that after the recovery of global medical manufacturing a very small fraction of medical equipment such as oxygen respirators, protective personal equipment, disinfectant are available to lower income countries. 35 Waiver of TRIPS would certainly help up in increase of production and supply. This down trend of vaccine shortage is clearly depicted in the figure below:
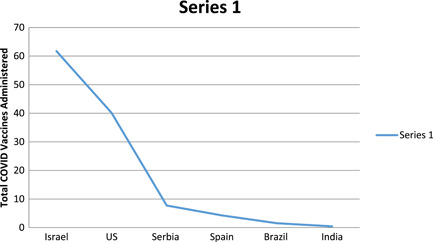
Source: Vaccinating the world: Amidst global shortages, India tries to strike a balance, Observer Research Foundation. [Color figure can be viewed at wileyonlinelibrary.com]
Public‐partnership is an important way for development of new vaccines and it attempts to amalgamate the system of both public and private sector into one single entity. This helps in accessing medicines for all regardless of developing or developed countries status. This must not only be seen as a promoter that will only benefit public sector rather an entity that is advantageous to private sector as well. The private industry can benefit from this partnership through better public relations, data, training and introduction to new markets. 36
IP can be managed both by public and private entities to promote development of new medicines. They provide an incentive for innovation and research into developing the medicines. When the consumers lacks the purchasing power no matter what size of their population is the public sector or charitable institutions must bridge the gap in purchasing power. 37 The private sector should provide the required capital and the labour required for the shift from basic research to medicine available on stocks. Finally, the access to developing countries should not be defined by the size of their financial pledges.
1.3. Implications for pharmaceutical companies
Some big pharmaceutical companies stated that if patent waiver happens on the demand of developing countries it will set as a dangerous precedent. 38 Pharmaceutical industry is often seen as a profit making system and more interested in the users of their product. 39 The question with regard to vaccine is that whether it is to be treated as a publicly funded business property or public goods accessible to all. The first argument put by these big pharmaceutical is that TRIPS waiver would have negative impact on the area of research and development. According to them for a pharmaceutical company to work it requires research and innovation based on long term commitments and resources. 40 Putting future medical research by waiving IP to jeopardy as they claim but it is to be reiterated that IP is a social product and the states have obligation to prevent high costs by such companies. Some people contended that the pharmaceutical can maximize its profits with the control on patent and other IPRs.
Another argument put forward that this would lead to more confusion both in public and private partners as some pharmaceutical firms are public‐ private partnership based. This would in turn leads to strained supply and even escalation of counterfeit vaccines. 41 The global manufacturers are already collaborating and working to increase production targets. Their shared goal is to vaccinate people effectively and is available to all. There would be a shortage of raw materials, quality control and distribution if TRIPS are waived as a lot of vaccines would be in demand. 42 The making of vaccines requires a lot of efforts and investment and years of research putting pressure on firms to produce them in outstanding number of supply will not help in maintaining the goal of accessibility. The quality of those vaccines would not be of up to standard as there will be less time left for quality assurance. This would rather be seen as a symbolic gesture from rich nations to show ethical approach in helping developing countries. IPR is said to become a tool impeding diagnosis, treatment and medical equipment. This can be seen as what happened during the AIDS epidemic in South Africa, the lack of the medicines was one of the main concerns against the pharmaceutical companies who were just busy making their profits. Physicians' group of Medicine Sans Frontieres (MSF) has compiled several cases of COVID‐19 prevention, diagnosis and treatment including threats of patents violation by patent holders against producer of 3D printed valve of ventilator. 43
The third argument would be there are already flexibilities expressed in TRIPS agreement. These are provided in the form of compulsory licensing and freedom to use parallel imports. These help any country be it developing or developed in having access to medicines. Certain Free Trade agreements are already being entered among various countries which prevent already patented medicine to not being in used again known as patent linkage. 44 Compulsory licensing is not effective in the case where countries are facing a huge amount of vaccine shortages. The present system of flexibilities is not able to support the present pandemic. Some report by MSF even points out that flexibilities of compulsory licensing are even time consuming and burdensome.
2. CONCLUSION
The waiver of TRIPS of COVID‐19 vaccine is a strong and effective commitment demanded by developing countries. This paper tried to analyse the impact of waiver on developing countries and pharmaceutical industries, and how does the dream of access to vaccine by all be achieved. The TRIPS when its first came into being the developing countries were far behind their assigned goal to comply with the norm. This is due to lack of fiscal and other resources, the unprecedented pandemic has further crippled it. There is a heavy shortage of vaccine not in just developing countries but other nations also. The relationship between TRIPS and public health is not a new one as AIDS pandemic is evident of that. But how TRIPS is to be governed in the individual nation still depends on its domestic law. The national legislation held as a guide to steer the provisions of TRIPS in any direction where one nation wants. There is a lack of manufacturing capacity in these developing countries the flexibilities provided in TRIPS regulation are not enough to increase the manufacturing capacity. The problem is not only of manufacturing but also of the prices fixed by the respective pharmaceutical companies. The access to vaccine is a social right that should be exercised by nations together. The TRIPS waiver helps in better cooperation and coordination between the nations and which further helps in research and development. The UN high level panel on access to medicine give instructions to governments to refrain from any activity that includes strategies or tactics that undermines the right of WTO members to use TRIPS. 45 Many of the countries make amendments in their domestic laws to use compulsory licensing. If it is already in process the TRIPS waiver to some extent will help in access to medicine easier and quicker. The developed countries should come forward and support the decision of TRIPS waiver in session WTO council meeting.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Biographies
Talat Chaudhary is a Fifth year student in the University Jamia Millia Islamia in Faculty of law. She is currently enrolled in Bachelor's degree B.A. LL.B. that includes study of law with subjects of humanities. Her current field is based on international law and she is interested in working in the area of Human rights and international law.
Arshi Chaudhary is a First year student in the University Jamia Hamdard in Department of Medicine. She is currently enrolled in Master's degree in the same department of medicine. She did her Bachelor's in medicine from the same university. Her current field of work is in Quality Assurance of Pharmaceutical products. She is interested in working in the area of Pharmaceutics.
Chaudhary, T. , Chaudhary, A. (2021). TRIPS waiver of COVID‐19 vaccines: Impact on pharmaceutical industry and what it means to developing countries. The Journal of World Intellectual Property. 24, 447–454. 10.1111/jwip.12198
ENDNOTES
Bruce Lehman, The Pharmaceutical Industry and the Patent System (International Intellectual Property Institute, 2003), 2–14. https://users.wfu.edu
The policy context for action on innovation and access, trilateral study, WTO. Retrieved from www.wto.in
Basma Ibrahim, Implications of WTO‐TRIPS Agreement from a National Innovation Systems Perspective (The School of Public Policy and Administration, 2003).
Ibid.
Article 7 of the TRIPS agreement. WTO.
Ibid.
Thomas Cottier, The Doha Waiver and its Effects on the Nature of the TRIPS System and on Competition Law: The Impact of Human Rights, Swiss National Centre of competence in Research. Working Paper no. 2006.
Prabhas Ranjan, The Case for Waiving Intellectual Property Protection for Covid‐19 Vaccines (Observer Research Foundation, 2021).
Ronald Lobante and Mira Johri, COVID‐19 Drug and Vaccine Patents Are Putting Profit Before People (2020).
Jessica L Greenbaum, 'Trips and Public Health: Solutions for Ensuring Global Access to Essential AIDS Medication in the Wake of Paragraph 6 Wake of paragraph 6 Waiver' (2008) 25 JCHLP 142.
Donald Harris, 'TRIPS After Fifteen Years: Success or Failure, as Measured by Compulsory Licensing' (2011) 18 JIPL. 369.
Haochen Sun, 'The Road to Doha & Beyond: Some Reflection on TRIPS Agreement and access to Public Health' (2004) 15 EJIL 123.
Ibid.
Sanjana Ganguly, Compulsory Licensing. IPTSE www.iptse.com
Benjamin Coriat, Fabienne Orsi and Cristina d'Almeida, 'TRIPS and the International Public Health Controversies: Issues And Challenges' (2006) 15 ICC 1033. https://doi.org/10.1093/icc/dtl029
Ibid.
Weinian Hu, Compulsory Licensing and Access to Future COVID 19 Vaccines, CEPS Research Report no. 2020 (2020).
Ibid.
Frederick M Abbott, 'The Doha Declaration on TRIPS and Public Health' (2002) 5 JIEL 469.
Kenneth C Shadlen, Patents and Pills, Power and Procedure: The North‐South Politics of Public Health in the WTO, Studies in Comparative International Development (Vol. 39, 2002).
Bradly Condon and Tapen Sinha, 'Global Diseases, Global Patents and Differential Treatment in WTO Law: Criteria for Suspending Patent Obligations in Developing Countries' (2006) 26 NJILB 1.
Alan O Sykes, TRIPs, Pharmaceuticals, Developing Countries, and the Doha "Solution", John M. Olin Program in Law and Economics Working Paper No. 140 (2002).
Ibid.
Supra note 22.
Kenneth C Shadlen, 'Patents and Pills, Power and Procedure: The North‐South Politi of Public Health in the WTO' (2004) 39 SCID 76.
Salla Sariola, 'Intellectual Property Rights Need to be Subverted to Ensure Global Vaccine Access' BMJ GHJ <www.bmj.com> accessed 24 June 2021.
Ibid.
Salla Sariola, 'Intellectual Property Rights Need to be Subverted to Ensure Global Vaccine Access' (2021) BMJ GHJ <www.bmj.com> accessed 24 June 2021.
Ibid.
Ibid.
Sushil Vachani and N. Craig Smith, Lessons from Pricing of AIDS Drugs in Developing Countries. Working Paper No. 03‐703b. London Business School (2004) <www.journals.sagepub.com> accessed 19 June 2021.
Ibid.
Human Rights Watch, Seven Reasons the EU is Wrong to Oppose the TRIPS Waiver (2021). <www.hrw.org> accessed 26 June 2021.
Ibid.
Richard Wilder and Eric M Solovy, The Development of Medicines for Developing Country Diseases: The Role of Intellectual Property. WIPO (2004) <www.wipo.int.com> accessed 21 June 2021.
Medicines Sans Fronteires (MSF), Access Campaign. WTO COVID‐19 TRIPS Waiver Proposal (2021) <www.msfaccess.org> accessed 25 June 2021.
Ibid.
Supra note, 29.
Infa.
AFP, Vaccine Patent Waiver Sets Dangerous Precedent, Says Big Pharma (2021) <www.deccanherald.com> accessed 22 June 2021.
Ashok Dhingra, Waiver Under TRIPS Agreement Needed More Urgently Now (2021). Mondaq <www.mondaq.com accessed 24 June 2021.
Guy Martin, TRIPS Waiver: Is the US Backing a Cause For Concern For Pharma? The Pharmaletter (2021). <www.pharmaletter.com> accessed 25 June 2021.
Ibid.
Art Gonzales, The TRIPS waiver on Covid‐19 Pharmaceuticals and the Big Pharma (2021). IBON <www.IBON.org> accessed 24 June 2021.
Catherine Saez, Main Recommendations of UN High‐Level Panel on Access to Medicines Presented at WTO (2017). Intellectual Property Watch <www.ip-watch.org> accessed 28 June 2021.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from corresponding author upon reasonable request.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from corresponding author upon reasonable request.


